Oxidation Reduction Reactions Redox Reactions Oxidation is the









- Slides: 11

Oxidation Reduction Reactions

Re-dox Reactions § Oxidation is the loss of electrons § Reduction is a gain of electrons § Leo goes Grr! § Lose Electrons Oxidation § Gain electrons Reduce

“Redox Reactions” Many reactions involve (or seem to involve) a transfer of electrons. Such reactions always involve both: § Oxidation Loss of electrons Na Na+ + e- § Reduction Gain of electrons Cl + e- Cl-

Determine a Redox Reaction § To determine if what you have is a redox reaction, first rewrite the equation to include the oxidation number for each element § The oxidation number is the charge an element has or appears to have when combining to form compounds

Oxidizing/Reducing Agents 1. The element that was oxidized is part of the reducing agent. 2. The element that was reduced is part of the oxidizing agent.

Assigning Oxidation Numbers 1. 2. 3. 4. 5. 6. 7. 8. All pure elements and homogeneous molecules = 0 Elements in group IA = +1 Elements in group IIA = +2 Ag+, Zn+2, Al+3 (unless it is a pure metal) In binary compounds the second element = anion charge Oxygen is almost always = -2 *unless it is O 2(g) Hydrogen is almost always = +1 *unless it is H 2(g) The total charge of a compound is always = 0

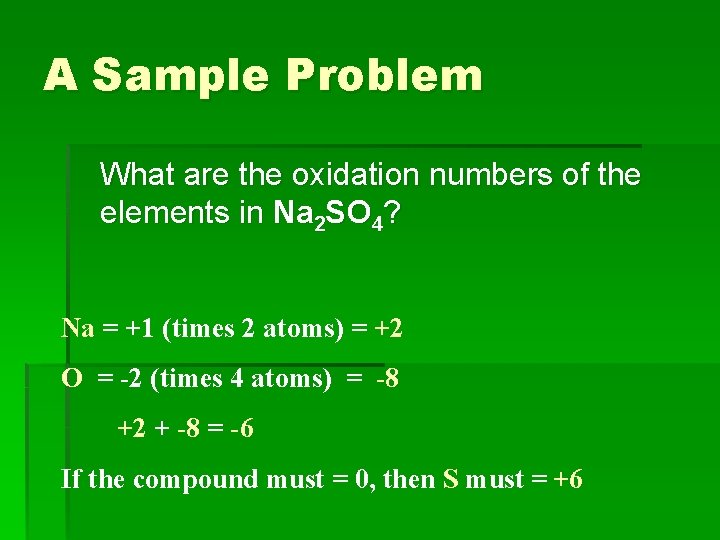
A Sample Problem What are the oxidation numbers of the elements in Na 2 SO 4? Na = +1 (times 2 atoms) = +2 O = -2 (times 4 atoms) = -8 +2 + -8 = -6 If the compound must = 0, then S must = +6

Give the oxidation number for: 1. 2. 3. 4. 5. 6. 7. 8. S Mn N C N S S Fe in Na 2 SO 3 in KMn. O 4 in Ca(NO 3)2 in Na 2 CO 3 in NO 2 in SO 4 -2 in H 2 S 2 O 7 in Fe(C 2 H 3 O 2)2 +4 +7 +5 +4 +3 +6 +6 +2

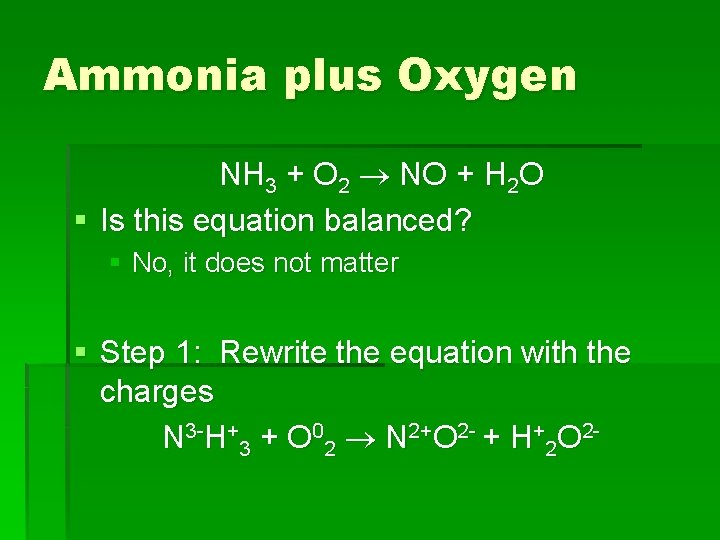
Ammonia plus Oxygen NH 3 + O 2 NO + H 2 O § Is this equation balanced? § No, it does not matter § Step 1: Rewrite the equation with the charges N 3 -H+3 + O 02 N 2+O 2 - + H+2 O 2 -

Ammonia plus Oxygen § Step 2: Determine the changes in the charges of each element N 3 -H+3 + O 02 N 2+O 2 - + H+2 O 2§ Nitrogen changes from 3 - to 2+ § it lost an electron and was oxidized § Oxygen changes from 0 to 2§ it gains electrons and so is reduced

Last Step § Step 3: If there is a species oxidized and one reduced, then it is a redox equation