Naming Compounds Names and Formulas Writing Chemical Formulas











- Slides: 26

Naming Compounds Names and Formulas


Writing Chemical Formulas n Chemical Formula: n n 1 – what elements 2 - ratios in compound Metals(positive ions) written first Polyatomic Ions: n n n group of atoms that act as an element has its own ionic charge Always look on the Data Table for n n name and ion charge

Ion Charge and the Formulas of Ionic Compounds n Law of Definite Proportions: n n n a specific compound elements are always in definite proportions; integers no decimals or fractions Ex: H 2 O – always 2 hydrogen atoms for one oxygen atom Ionic compounds: n n metal + non-metal Electron(s) transferred bonded by electrical force (opp. charges attract) electrically neutral

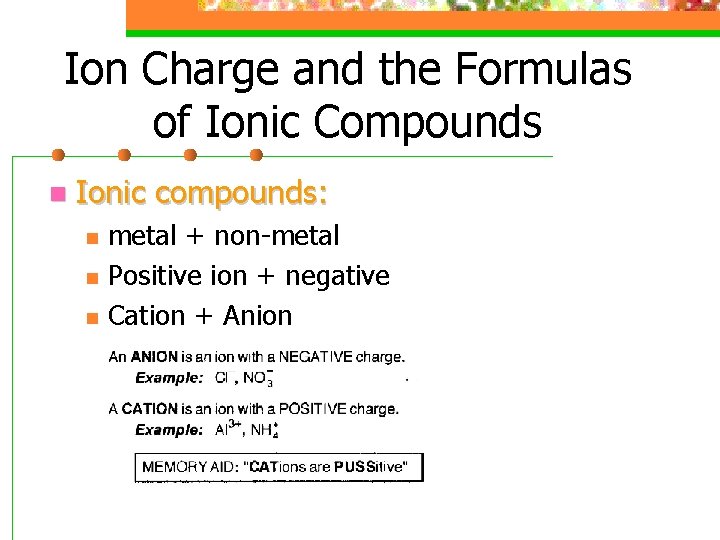
Ion Charge and the Formulas of Ionic Compounds n Ionic compounds: n n n metal + non-metal Positive ion + negative Cation + Anion

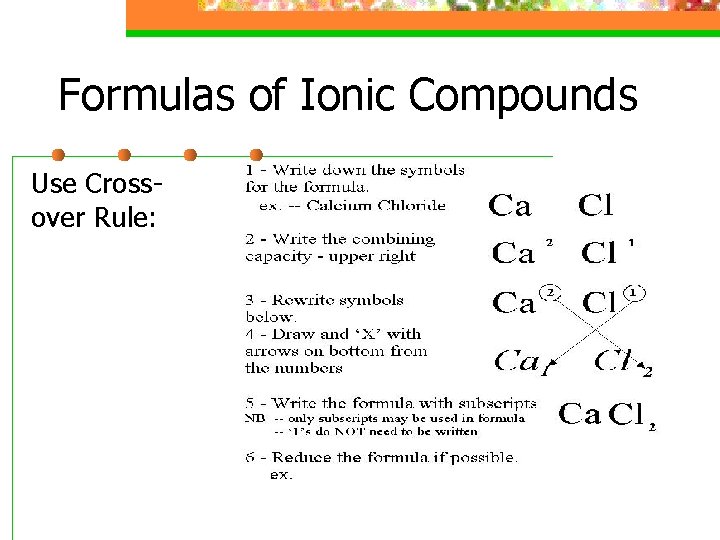
Formulas of Ionic Compounds Use Crossover Rule:

Ion Charge and the Formulas of Ionic Compounds Formulas for Ionic Compounds with Polyatomic Ions: n Use crossover rule but n n n Polyatomic Ions --- MUST BE KEPT TOGETHER Use brackets around polyatomic groups if there is more than 1

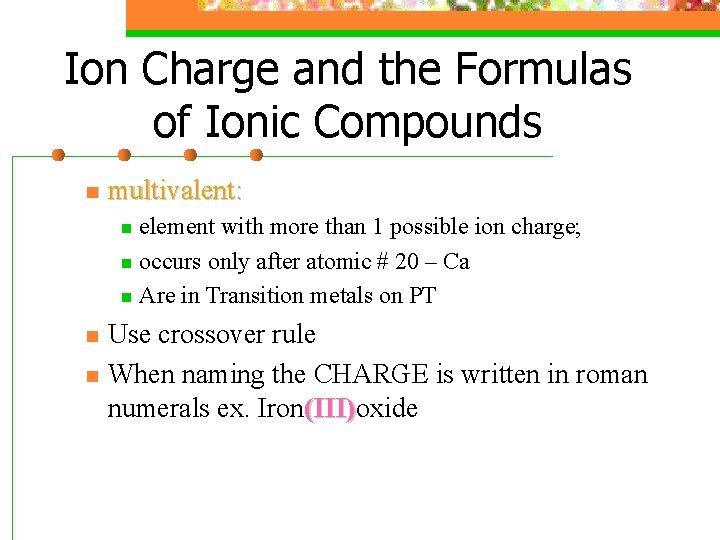
Ion Charge and the Formulas of Ionic Compounds n multivalent: element with more than 1 possible ion charge; n occurs only after atomic # 20 – Ca n Are in Transition metals on PT n n n Use crossover rule When naming the CHARGE is written in roman numerals ex. Iron(III)oxide (III)

Naming Ionic Compounds n Rules for Naming Ionic Compounds: n n Metal(pos) ion written 1 st name spelled same in lower case non-metal ion written 2 nd given suffix “–ide”

Naming Ionic Compounds n Naming Polyatomic Ions: n n n pos. polyatomic ions written 1 st neg. polyatomic ions written 2 nd; name of ion not changed Use the data table Do Exercise #4

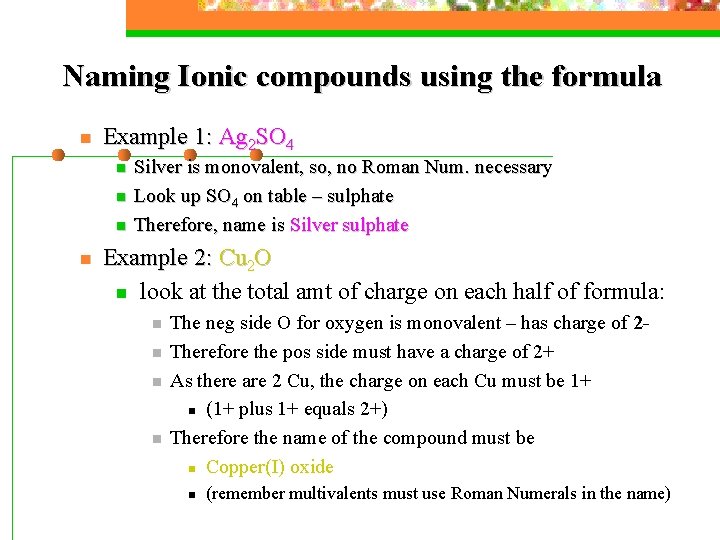
Naming Ionic compounds using the formula n Example 1: Ag 2 SO 4 n n Silver is monovalent, so, no Roman Num. necessary Look up SO 4 on table – sulphate Therefore, name is Silver sulphate Example 2: Cu 2 O n look at the total amt of charge on each half of formula: n n The neg side O for oxygen is monovalent – has charge of 2 Therefore the pos side must have a charge of 2+ As there are 2 Cu, the charge on each Cu must be 1+ n (1+ plus 1+ equals 2+) Therefore the name of the compound must be n Copper(I) oxide n (remember multivalents must use Roman Numerals in the name)

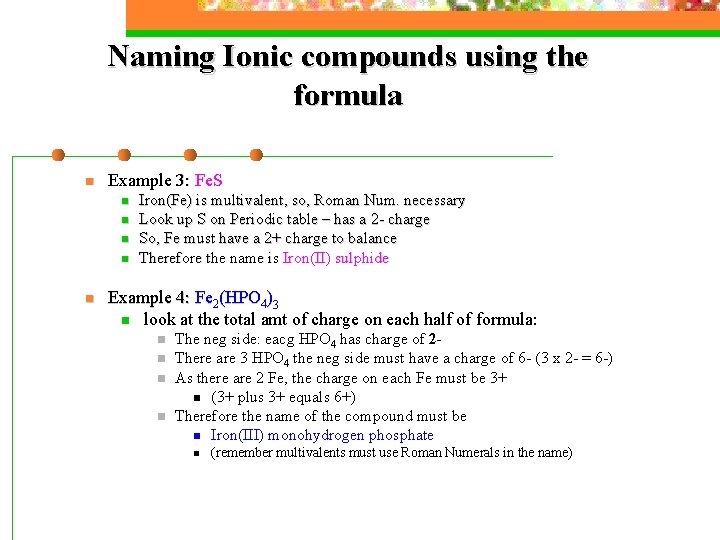
Naming Ionic compounds using the formula n Example 3: Fe. S n n n Iron(Fe) is multivalent, so, Roman Num. necessary Look up S on Periodic table – has a 2 - charge So, Fe must have a 2+ charge to balance Therefore the name is Iron(II) sulphide Example 4: Fe 2(HPO 4)3 n look at the total amt of charge on each half of formula: n n The neg side: eacg HPO 4 has charge of 2 There are 3 HPO 4 the neg side must have a charge of 6 - (3 x 2 - = 6 -) As there are 2 Fe, the charge on each Fe must be 3+ n (3+ plus 3+ equals 6+) Therefore the name of the compound must be n Iron(III) monohydrogen phosphate n (remember multivalents must use Roman Numerals in the name)

Naming Ionic compounds using the formula n Try It: n n n n n 1 2 3 4 5 6 7 8 9 – – – – – Ag 3 PO 4 Al 2(SO 4)3 Fe 2 S 3 Cu. Cl (NH 4)2 CO 3 VCl 3 Hg 2 CO 3 Cu. SO 4 (NH 4)2 S n Answers: n n n n n 1 2 3 4 5 6 7 8 9 – – – – – Silver phosphate Aluminum sulphate Iron(III) sulphide Copper(I) chloride Ammonium carbonate Vanadium(III) chloride Mercury(I) carbonate copper(II) sulphate Ammonium sulphide

Naming Ionic compounds using the formula Do Exercise #5 n Do Handout: Writing formulas Quiz n n n Do Handout: Formula Writing n n (exercise in Names -> Formulas) Do Handout: Inorganic Nomenclature Test (may do as Test or Quiz)

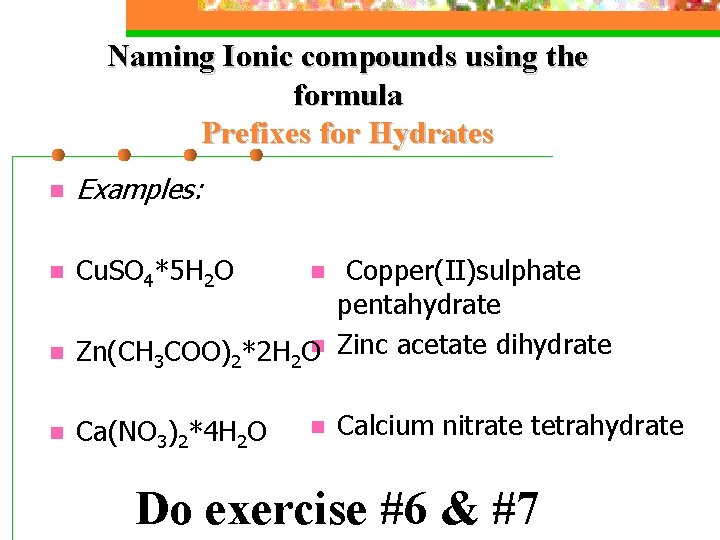
Naming Ionic compounds using the formula Hydrates n n n When a crystal of an ionic compound is grown by evaporation from aqueous solution, frequently it is found that the crystal structure will include water molecules Molecules which include water molecules are called HYDRATES Use prefixes to tell how many water molecules are attached;

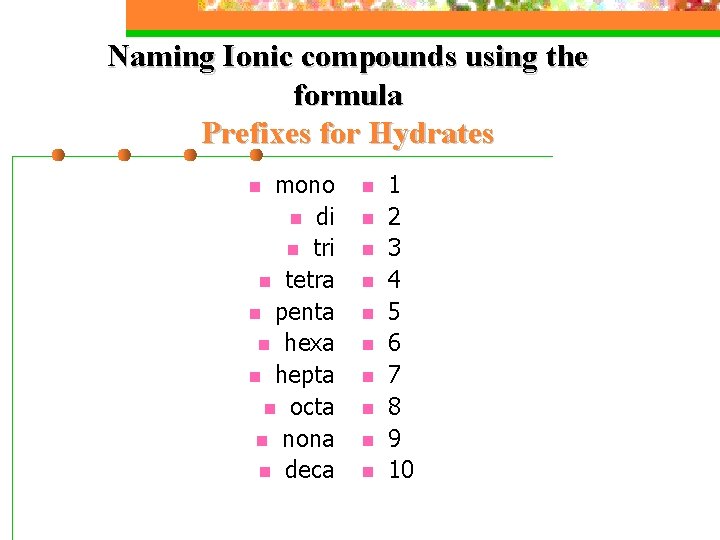
Naming Ionic compounds using the formula Prefixes for Hydrates mono n di n tri n tetra n penta n hexa n hepta n octa n nona n deca n n n 1 2 3 4 5 6 7 8 9 10

Naming Ionic compounds using the formula Prefixes for Hydrates n Examples: n Cu. SO 4*5 H 2 O n n Copper(II)sulphate pentahydrate Zn(CH 3 COO)2*2 H 2 On Zinc acetate dihydrate n Ca(NO 3)2*4 H 2 O n Calcium nitrate tetrahydrate Do exercise #6 & #7

Naming Covalent Compounds n Unlike Ionic compounds there are several various combinations of elements possible. n n n Ionic example: sodium and chlorine always forms Na. Cl It is the ONLY possible combination This combination is called a formula unit; NOT a molecule Covalent example: carbon and oxygen

Naming Covalent Compounds n Unlike Ionic compounds, Covalent compounds has numerous possibilities in how the elements can combine: n n n Example: carbon and oxygen Can form CO or CO 2 or CO 3 Each has unique properties: CO will kill you while CO 2 will not You cannot all these variations carbon oxide A different naming system is necessary

Naming Covalent Compounds n n n Prefix naming system – use prefixes to name the various covalent molecules Note: these are molecules not formula units as with Ionic compounds Examples of naming covalent molecules: n n carbon and oxygen can form CO or CO 2 or CO 3 CO is named carbon monoxide CO 2 is named carbon dioxide CO 3 has a special name carbonate – a polyatomic structure

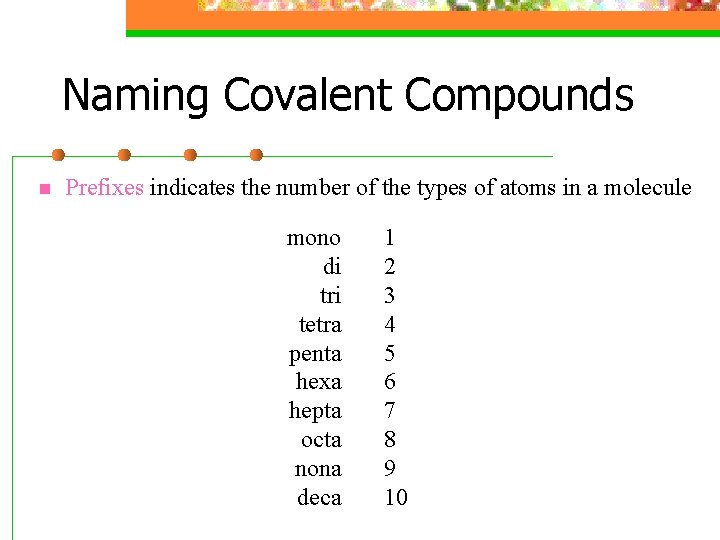
Naming Covalent Compounds n Prefixes indicates the number of the types of atoms in a molecule mono di tri tetra penta hexa hepta octa nona deca 1 2 3 4 5 6 7 8 9 10

Naming Covalent Compounds n Rules for prefixes: n n n 1 - don’t use mono for the first element if there is only one; 2 - always use mono for second element if there is only one atom in the molecule 3 - mono drops the 2 nd “o” as in carbon monoxide 4 - second element ends with ‘ide’ 5 – add the appropriate prefix according to number of atoms present in molecule

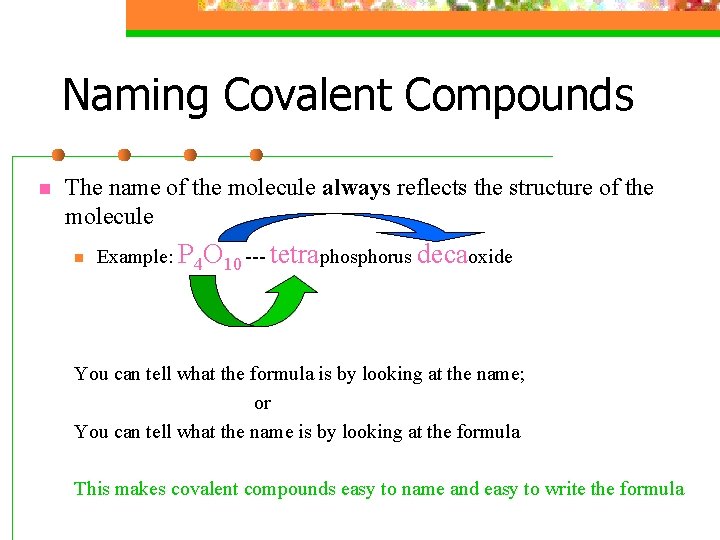
Naming Covalent Compounds n The name of the molecule always reflects the structure of the molecule n Example: P 4 O 10 --- tetraphosphorus decaoxide You can tell what the formula is by looking at the name; or You can tell what the name is by looking at the formula This makes covalent compounds easy to name and easy to write the formula

Naming Covalent Compounds n REMEMBER: always determine to see if the compound is n. IONIC or n. COVALENT n Then follow the appropriate rules n Do Exercise #8 & 9 Handout: Nomenclature Flow Chart

Naming Acids n 2 types of acids: n 1 -- has NO oxygen name begins with ‘hydro’ ends with ‘ic’ n n n examples: n HF – hydrogen fluoride hydrofluoric acid HCl – hydrogen chloride hydrochloric acid n n

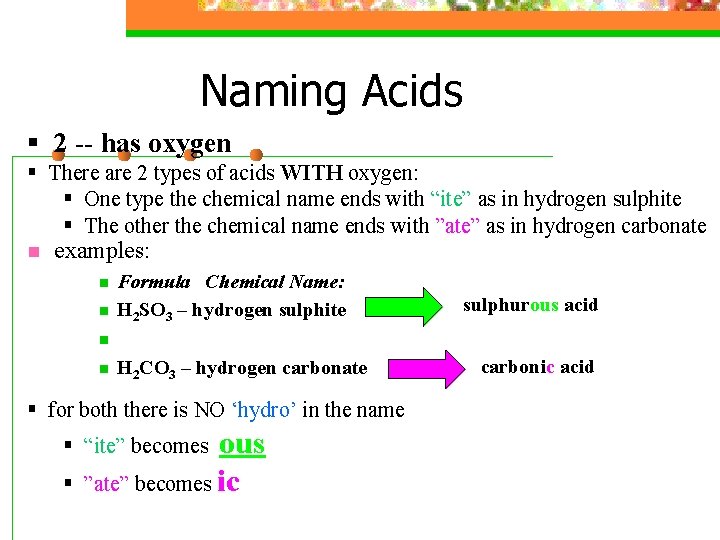
Naming Acids § 2 -- has oxygen § There are 2 types of acids WITH oxygen: § One type the chemical name ends with “ite” as in hydrogen sulphite § The other the chemical name ends with ”ate” as in hydrogen carbonate n examples: n n Formula Chemical Name: H 2 SO 3 – hydrogen sulphite sulphurous acid n n H 2 CO 3 – hydrogen carbonate § for both there is NO ‘hydro’ in the name ous ”ate” becomes ic § “ite” becomes § carbonic acid