Ionic Compounds Chapter 5 Ionic Compounds Ionic compounds

























- Slides: 35

Ionic Compounds Chapter 5

Ionic Compounds § Ionic compounds are made up by the chemical combination of metallic and non-metallic elements. § Most rocks, minerals and gemstones are ionic compounds.

Properties of Ionic Compounds § Think of the properties of rocks, bricks, and table salt. What properties do they share? § Have high melting and boiling temperatures. § Are hard but brittle § They also: § Do NOT conduct electricity in the solid state § They will only conduct electricity if they are melted or dissolved in water

Structure of ionic compounds § The physical properties of ionic compounds are very different from metals. § The structure of ionic compounds must therefore be very different from those present in metals.

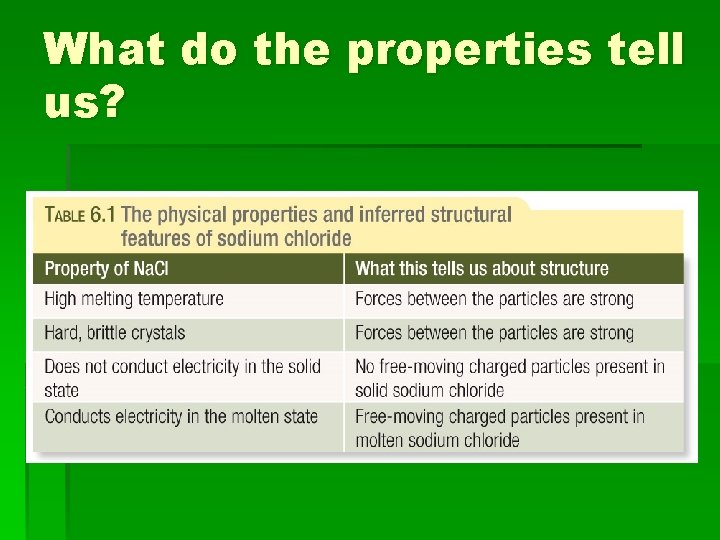
What do the properties tell us?

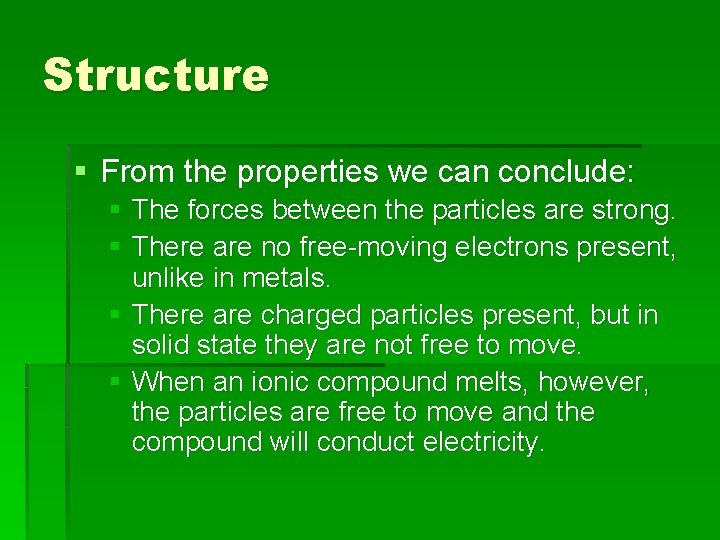
Structure § From the properties we can conclude: § The forces between the particles are strong. § There are no free-moving electrons present, unlike in metals. § There are charged particles present, but in solid state they are not free to move. § When an ionic compound melts, however, the particles are free to move and the compound will conduct electricity.

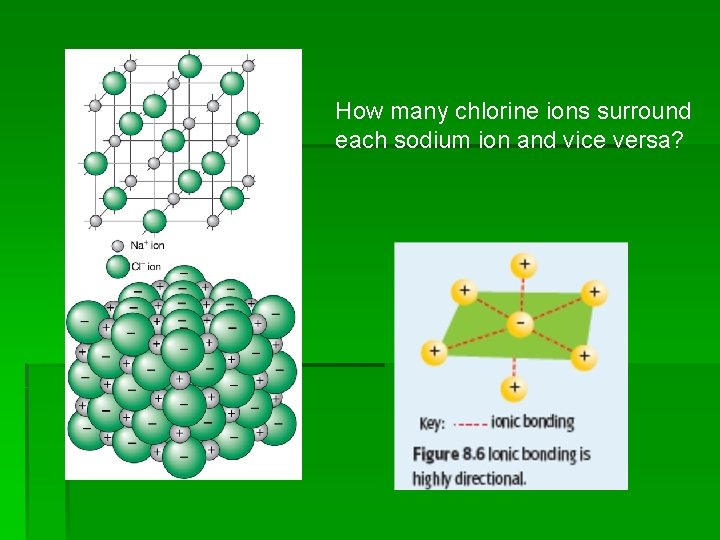
The ionic bonding model Chemists believe that when metallic and non-metallic atoms react to form ionic compounds the following steps occur: § Metal atoms lose electrons to non-metallic atoms and become positively charged metal ions. § Non-metal atoms gain electrons from the metal atoms and so become negatively charged non-metal ions. § Large numbers of positive and negative ions formed in this way then combine to form a three-dimensional lattice. § The three dimensional lattice is held together strongly by electrostatic forces of attraction between positive and negative ions. This electrostatic force is called ionic bonding.

How many chlorine ions surround each sodium ion and vice versa?

Using the ionic bonding model to explain the properties of sodium chloride

High Melting Temperature § Ever noticed that when you eat fish and chips the food may be hot but the salt does not melt. § This is because to melt and ionic solid energy must be provided to allow the ions to break free and move. § Na. Cl has a high melting temp, this indicates a large amount of energy is needed to reduce the electrostatic attraction between the oppositely charged ions and allow them to move freely.

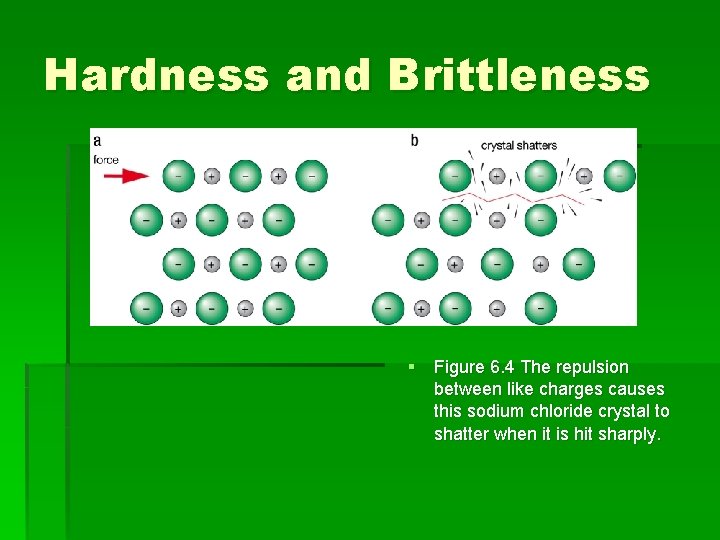
Hardness and Brittleness § Unlike metals ionic compounds are not malleable. They break when beaten. § A force can disrupt the strong electrostatic forces holding the lattice in place. § A sodium chloride crystal cannot be scratched easily but if a strong force (a hammer blow) is applied it will shatter. § This is because the layers of ions will move relative to each other due to the force. § During this movement, ions of like charge will become adjacent to each other. Resulting in repulsion

Hardness and Brittleness § Figure 6. 4 The repulsion between like charges causes this sodium chloride crystal to shatter when it is hit sharply.

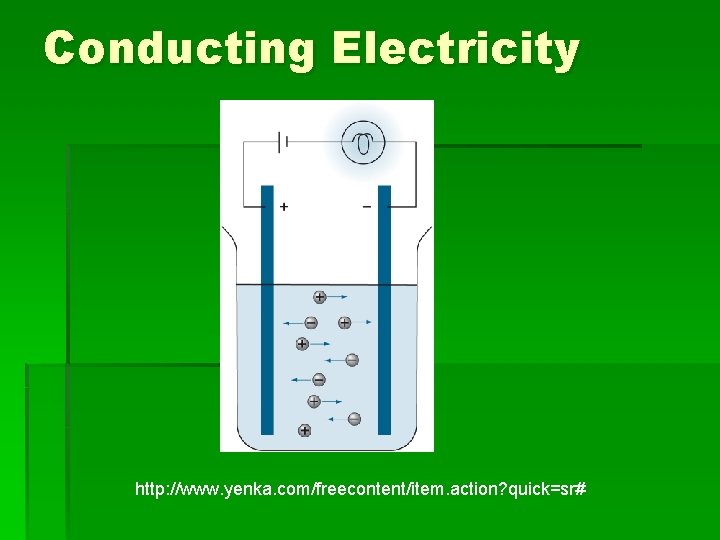
Electrical Conductivity § In the solid form, ions in sodium chloride are held in the crystal lattice and are not free to move so cannot conduct electricity. § When the solid melts the ions are free to move. § The movement of these charged particles to an electrode completes an electrical circuit. § In a similar way, when sodium chloride dissolves in water, the ions separate and are free to move towards the opposite charge.

Conducting Electricity http: //www. yenka. com/freecontent/item. action? quick=sr#

Reactions of metals with non-metals § Metallic atoms have low ionisation energies and low electronegativities. § Non-metallic atoms have high ionisation energies and high electronegativities. § In other words metallic atoms lose electrons easily and non-metallic atoms gain electrons easily.

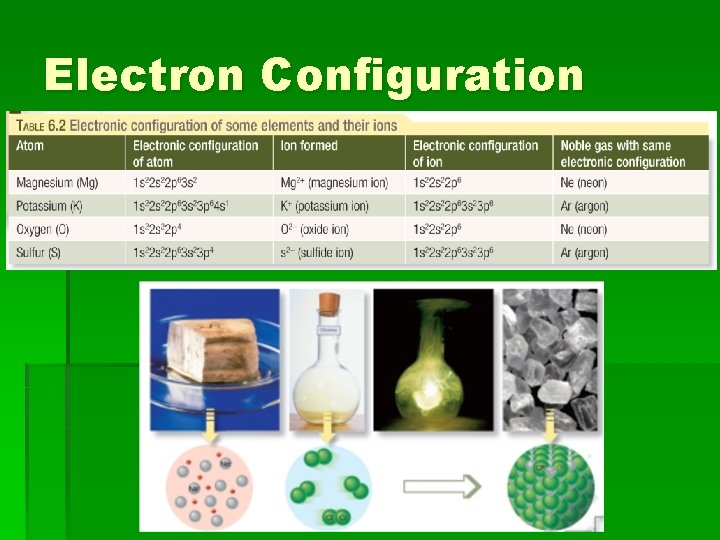
Ionic Compounds § So the metal atoms lose an electron to the non-metal atoms. § In doing so, both atoms will often achieve the electronic configuration of the nearest noblest gas, which is particularly stable.

Sodium Chloride § When sodium reacts with chlorine: § Na atom (1 s 2 2 p 6 3 s 1) loses an electron to become 1 s 2 2 p 6 (the same as Neon) § Cl atom (1 s 2 2 p 6 3 s 1 3 p 5) gains an electron to become 1 s 2 2 p 6 3 s 1 3 p 6 (the same as argon)

Electron Configuration

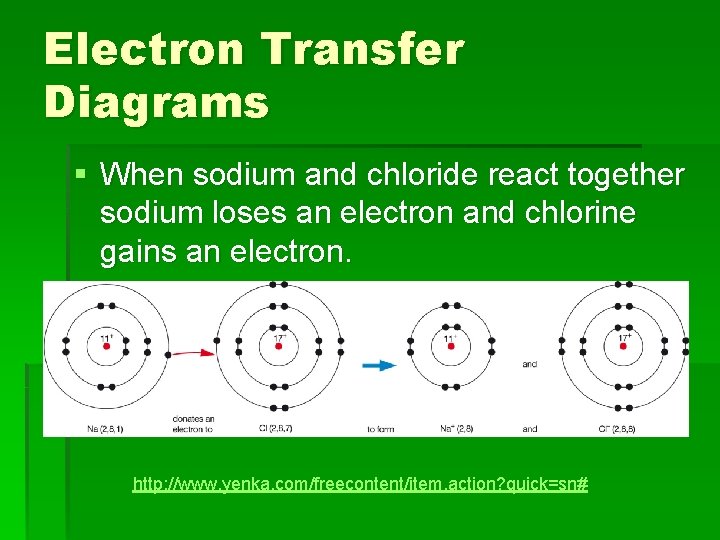
Electron Transfer Diagrams § When sodium and chloride react together sodium loses an electron and chlorine gains an electron. http: //www. yenka. com/freecontent/item. action? quick=sn#

Sodium Chloride What is happening: § Chlorine molecules splitting into separate chlorine atoms § Electrons being transferred from sodium atoms to chlorine atoms – positively charged sodium and negatively charged chlorine ions are being formed. § Sodium and chloride ions combining to form a three dimensional lattice.

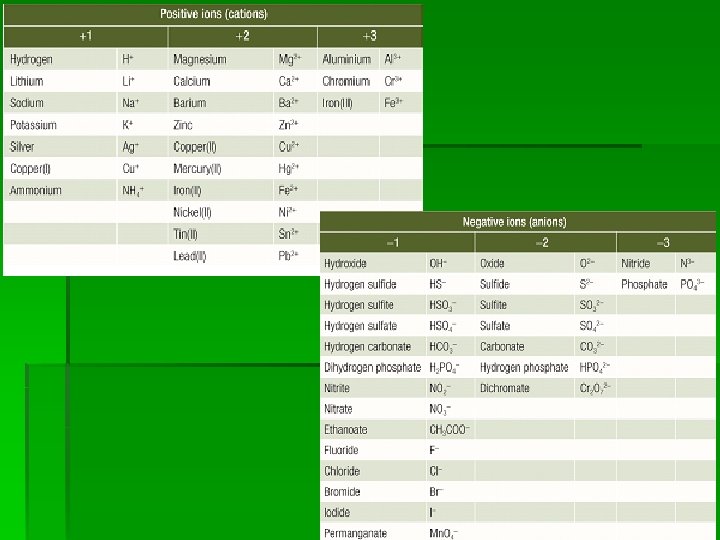
Notes: § When a non-metal atom gains one or more electrons, the name of the negative ion ends in –ide. § When a metal atom loses one or more electrons the name of the positive ion is the same as the metal and is always named first. § For example: sodium chloride

Electrovalency § The charge on an ion is known as its electrovalency. § That is the little positive or negative number to the top right of a chemical symbol. § Sodium has an electrovalency of +1 whilst chlorine has an electrovalency of -1 § Na+1 and Cl-1

Magnesium Oxide § What are the electron configurations for Magnesium and Oxygen? § How many electrons does magnesium need to lose to get a full outer shell? § How many electrons does oxygen need to gain to get a full outer shell? § Draw an electron transfer diagram. § What is the electrovalency of a magnesium ion and an oxide ion?

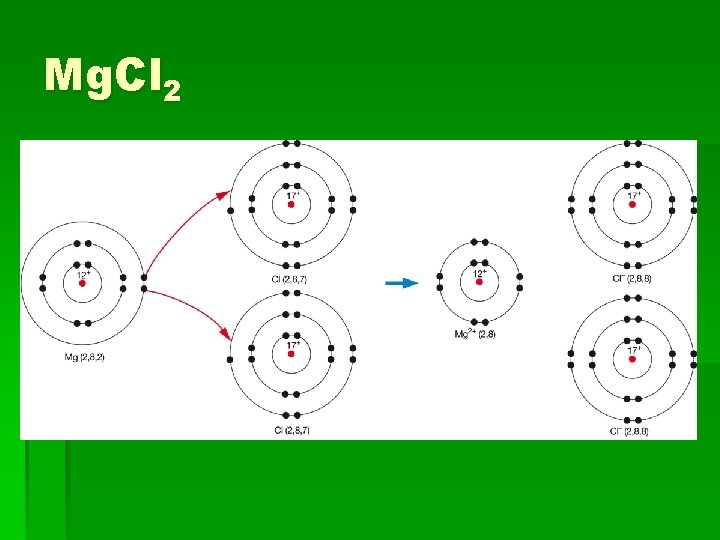
Magnesium Chloride § What are the electron configurations for Mg and Cl? § So a Mg atom will have a stable outer shell if 2 electrons are removed. § A Cl atom only needs to gain one electron. § So how can this work? http: //www. yenka. com/freecontent/item. action? quick=so#

Mg. Cl 2

Your Turn § § Page 100 Question 6 Question 7 Question 8

Chemical Formulas § Almost every compound in which a metal is combined with a non-metal displays ionic bonding. § The formulas of simple ionic compounds, such as Na. Cl and Mg. Cl 2 can be predicted from the electron configurations of the atoms.

Electrovalencies § Elements in groups 1 all have an electrovalency of +1 (they all have only one electron to lose) § Elements in group 17 all have an electrovalency of -1 § What about groups 2 and groups 16? § Does this formula work for all atoms?

Writing Formulas: Rules § Chemical formulas are part of the language of chemists. To understand use this language, you need to follow a number of fules.

Writing Formulas: Rules Simple Ions § The positive ion is place first in the formula, the negative ion is second. § For example, Kf, Cu. O § Positive and negative ions are combined so that the total number of positive charges is balanced by the total number of negative charges. § For example, Cu. S, Cu. Cl 2, Al. Cl 3 and Al 2 O 3 § When there are two or more of a particular ion in a compound, then in the chemical formula the number is written as a subscript after the chemical symbol. For example, Al 2 O 3


Polyatomic ions § § § Some ions contain more than one atom. These are called polyatomic ions. They include nitrate (NO 3 -) and hydroxide (OH-). What else? § If more than one of these ions is used to balance the charge of a compound, then it is placed in brackets with the required number written as a subscript after the brackets. For example Mg(NO 3)2 and Al(OH)3 § Brackets are not required for the formula of sodium nitrate Na. NO 3, where there is only one nitrate ion present for each sodium ion.

Different Electrovalencies § § § § Some elements form ions with different charges. Iron ions can have a charge of +2 or +3. In this situation you need to specify the electrovalency when naming the compound. This is done by placing a Roman numeral representing the electrovalency of the ion immediately after the metal in the name of the compound. For example Iron(II) chloride contains Fe 2+ ions and so the formula is Fe. Cl 2 Iron(III) chloride contains Fe 3+ ions and so the forumla is Fe. Cl 3

Your Turn § Page 102 § Question 9 - 12
