Lecture 16 The Redox Reactions Oxidation State HalfReactions



- Slides: 15

Lecture 16 The Redox Reactions Oxidation State Half-Reactions Balanced Oxidation-Reduction reactions Predicted Sequence of Redox Reactions Tracers for these reactions Distributions in Nature

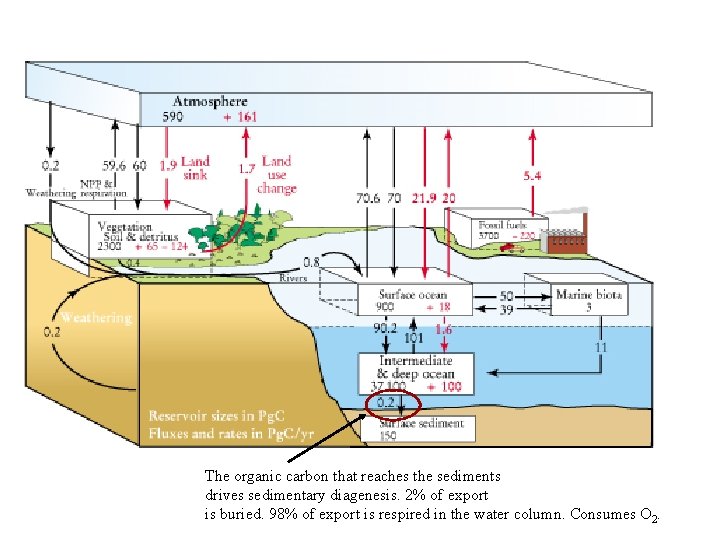
The organic carbon that reaches the sediments drives sedimentary diagenesis. 2% of export is buried. 98% of export is respired in the water column. Consumes O 2.

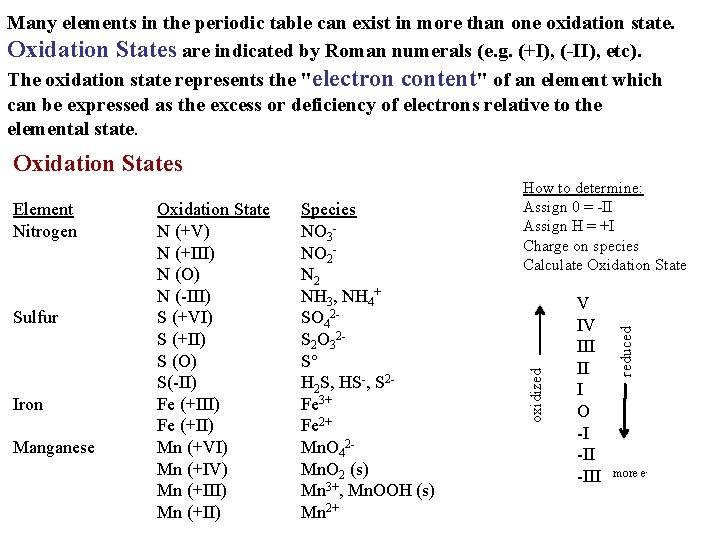
Many elements in the periodic table can exist in more than one oxidation state. Oxidation States are indicated by Roman numerals (e. g. (+I), (-II), etc). The oxidation state represents the "electron content" of an element which can be expressed as the excess or deficiency of electrons relative to the elemental state. Oxidation States Iron Manganese Species NO 3 NO 2 N 2 NH 3, NH 4+ SO 42 S 2 O 32 S H 2 S, HS-, S 2 Fe 3+ Fe 2+ Mn. O 42 Mn. O 2 (s) Mn 3+, Mn. OOH (s) Mn 2+ V IV III II I O -I -III reduced Sulfur Oxidation State N (+V) N (+III) N (O) N (-III) S (+VI) S (+II) S (O) S(-II) Fe (+II) Mn (+VI) Mn (+IV) Mn (+III) Mn (+II) oxidized Element Nitrogen How to determine: Assign 0 = -II Assign H = +I Charge on species Calculate Oxidation State more e-

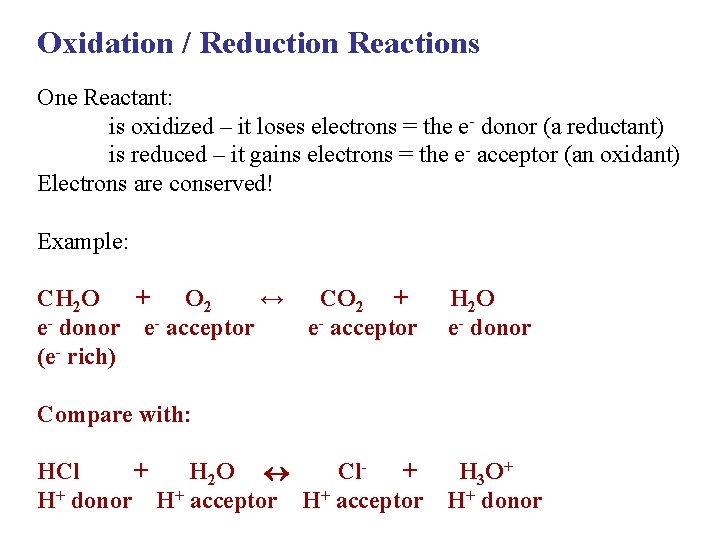
Oxidation / Reduction Reactions One Reactant: is oxidized – it loses electrons = the e- donor (a reductant) is reduced – it gains electrons = the e- acceptor (an oxidant) Electrons are conserved! Example: CH 2 O + O 2 ↔ e- donor e- acceptor (e- rich) CO 2 + e- acceptor H 2 O e- donor Compare with: HCl + H 2 O Cl+ H+ donor H+ acceptor H 3 O + H+ donor

The driver of ocean redox chemistry is organic matter. Why is organic matter an electron donor? photosynthesis

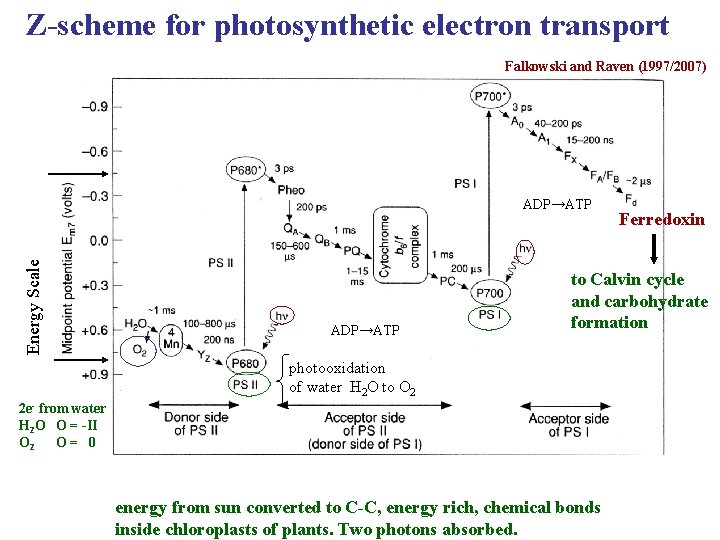
Z-scheme for photosynthetic electron transport Falkowski and Raven (1997/2007) Energy Scale ADP→ATP Ferredoxin to Calvin cycle and carbohydrate formation photooxidation of water H 2 O to O 2 2 e- from water H 2 O O = -II O 2 O= 0 energy from sun converted to C-C, energy rich, chemical bonds inside chloroplasts of plants. Two photons absorbed.

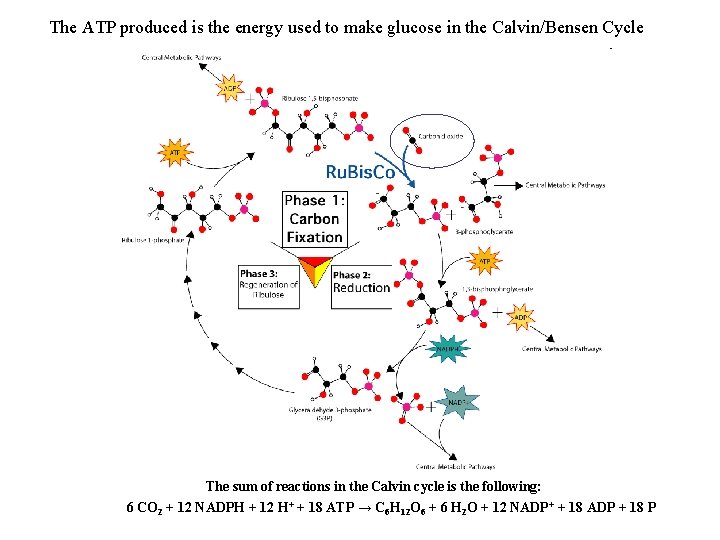
The ATP produced is the energy used to make glucose in the Calvin/Bensen Cycle The sum of reactions in the Calvin cycle is the following: 6 CO 2 + 12 NADPH + 12 H+ + 18 ATP → C 6 H 12 O 6 + 6 H 2 O + 12 NADP+ + 18 ADP + 18 P

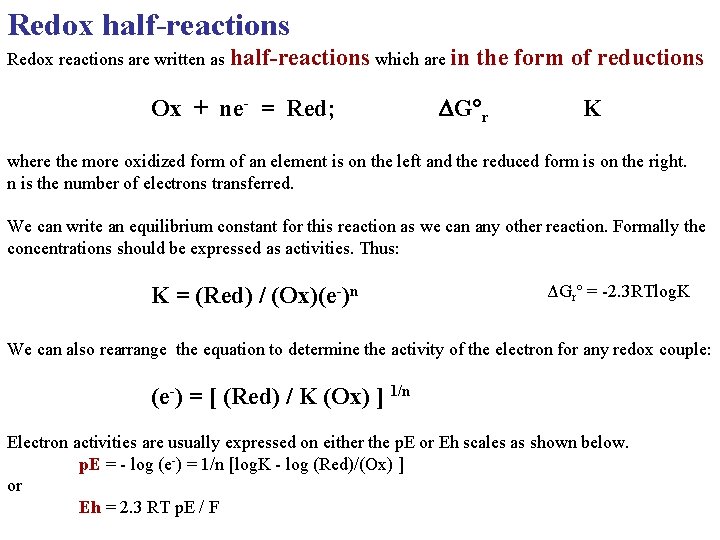
Redox half-reactions Redox reactions are written as half-reactions which are in Ox + ne- = Red; the form of reductions DG r K where the more oxidized form of an element is on the left and the reduced form is on the right. n is the number of electrons transferred. We can write an equilibrium constant for this reaction as we can any other reaction. Formally the concentrations should be expressed as activities. Thus: K = (Red) / (Ox)(e-)n ΔGr° = -2. 3 RTlog. K We can also rearrange the equation to determine the activity of the electron for any redox couple: (e-) = [ (Red) / K (Ox) ] 1/n Electron activities are usually expressed on either the p. E or Eh scales as shown below. p. E = - log (e-) = 1/n [log. K - log (Red)/(Ox) ] or Eh = 2. 3 RT p. E / F

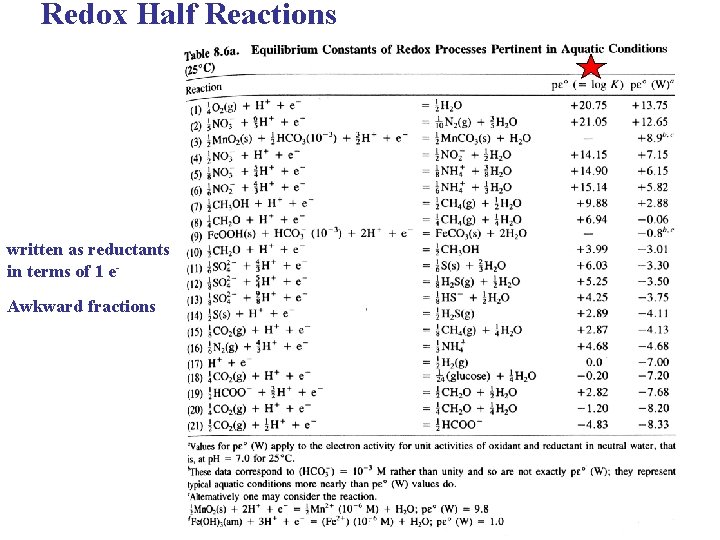
Redox Half Reactions written as reductants in terms of 1 e. Awkward fractions

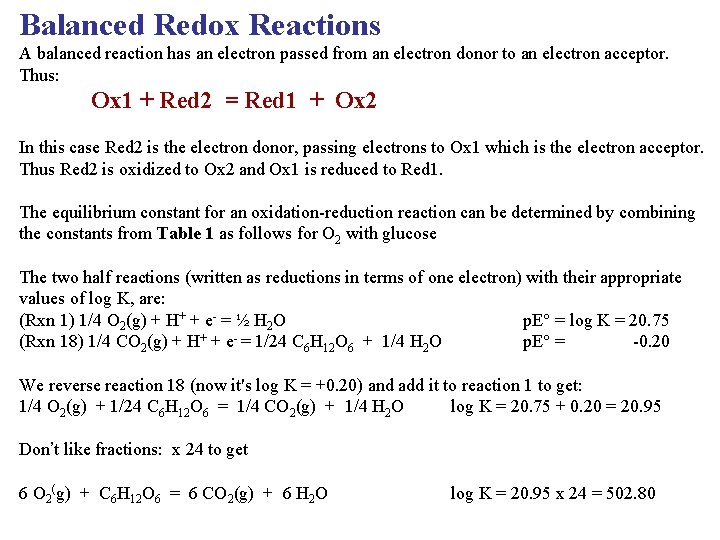
Balanced Redox Reactions A balanced reaction has an electron passed from an electron donor to an electron acceptor. Thus: Ox 1 + Red 2 = Red 1 + Ox 2 In this case Red 2 is the electron donor, passing electrons to Ox 1 which is the electron acceptor. Thus Red 2 is oxidized to Ox 2 and Ox 1 is reduced to Red 1. The equilibrium constant for an oxidation-reduction reaction can be determined by combining the constants from Table 1 as follows for O 2 with glucose The two half reactions (written as reductions in terms of one electron) with their appropriate values of log K, are: (Rxn 1) 1/4 O 2(g) + H+ + e- = ½ H 2 O p. E = log K = 20. 75 (Rxn 18) 1/4 CO 2(g) + H+ + e- = 1/24 C 6 H 12 O 6 + 1/4 H 2 O p. E = -0. 20 We reverse reaction 18 (now it's log K = +0. 20) and add it to reaction 1 to get: 1/4 O 2(g) + 1/24 C 6 H 12 O 6 = 1/4 CO 2(g) + 1/4 H 2 O log K = 20. 75 + 0. 20 = 20. 95 Don’t like fractions: x 24 to get 6 O 2(g) + C 6 H 12 O 6 = 6 CO 2(g) + 6 H 2 O log K = 20. 95 x 24 = 502. 80

Ideal Redox Sequence There is an ideal sequence of redox reactions driven by e- rich organic matter that is based on the energy available for the microbes that mediate the reactions. In this sequence organic matter is combusted in order by O 2 → NO 3 → Mn. O 2 → Fe 2 O 3 → SO 42 - (decreasing energy yield). Most of these reactions have slow kinetics if not mediated by bacteria. Bacteria mediate most of these reactions and get the energy for their life processes. Because the energy of the sun is trapped in the C-C bonds, bacteria are indirectly using sunlight when they combust natural organic matter to CO 2. Bacteria use the electron acceptors in the order of decreasing energy availability.

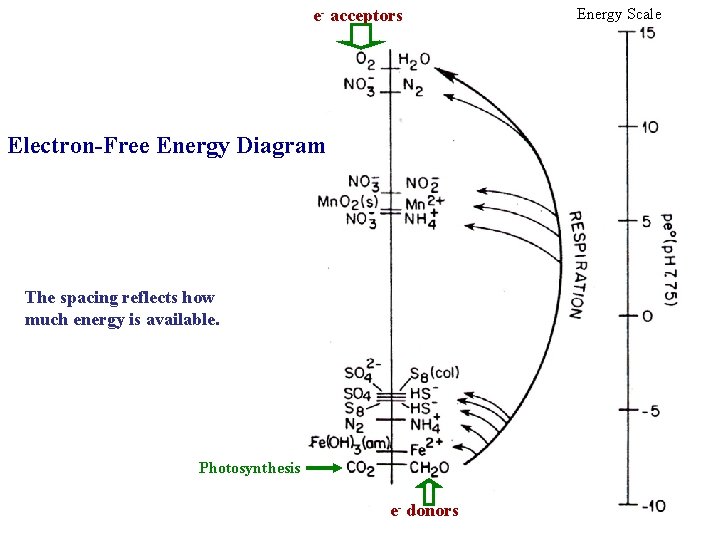
e- acceptors Electron-Free Energy Diagram The spacing reflects how much energy is available. Photosynthesis e- donors Energy Scale

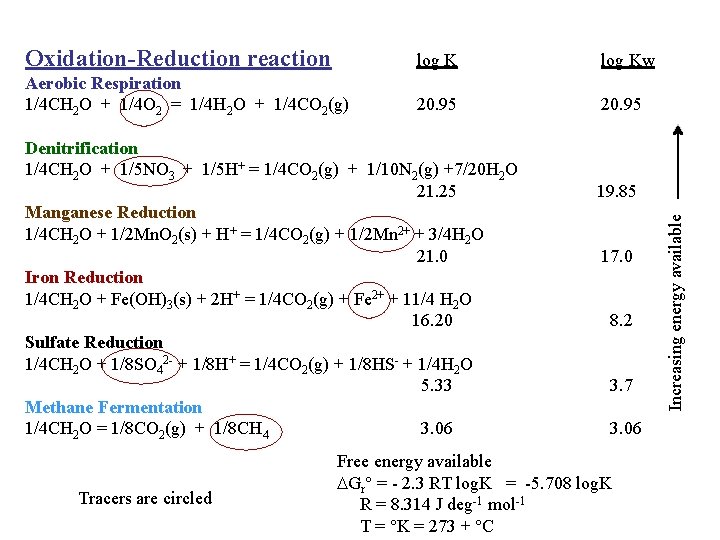
Oxidation-Reduction reaction log Kw Aerobic Respiration 1/4 CH 2 O + 1/4 O 2 = 1/4 H 2 O + 1/4 CO 2(g) 20. 95 Tracers are circled 19. 85 17. 0 8. 2 3. 7 3. 06 Free energy available DGr° = - 2. 3 RT log. K = -5. 708 log. K R = 8. 314 J deg-1 mol-1 T = °K = 273 + °C Increasing energy available Denitrification 1/4 CH 2 O + 1/5 NO 3 + 1/5 H+ = 1/4 CO 2(g) + 1/10 N 2(g) +7/20 H 2 O 21. 25 Manganese Reduction 1/4 CH 2 O + 1/2 Mn. O 2(s) + H+ = 1/4 CO 2(g) + 1/2 Mn 2+ + 3/4 H 2 O 21. 0 Iron Reduction 1/4 CH 2 O + Fe(OH)3(s) + 2 H+ = 1/4 CO 2(g) + Fe 2+ + 11/4 H 2 O 16. 20 Sulfate Reduction 1/4 CH 2 O + 1/8 SO 42 - + 1/8 H+ = 1/4 CO 2(g) + 1/8 HS- + 1/4 H 2 O 5. 33 Methane Fermentation 1/4 CH 2 O = 1/8 CO 2(g) + 1/8 CH 4 3. 06

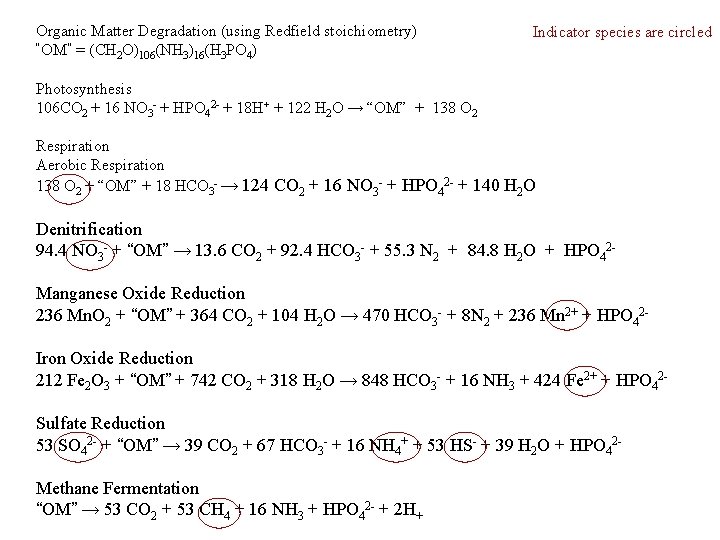
Organic Matter Degradation (using Redfield stoichiometry) “OM” = (CH 2 O)106(NH 3)16(H 3 PO 4) Indicator species are circled Photosynthesis 106 CO 2 + 16 NO 3 - + HPO 42 - + 18 H+ + 122 H 2 O → “OM” + 138 O 2 Respiration Aerobic Respiration 138 O 2 + “OM” + 18 HCO 3 - → 124 CO 2 + 16 NO 3 - + HPO 42 - + 140 H 2 O Denitrification 94. 4 NO 3 - + “OM” → 13. 6 CO 2 + 92. 4 HCO 3 - + 55. 3 N 2 + 84. 8 H 2 O + HPO 42 Manganese Oxide Reduction 236 Mn. O 2 + “OM” + 364 CO 2 + 104 H 2 O → 470 HCO 3 - + 8 N 2 + 236 Mn 2+ + HPO 42 Iron Oxide Reduction 212 Fe 2 O 3 + “OM” + 742 CO 2 + 318 H 2 O → 848 HCO 3 - + 16 NH 3 + 424 Fe 2+ + HPO 42 Sulfate Reduction 53 SO 42 - + “OM” → 39 CO 2 + 67 HCO 3 - + 16 NH 4+ + 53 HS- + 39 H 2 O + HPO 42 Methane Fermentation “OM” → 53 CO 2 + 53 CH 4 + 16 NH 3 + HPO 42 - + 2 H+
