Ionic Nomenclature Naming Ionic Compounds Ionic Compounds Review







- Slides: 15

Ionic Nomenclature Naming Ionic Compounds

Ionic Compounds Review n Ionic compounds are made of positive and negative ions. ¡ n Also known as “salts”. Polyatomic ions are groups of atoms that behave as a single ion: ¡ Examples: n n n CO 3 -2 CN-1 SO 4 -2

Naming Ionic Compounds n Two types of ionic compounds: ¡ Binary, contains 2 elements n ¡ Na. Cl, Mg. Br 2, Fe 2 O 3 Ternary, contains 3 elements n n Two of the elements are in a polyatomic ion Na. NO 3, NH 4 Cl, Cr 2(SO 4)3

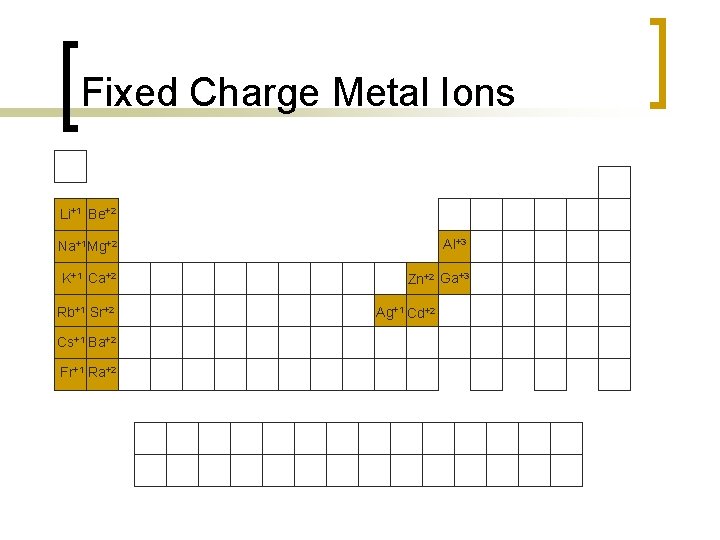
Naming Binary Ionic Compounds n Some metal ions have only one possible charge: ¡ ¡ ¡ n For fixed charge cations, name as element. ¡ ¡ n Group 1 A metal ions all have +1 charge. Group 2 A metal ions all have +2 charge. Ag+1, Zn+2, Cd+2, Al+3, Ga+3 Na+1 = sodium ion Ca+2 = calcium ion Ag+1 = silver ion Ga+3 = gallium ion Name anion as stem of element’s name followed by suffix –ide. ¡ ¡ ¡ Cl-1 = chloride ion S-2 = sulfide ion N-3 = nitride ion

Fixed Charge Metal Ions Li+1 Be+2 Na+1 Mg+2 Al+3 K+1 Ca+2 Zn+2 Ga+3 Rb+1 Sr+2 Cs+1 Ba+2 Fr+1 Ra+2 Ag+1 Cd+2

Naming Binary Ionic Compounds n n n n Na. Cl KBr Mg. F 2 Ca. O Ag. I Al 2 S 3 Zn 3 P 2 sodium chloride potassium bromide magnesium fluoride calcium oxide silver iodide aluminum sulfide zinc phosphide

Naming Binary Ionic Compounds n Most metals can form multiple cations. ¡ ¡ n Iron can form Fe+2 ions or Fe+3 ions. Copper can form Cu+1 ions or Cu+2 ions. For variable charge cations, name as element w/ Roman numeral in ( ) to indicate charge. ¡ ¡ ¡ Fe+2 = iron(II) ion Fe+3 = iron(III) ion Cu+1 = copper(I) ion Cu+2 = copper(II) ion Pb+2 = lead(II) ion Pb+4 = lead(IV) ion

Variable Charge Metal Ions

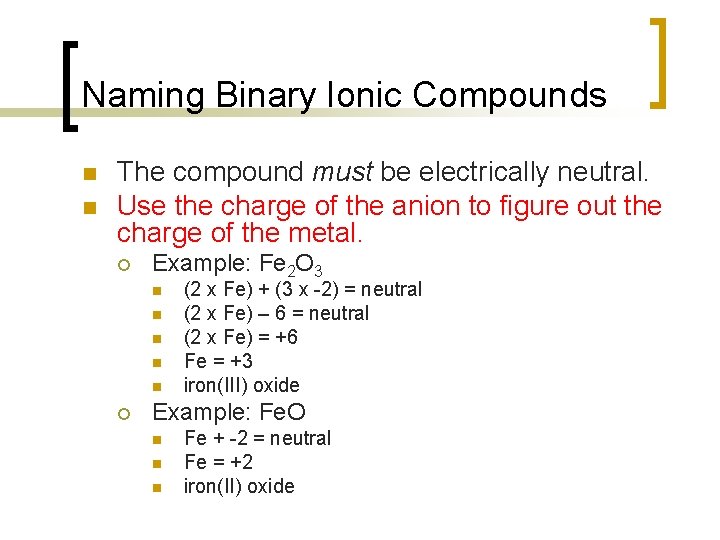
Naming Binary Ionic Compounds n n The compound must be electrically neutral. Use the charge of the anion to figure out the charge of the metal. ¡ Example: Fe 2 O 3 n n n ¡ (2 x Fe) + (3 x -2) = neutral (2 x Fe) – 6 = neutral (2 x Fe) = +6 Fe = +3 iron(III) oxide Example: Fe. O n n n Fe + -2 = neutral Fe = +2 iron(II) oxide

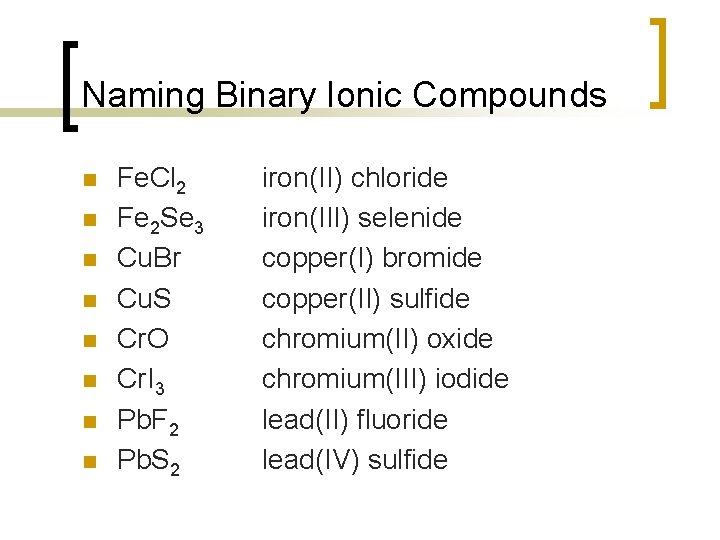
Naming Binary Ionic Compounds n n n n Fe. Cl 2 Fe 2 Se 3 Cu. Br Cu. S Cr. O Cr. I 3 Pb. F 2 Pb. S 2 iron(II) chloride iron(III) selenide copper(I) bromide copper(II) sulfide chromium(II) oxide chromium(III) iodide lead(II) fluoride lead(IV) sulfide

Naming Ternary Ionic Compounds n n Name metal as you would for a binary, with charge as a Roman numeral if necessary. Name polyatomic anions as themselves. Know these: ¡ ¡ ¡ CO 3 -2 = carbonate NO 3 -1 = nitrate PO 4 -3 = phosphate SO 4 -2 = sulfate OH-1 = hydroxide ¡ ¡ ¡ Cl. O-1 = hypochlorite Cl. O 3 -1 = chlorate C 2 H 3 O 2 -1 = acetate CH 3 COO-1 = acetate CN-1 = cyanide

Naming Ternary Ionic Compounds n Only one polyatomic cation: ¡ n NH 4+1 = ammonium Name it, then name the anion properly.

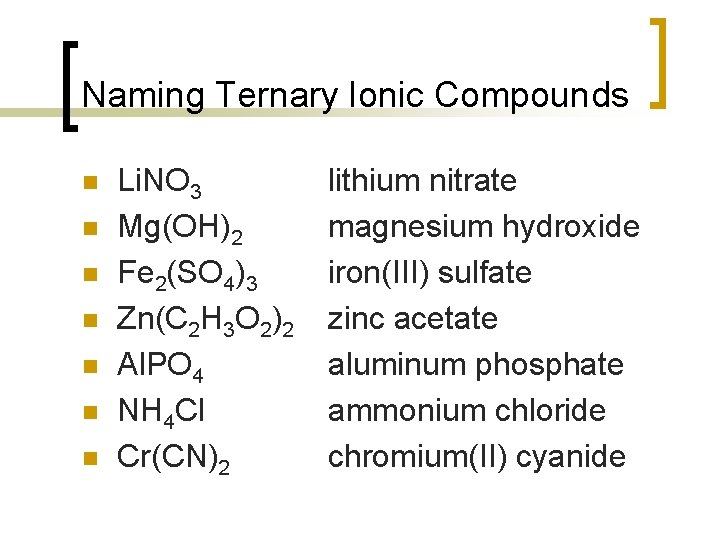
Naming Ternary Ionic Compounds n n n n Li. NO 3 Mg(OH)2 Fe 2(SO 4)3 Zn(C 2 H 3 O 2)2 Al. PO 4 NH 4 Cl Cr(CN)2 lithium nitrate magnesium hydroxide iron(III) sulfate zinc acetate aluminum phosphate ammonium chloride chromium(II) cyanide

Writing Formulas From Names n To write an ionic formula from a name, write the ions with their proper charges, then criss-cross. ¡ n Write the formula for iron(III) hydroxide ¡ ¡ ¡ n Don’t forget that polyatomic ions need parentheses if there’s more than one of them. iron(III) = Fe+3 hydroxide = OH-1 iron(III) hydroxide Fe+3 + OH-1 Fe(OH)3 Write the formula for potassium sulfate ¡ ¡ ¡ potassium = K+1 sulfate = SO 4 -2 potassium sulfate K+1 + SO 4 -2 K 2 SO 4

Writing Formulas From Names n n n calcium carbonate sodium oxide aluminum cyanide potassium acetate copper(II) hydroxide gold(III) sulfide Ca+2 + CO 3 -2 Na+ + O-2 Al+3 + CN-1 K+1 + C 2 H 3 O 2 -1 Cu+2 + OH-1 Au+3 + S-2 Ca. CO 3 Na 2 O Al(CN)3 KC 2 H 3 O 2 Cu(OH)2 Au 2 S 3