Chapter 18 Electrochemistry OxidationReduction REDOX Reactions in which

- Slides: 34

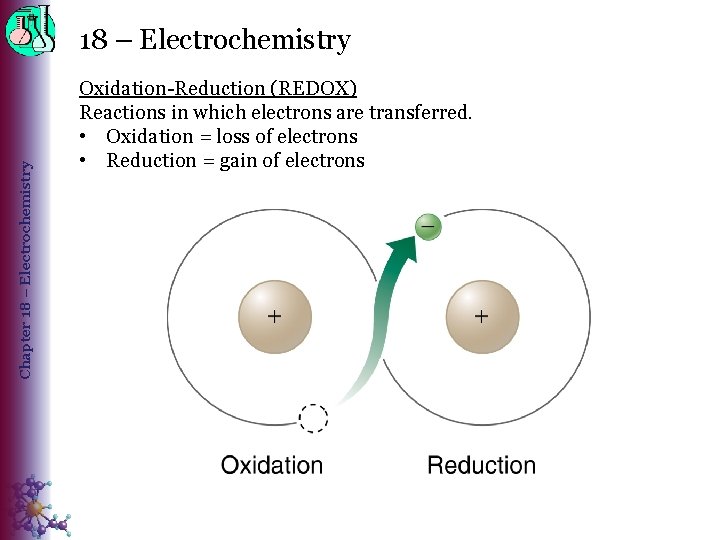
Chapter 18 – Electrochemistry Oxidation-Reduction (REDOX) Reactions in which electrons are transferred. • Oxidation = loss of electrons • Reduction = gain of electrons

Chapter 18 – Electrochemistry 18. 1 – Oxidation States & Redox Reactions Assigning Oxidation Numbers • The sum of the oxidation numbers is 0 for a neutral compound. • For a polyatomic ion, the sum of the oxidation numbers is the charge of a the ion. Uncombined Element 0 Monatomic Ions Charge of ion. Group 1 Elements +1 Group 2 Elements +2 Hydrogen (H) +1 when bonded to nonmetals -1 when bonded to metals Oxygen (O) -2 almost always -1 in peroxides (O 22 -) +2 with F in a binary compound Fluorine (F) -1

Chapter 18 – Electrochemistry 18. 1 – Oxidation States & Redox Reactions A redox reaction is a reaction in which oxidation numbers change • If one substance loses electrons, another substance must gain electrons Oxidation = loss of electrons • When a substance is oxidized, its oxidation number increases • The species that is oxidized is the REDUCING AGENT Reduction = gain of electrons • When a substance is reduced, its oxidation number decreases. • The species that is reduced is the OXIDIZING AGENT Mnemonics:

Chapter 18 – Electrochemistry 18. 1 – Oxidation States & Redox Reactions Balancing Equations are balanced primarily to ensure mass is conserved. • Electrons are part of that mass and must also be balanced. ◊ Mass and charge must both be conserved in a reaction. Half Reactions Method of balancing equations by separating the equation into 2 “half reactions”, one showing oxidation and one showing reduction Sn 2+(aq) + 2 Fe 3+(aq) → Sn 4+(aq) + 2 Fe 2+(aq) Oxidation = Sn 2+(aq) → Sn 4+(aq) + 2 e–(aq) Reduction = 2 Fe 3+(aq) + 2 e–(aq) → 2 Fe 2+(aq) • The number of electrons lost must equal the number of electrons gained.

18. 1 – Oxidation States & Redox Reactions Chapter 18 – Electrochemistry Balancing Redox Reactions* Redox Reactions may occur in acidic, basic, or neutral solution. • Same basic process for each solution type. (Basic has extra step. ) 1. Write skeleton equations for the oxidation and reduction half reactions. 2. For each half reaction: a. Balance the elements other than H and O. b. Add H 2 O to balance O atoms. c. Add H+ to balance H atoms. d. If balancing in basic conditions, then add OH- to neutralize any H+ and simplify. e. Add e- to balance charges; the sum of the charges should be the same on both sides. 3. Multiply the half-reactions by integers to equal the numbers of electrons in both half reactions. 4. Add the two half-reactions and simplify.

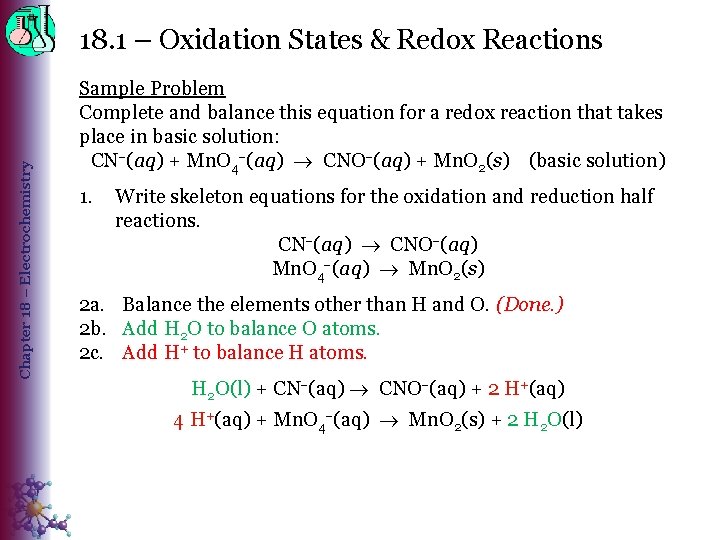
Chapter 18 – Electrochemistry 18. 1 – Oxidation States & Redox Reactions Sample Problem Complete and balance this equation for a redox reaction that takes place in basic solution: CN (aq) + Mn. O 4 (aq) CNO (aq) + Mn. O 2(s) (basic solution) 1. Write skeleton equations for the oxidation and reduction half reactions. CN (aq) CNO (aq) Mn. O 4 (aq) Mn. O 2(s) 2 a. Balance the elements other than H and O. (Done. ) 2 b. Add H 2 O to balance O atoms. 2 c. Add H+ to balance H atoms. H 2 O(l) + CN (aq) CNO (aq) + 2 H+(aq) 4 H+(aq) + Mn. O 4 (aq) Mn. O 2(s) + 2 H 2 O(l)

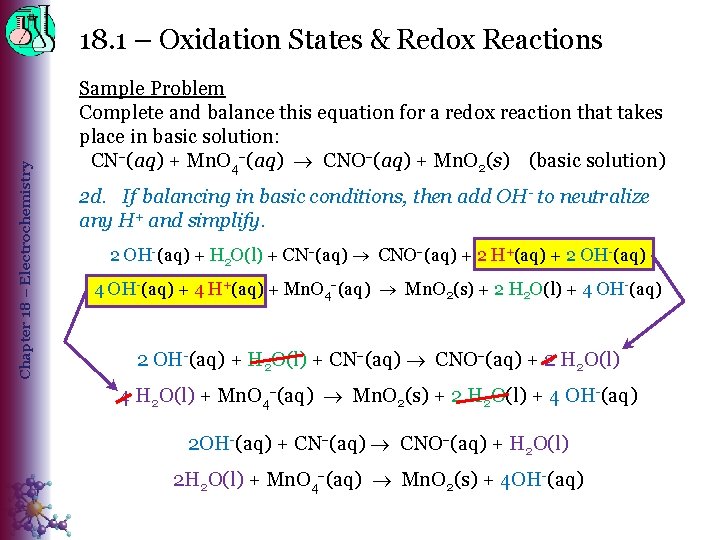
Chapter 18 – Electrochemistry 18. 1 – Oxidation States & Redox Reactions Sample Problem Complete and balance this equation for a redox reaction that takes place in basic solution: CN (aq) + Mn. O 4 (aq) CNO (aq) + Mn. O 2(s) (basic solution) 2 d. If balancing in basic conditions, then add OH- to neutralize any H+ and simplify. 2 OH-(aq) + H 2 O(l) + CN (aq) CNO (aq) + 2 H+(aq) + 2 OH-(aq) 4 OH-(aq) + 4 H+(aq) + Mn. O 4 (aq) Mn. O 2(s) + 2 H 2 O(l) + 4 OH-(aq) 2 OH-(aq) + H 2 O(l) + CN (aq) CNO (aq) + 2 H 2 O(l) 4 H 2 O(l) + Mn. O 4 (aq) Mn. O 2(s) + 2 H 2 O(l) + 4 OH-(aq) 2 OH-(aq) + CN (aq) CNO (aq) + H 2 O(l) 2 H 2 O(l) + Mn. O 4 (aq) Mn. O 2(s) + 4 OH-(aq)

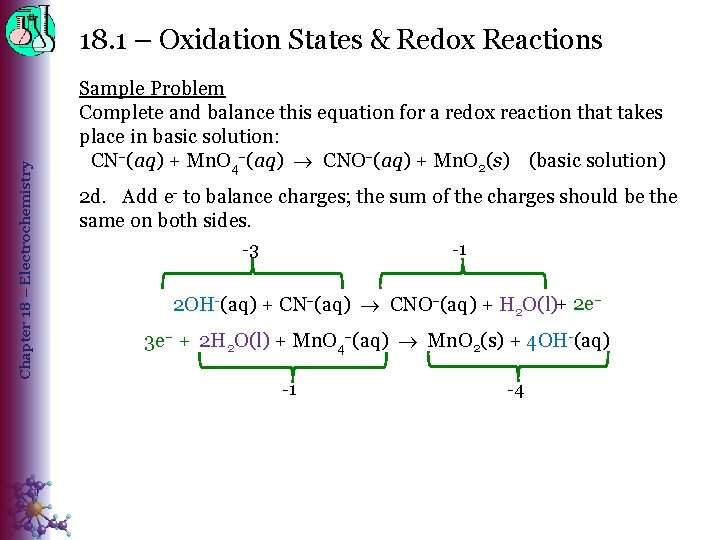
Chapter 18 – Electrochemistry 18. 1 – Oxidation States & Redox Reactions Sample Problem Complete and balance this equation for a redox reaction that takes place in basic solution: CN (aq) + Mn. O 4 (aq) CNO (aq) + Mn. O 2(s) (basic solution) 2 d. Add e- to balance charges; the sum of the charges should be the same on both sides. -3 -1 2 OH-(aq) + CN (aq) CNO (aq) + H 2 O(l)+ 2 e– 3 e– + 2 H 2 O(l) + Mn. O 4 (aq) Mn. O 2(s) + 4 OH-(aq) -1 -4

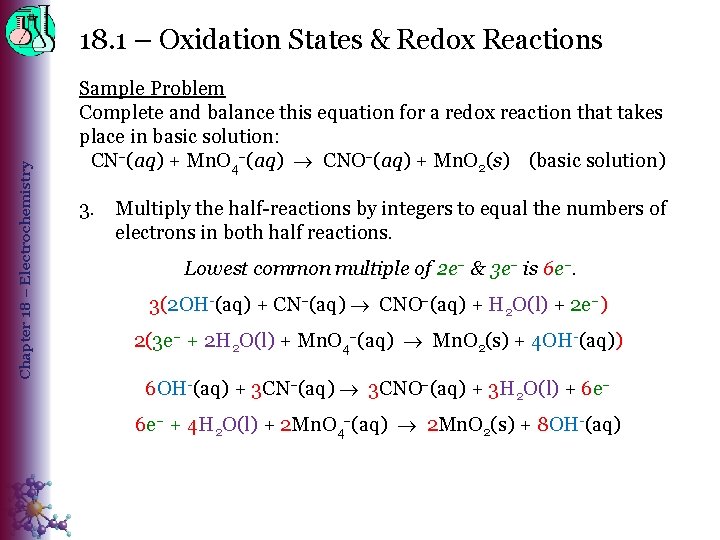
Chapter 18 – Electrochemistry 18. 1 – Oxidation States & Redox Reactions Sample Problem Complete and balance this equation for a redox reaction that takes place in basic solution: CN (aq) + Mn. O 4 (aq) CNO (aq) + Mn. O 2(s) (basic solution) 3. Multiply the half-reactions by integers to equal the numbers of electrons in both half reactions. Lowest common multiple of 2 e– & 3 e– is 6 e–. 3(2 OH-(aq) + CN (aq) CNO (aq) + H 2 O(l) + 2 e–) 2(3 e– + 2 H 2 O(l) + Mn. O 4 (aq) Mn. O 2(s) + 4 OH-(aq)) 6 OH-(aq) + 3 CN (aq) 3 CNO (aq) + 3 H 2 O(l) + 6 e– + 4 H 2 O(l) + 2 Mn. O 4 (aq) 2 Mn. O 2(s) + 8 OH-(aq)

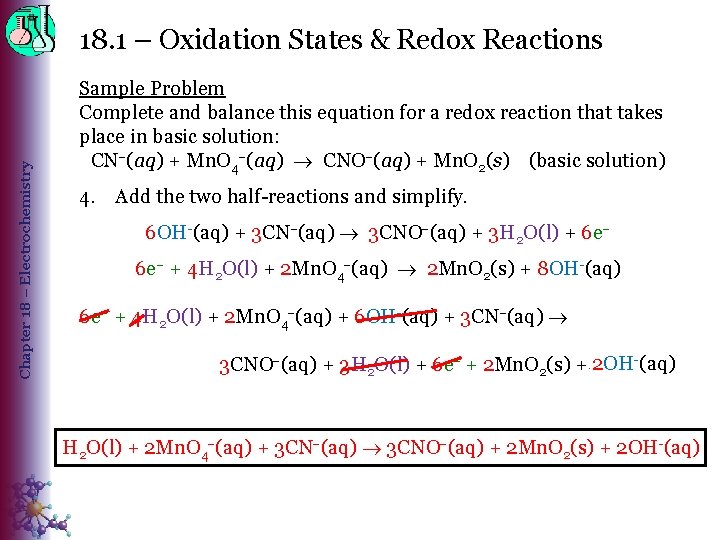
Chapter 18 – Electrochemistry 18. 1 – Oxidation States & Redox Reactions Sample Problem Complete and balance this equation for a redox reaction that takes place in basic solution: CN (aq) + Mn. O 4 (aq) CNO (aq) + Mn. O 2(s) (basic solution) 4. Add the two half-reactions and simplify. 6 OH-(aq) + 3 CN (aq) 3 CNO (aq) + 3 H 2 O(l) + 6 e– + 4 H 2 O(l) + 2 Mn. O 4 (aq) 2 Mn. O 2(s) + 8 OH-(aq) 6 e– + 4 H 2 O(l) + 2 Mn. O 4 (aq) + 6 OH-(aq) + 3 CN (aq) 3 CNO (aq) + 3 H 2 O(l) + 6 e– + 2 Mn. O 2(s) + 2 OH 8 OH--(aq) H 2 O(l) + 2 Mn. O 4 (aq) + 3 CN (aq) 3 CNO (aq) + 2 Mn. O 2(s) + 2 OH-(aq)

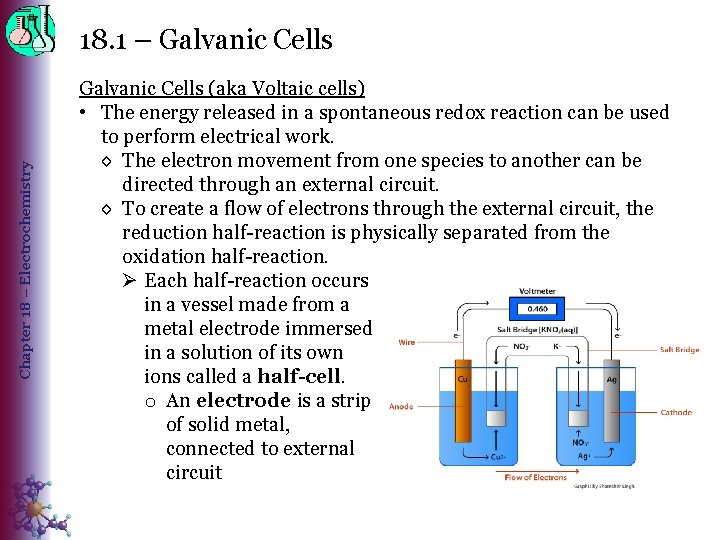
Chapter 18 – Electrochemistry 18. 1 – Galvanic Cells (aka Voltaic cells) • The energy released in a spontaneous redox reaction can be used to perform electrical work. ◊ The electron movement from one species to another can be directed through an external circuit. ◊ To create a flow of electrons through the external circuit, the reduction half-reaction is physically separated from the oxidation half-reaction. Ø Each half-reaction occurs in a vessel made from a metal electrode immersed in a solution of its own ions called a half-cell. o An electrode is a strip of solid metal, connected to external circuit

Chapter 18 – Electrochemistry 18. 1 – Galvanic Cells (aka Voltaic cells) • Anode Half-Cell ◊ Contains the electrode where oxidation occurs Ø Negative electrode (by convention) Ø During reaction, the anode loses mass (metal turns into ions in solution) o anode solution becomes more concentrated during reaction • Cathode Half-Cell ◊ Contains the electrode where reduction occurs Ø Positive electrode (by convention) Ø During reduction, the cathode gains mass (as ions gaining electrons deposit on electrode) o cathode solution becomes less concentrated during reaction

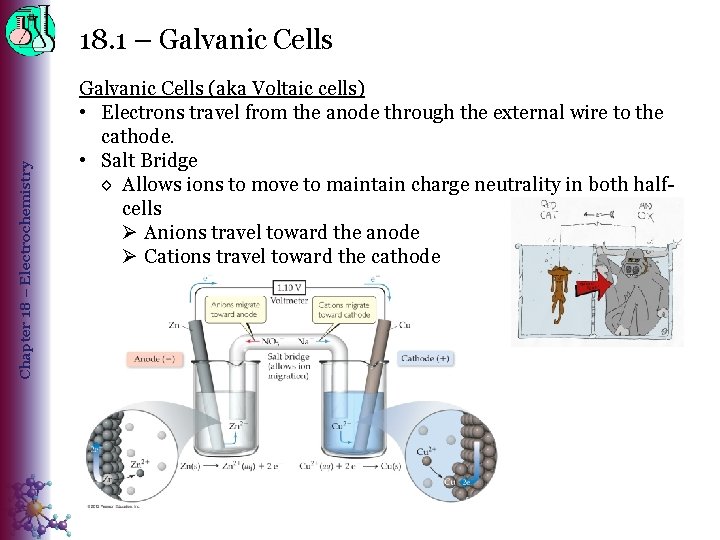
Chapter 18 – Electrochemistry 18. 1 – Galvanic Cells (aka Voltaic cells) • Electrons travel from the anode through the external wire to the cathode. • Salt Bridge ◊ Allows ions to move to maintain charge neutrality in both halfcells Ø Anions travel toward the anode Ø Cations travel toward the cathode

Chapter 18 – Electrochemistry 18. 1 – Galvanic Cells Electrical Potential • Electrons flow from the anode to the cathode because of a difference in potential energy. ◊ Potential energy of electrons is higher in the anode than in the cathode. Ø Whichever electrode increases most in stability from the loss of electrons will be the anode. Ø Related to ionization energy/electron affinity ◊ Differences in potential between electrodes are measured in volts. 1 volt = 1 joule 1 coulomb or 1 V= 1 J 1 C Ø This definition is important for canceling units in cell potential calculations.

Chapter 18 – Electrochemistry 18. 1 – Galvanic Cells Electrical Potential The difference in potential between electrodes in a voltaic cell is called: • Cell potential (Ecell) • Electromotive force (EMF) ◊ “force that moves the electrons” • or simply Voltage (V) In any voltaic cell, the cell potential is ALWAYS positive. (Ecell >0) • Useful for identifying oxidation rxn & reduction rxn. • A voltaic cell is a spontaneous process. (∆G is negative. ) Standard cell potential (Eocell) • Cell potential under standard conditions • 1 M concentration for solutions • 1 atm (for gases) • 25 o. C (298 K)

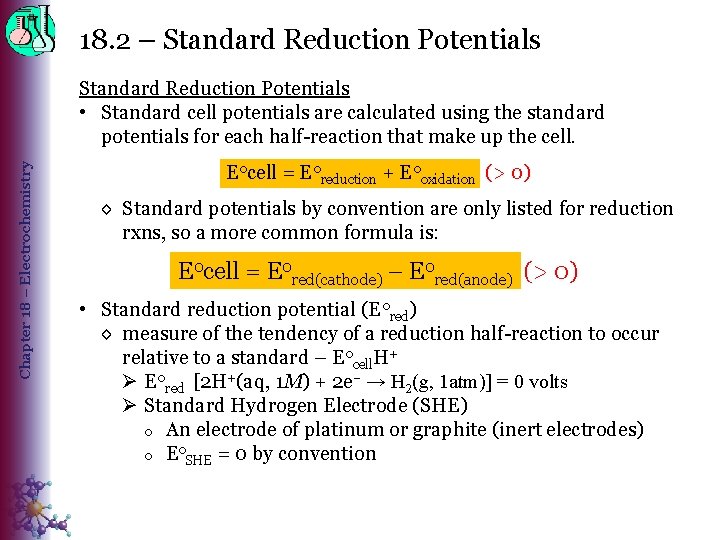
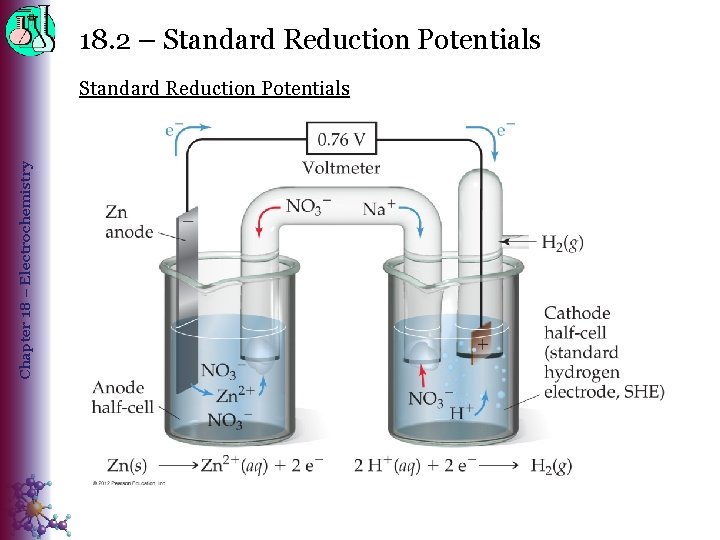
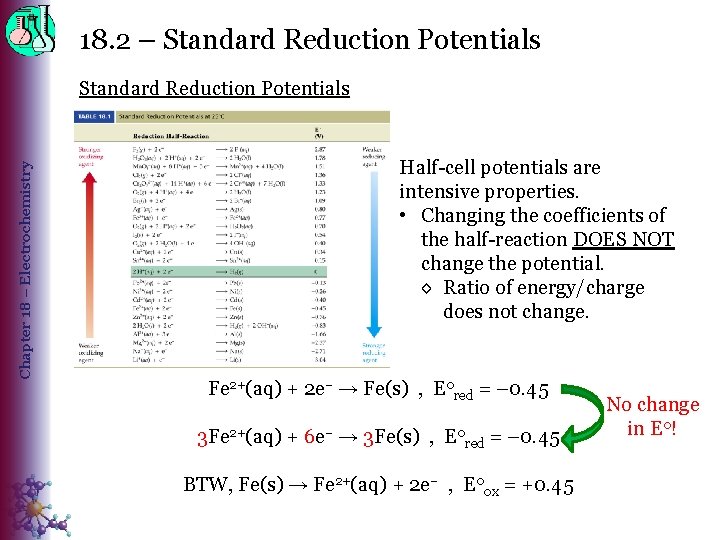
18. 2 – Standard Reduction Potentials Chapter 18 – Electrochemistry Standard Reduction Potentials • Standard cell potentials are calculated using the standard potentials for each half-reaction that make up the cell. E 0 cell = E 0 reduction + E 0 oxidation (> 0) ◊ Standard potentials by convention are only listed for reduction rxns, so a more common formula is: E 0 cell = E 0 red(cathode) – E 0 red(anode) (> 0) • Standard reduction potential (E 0 red) ◊ measure of the tendency of a reduction half-reaction to occur relative to a standard – E 0 cell. H+ Ø E 0 red [2 H+(aq, 1 M) + 2 e– → H 2(g, 1 atm)] = 0 volts Ø Standard Hydrogen Electrode (SHE) o An electrode of platinum or graphite (inert electrodes) o Eo. SHE = 0 by convention

18. 2 – Standard Reduction Potentials Chapter 18 – Electrochemistry Standard Reduction Potentials

18. 2 – Standard Reduction Potentials Chapter 18 – Electrochemistry Standard Reduction Potentials Half-cell potentials are intensive properties. • Changing the coefficients of the half-reaction DOES NOT change the potential. ◊ Ratio of energy/charge does not change. Fe 2+(aq) + 2 e– → Fe(s) , E 0 red = – 0. 45 3 Fe 2+(aq) + 6 e– → 3 Fe(s) , E 0 red = – 0. 45 BTW, Fe(s) → Fe 2+(aq) + 2 e– , E 0 ox = +0. 45 No change in E 0!

18. 2 – Standard Reduction Potentials Chapter 18 – Electrochemistry Sample Problem Use Table 20. 1 to calculate E 0 cellfor the voltaic cell described by the equation: Cr 2 O 72 (aq) + 14 H+(aq) + 6 I (aq) 2 Cr 3+(aq) + 3 I 2(s) + 7 H 2 O(l) 1. Separate the equation into half-reactions: a. Reduction occurs at the cathode: Cr 2 O 72 (aq) + 14 H+(aq) + 6 e– 2 Cr 3+(aq) + 7 H 2 O(l) Eº = +1. 33 V b. Oxidation occurs at the anode: 6 I (aq) 3 I 2(s) + 6 e– Eº = +0. 54 V 2. Subtract and solve. (Remember Eºcell must be positive. ) E 0 cell = E 0 red(cathode) – E 0 red(anode) E 0 cell = 1. 33 V – 0. 54 V = 0. 79 V

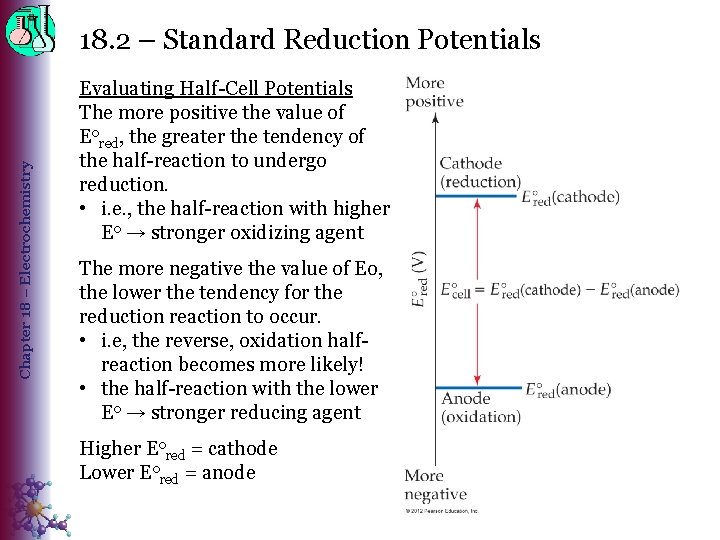
Chapter 18 – Electrochemistry 18. 2 – Standard Reduction Potentials Evaluating Half-Cell Potentials The more positive the value of Eored, the greater the tendency of the half-reaction to undergo reduction. • i. e. , the half-reaction with higher Eo → stronger oxidizing agent The more negative the value of Eo, the lower the tendency for the reduction reaction to occur. • i. e, the reverse, oxidation halfreaction becomes more likely! • the half-reaction with the lower Eo → stronger reducing agent Higher Eored = cathode Lower Eored = anode

18. 2 – Standard Reduction Potentials Sample Problem A voltaic cell is based on the two standard half-reactions: Chapter 18 – Electrochemistry Cd 2+(aq) + 2 e Cd(s) & Sn 2+(aq) + 2 e– Sn(s) Determine (a) which half-reaction occurs at the cathode and which occurs at the anode and (b) the standard cell potential. 1. Identify the anode & cathode by looking up E 0 red for each. E 0 red (Cd 2+/Cd) = 0. 403 V & E 0 red (Sn 2+/Sn) = 0. 136 V (Sn 2+/Sn) has a greater Eored & is cathode, so (Cd 2+/Cd) = anode. 2. Write half-reactions as they occur (reversing oxidation rxn) Cathode: Sn 2+(aq) + 2 e– Sn(s) Anode: Cd(s) Cd 2+(aq) + 2 e

18. 2 – Standard Reduction Potentials Sample Problem A voltaic cell is based on the two standard half-reactions: Chapter 18 – Electrochemistry Cd 2+(aq) + 2 e Cd(s) & Sn 2+(aq) + 2 e– Sn(s) Determine (a) which half-reaction occurs at the cathode and which occurs at the anode and (b) the standard cell potential. 3. Combine the reactions into a net equation by canceling e–. Cathode: Anode: Sn 2+(aq) + 2 e– Sn(s) Cd(s) Cd 2+(aq) + 2 e Sn 2+(aq) + Cd(s) Cd 2+(aq) + Sn(s) 4. Subtract and solve. (Remember E 0 cell must be positive. ) E 0 cell = E 0 red(cathode) – E 0 red(anode) E 0 cell = 0. 136 V – ( 0. 403 V) = 0. 267 V

Chapter 18 – Electrochemistry 18. 2 – Standard Reduction Potentials Line Notation • The anode (oxidation) is written before the cathode (reduction). • Within a half-cell, the reactants are listed before the products. • A single vertical line indicates a change in state or phase. ◊ If two species in a half reaction are the same phase, comma may be used in place of vertical line. • Concentrations of aqueous solutions are written in parentheses after the symbol for the ion or molecule. • A double vertical line is used to indicate the junction between the half-cells (salt bridge). Anode | oxidation half cell || reduction half cell | Cathode Ni (s) | Ni 2+ (0. 180 M) || Cu 2+ (0. 2 M) | Cu (s) Anode Half Cell Cathode Half Cell

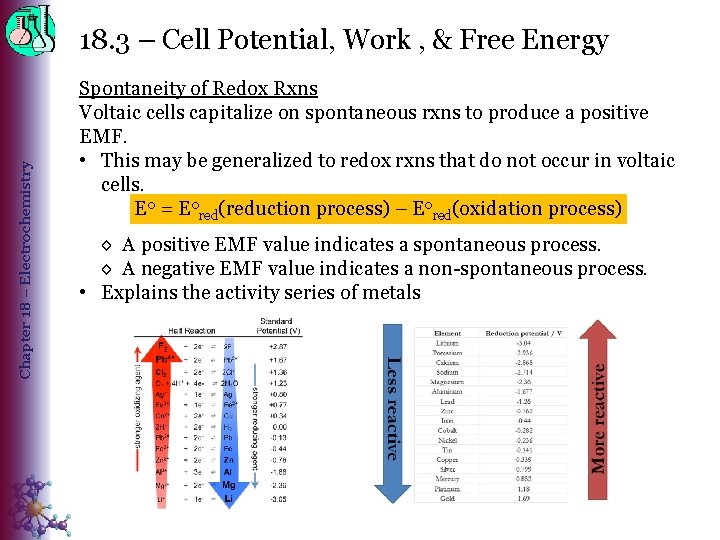
Chapter 18 – Electrochemistry 18. 3 – Cell Potential, Work , & Free Energy Spontaneity of Redox Rxns Voltaic cells capitalize on spontaneous rxns to produce a positive EMF. • This may be generalized to redox rxns that do not occur in voltaic cells. E 0 = E 0 red(reduction process) – Eored(oxidation process) ◊ A positive EMF value indicates a spontaneous process. ◊ A negative EMF value indicates a non-spontaneous process. • Explains the activity series of metals

Chapter 18 – Electrochemistry 18. 3 – Cell Potential, Work , & Free Energy Sample Problem Determine whether the following reactions are spontaneous under standard conditions. a. Cu(s) + 2 H+(aq) Cu 2+(aq) + H 2(g) b. Cl 2(g) + 2 I (aq) 2 Cl (aq) + I 2(s) a. Reduction: 2 H+(aq) + 2 e– H 2(g) Eºred = 0 V =-0. 34 V +0. 34 V Oxidation: Cu(s) Cu 2+(aq) + 2 e– + Eºred ox = Eº = -0. 34 V Forward reaction is not thermodynamically favored, reverse is. b. Reduction: Cl 2(g) + 2 e– 2 Cl (aq) Eºred = +1. 36 V Oxidation: 2 I (aq) I 2(s) + 2 e– =-0. 54 V + Eºox red= Eº = 0. 82 V Forward reaction is thermodynamically favored.

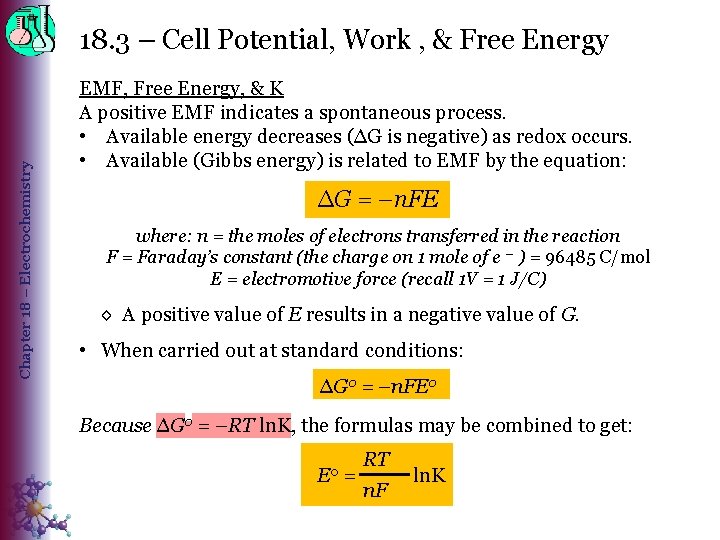
Chapter 18 – Electrochemistry 18. 3 – Cell Potential, Work , & Free Energy EMF, Free Energy, & K A positive EMF indicates a spontaneous process. • Available energy decreases (∆G is negative) as redox occurs. • Available (Gibbs energy) is related to EMF by the equation: ∆G = –n. FE where: n = the moles of electrons transferred in the reaction F = Faraday’s constant (the charge on 1 mole of e – ) = 96485 C/mol E = electromotive force (recall 1 V = 1 J/C) ◊ A positive value of E results in a negative value of G. • When carried out at standard conditions: ∆G 0 = –n. FE 0 Because ∆G 0 = –RT ln. K, the formulas may be combined to get: E 0 = RT n. F ln. K

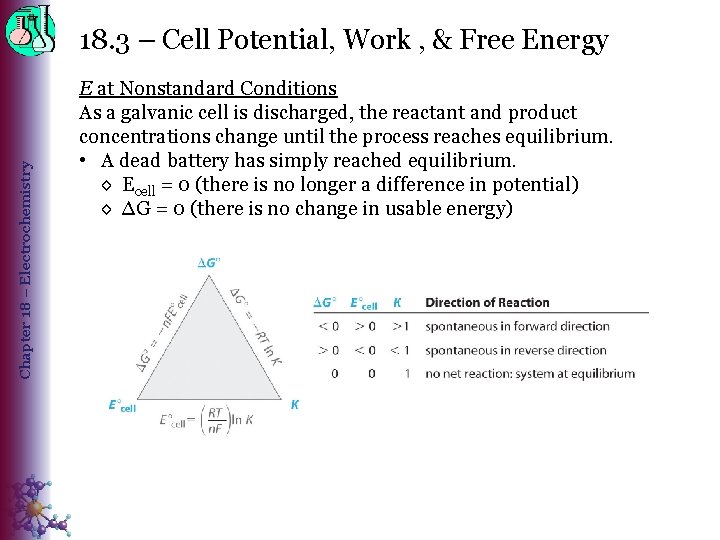
Chapter 18 – Electrochemistry 18. 3 – Cell Potential, Work , & Free Energy E at Nonstandard Conditions As a galvanic cell is discharged, the reactant and product concentrations change until the process reaches equilibrium. • A dead battery has simply reached equilibrium. ◊ Ecell = 0 (there is no longer a difference in potential) ◊ ∆G = 0 (there is no change in usable energy)

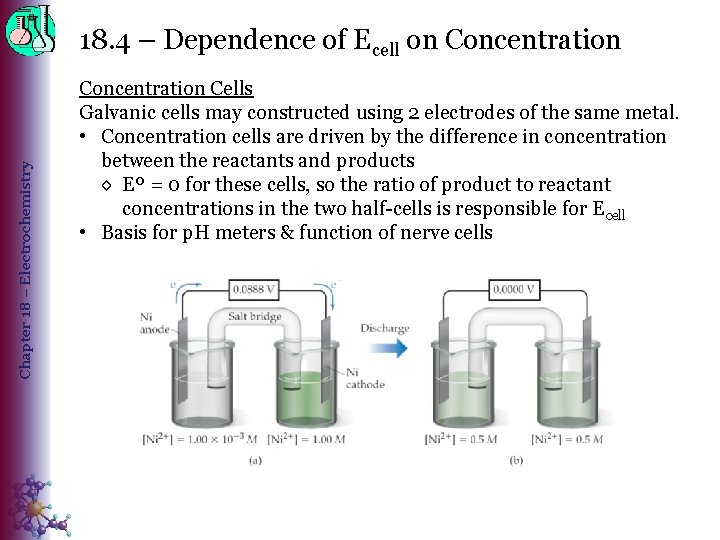
Chapter 18 – Electrochemistry 18. 4 – Dependence of Ecell on Concentration Cells Galvanic cells may constructed using 2 electrodes of the same metal. • Concentration cells are driven by the difference in concentration between the reactants and products ◊ Eº = 0 for these cells, so the ratio of product to reactant concentrations in the two half-cells is responsible for Ecell • Basis for p. H meters & function of nerve cells

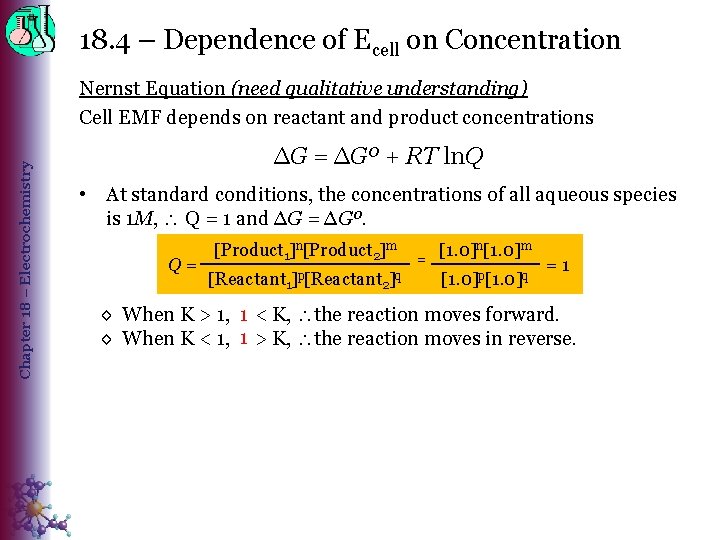
18. 4 – Dependence of Ecell on Concentration Chapter 18 – Electrochemistry Nernst Equation (need qualitative understanding) Cell EMF depends on reactant and product concentrations ∆G = ∆Gº + RT ln. Q • At standard conditions, the concentrations of all aqueous species is 1 M, Q = 1 and ∆G = ∆Gº. Q= [Product 1]n[Product 2]m [Reactant 1 ]p[Reactant q 2] = [1. 0]n[1. 0]m [1. 0]p[1. 0]q =1 1 < K, the reaction moves forward. ◊ When K > 1, Q 1 > K, the reaction moves in reverse. ◊ When K < 1, Q

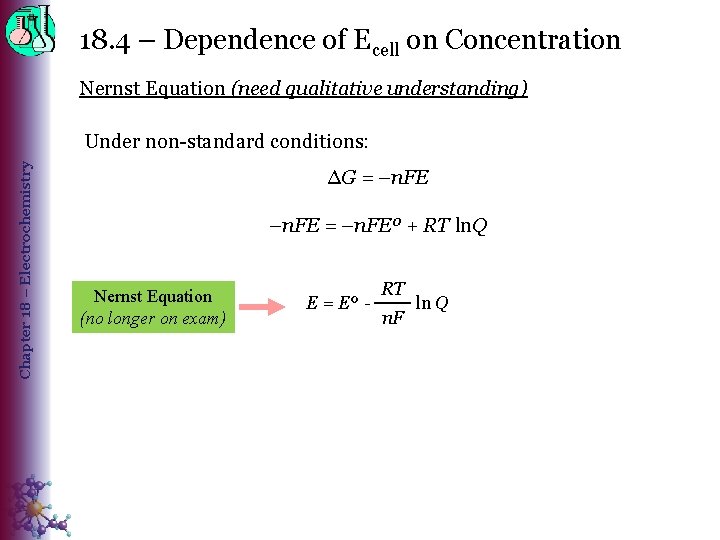
18. 4 – Dependence of Ecell on Concentration Nernst Equation (need qualitative understanding) Chapter 18 – Electrochemistry Under non-standard conditions: ∆G = –n. FEº + RT ln. Q Nernst Equation (no longer on exam) E = Eº - RT n. F ln Q

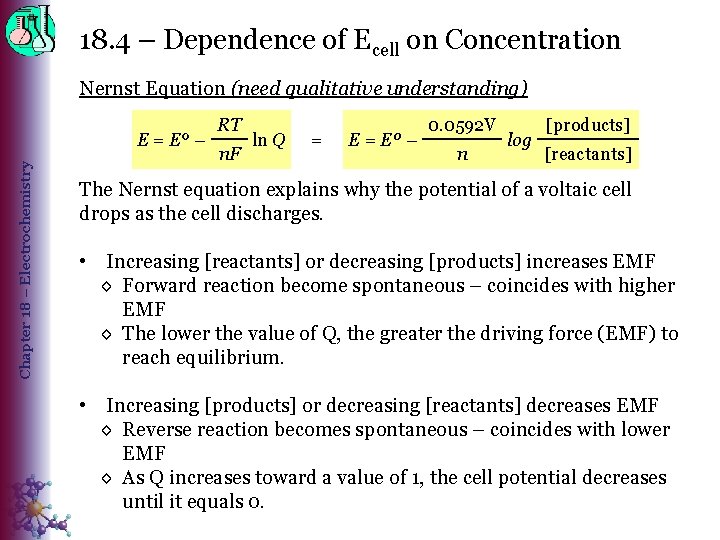
18. 4 – Dependence of Ecell on Concentration Nernst Equation (need qualitative understanding) Chapter 18 – Electrochemistry E = Eº – RT n. F ln Q = Eº – 0. 0592 V n log [products] [reactants] The Nernst equation explains why the potential of a voltaic cell drops as the cell discharges. • Increasing [reactants] or decreasing [products] increases EMF ◊ Forward reaction become spontaneous – coincides with higher EMF ◊ The lower the value of Q, the greater the driving force (EMF) to reach equilibrium. • Increasing [products] or decreasing [reactants] decreases EMF ◊ Reverse reaction becomes spontaneous – coincides with lower EMF ◊ As Q increases toward a value of 1, the cell potential decreases until it equals 0.

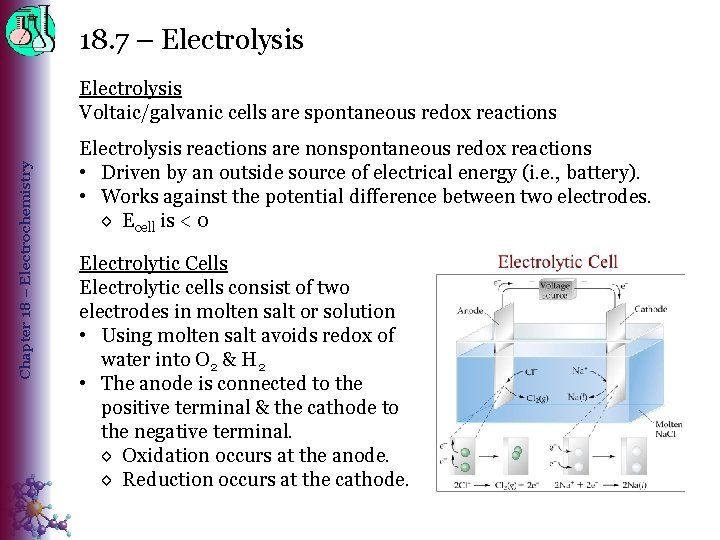
18. 7 – Electrolysis Chapter 18 – Electrochemistry Electrolysis Voltaic/galvanic cells are spontaneous redox reactions Electrolysis reactions are nonspontaneous redox reactions • Driven by an outside source of electrical energy (i. e. , battery). • Works against the potential difference between two electrodes. ◊ Ecell is < 0 Electrolytic Cells Electrolytic cells consist of two electrodes in molten salt or solution • Using molten salt avoids redox of water into O 2 & H 2 • The anode is connected to the positive terminal & the cathode to the negative terminal. ◊ Oxidation occurs at the anode. ◊ Reduction occurs at the cathode.

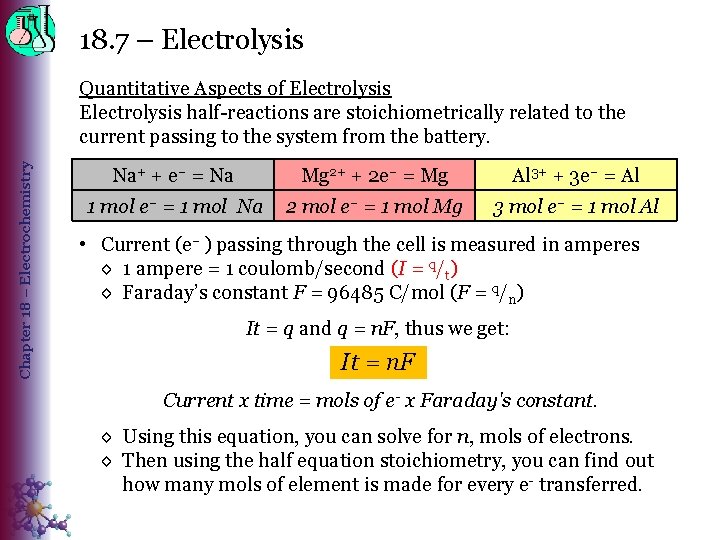
18. 7 – Electrolysis Chapter 18 – Electrochemistry Quantitative Aspects of Electrolysis half-reactions are stoichiometrically related to the current passing to the system from the battery. Na+ + e– = Na Mg 2+ + 2 e– = Mg Al 3+ + 3 e– = Al 1 mol e– = 1 mol Na 2 mol e– = 1 mol Mg 3 mol e– = 1 mol Al • Current (e– ) passing through the cell is measured in amperes ◊ 1 ampere = 1 coulomb/second (I = q/t) ◊ Faraday’s constant F = 96485 C/mol (F = q/n) It = q and q = n. F, thus we get: It = n. F Current x time = mols of e- x Faraday's constant. ◊ Using this equation, you can solve for n, mols of electrons. ◊ Then using the half equation stoichiometry, you can find out how many mols of element is made for every e- transferred.

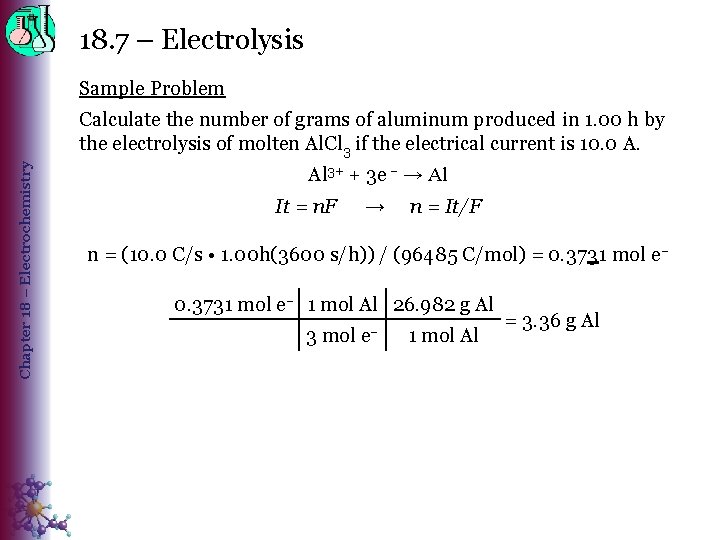
18. 7 – Electrolysis Sample Problem Chapter 18 – Electrochemistry Calculate the number of grams of aluminum produced in 1. 00 h by the electrolysis of molten Al. Cl 3 if the electrical current is 10. 0 A. Al 3+ + 3 e – → Al It = n. F → n = It/F n = (10. 0 C/s • 1. 00 h(3600 s/h)) / (96485 C/mol) = 0. 3731 mol e– 1 mol Al 26. 982 g Al 3 mol e– 1 mol Al = 3. 36 g Al