ELECTROCHEMISTRY Chapter 18 Introduction Electrochemistry the study of


















- Slides: 53

ELECTROCHEMISTRY Chapter 18

Introduction * Electrochemistry: the study of the relationship between electron flow and redox reactions. Significance and Applications? What is a redox reaction? An oxidation – reduction reaction in which electrons are transferred (same # e- lost and gained). Zn(s) + Cu 2+(aq) → Zn 2+(aq) + Cu(s)

I. Oxidation and Reduction Reactions A. Introduction 1. Reactions involving the transfer of electrons. 2. One species has a desire to lose electrons an another species has a desire to gain electrons. With the correct conditions, energy in the form of current flow is produced. 3. Driving force is tendency for electron flow. 4. Examples: batteries, combustion, rusting of iron, metabolism of food in the body.

B. Definitions 1. Oxidation - The loss of electron(s). Oxidation Reaction M M n+ + n e While M is oxidized, it acts as an reducing agent because it causes something else to be reduced (i. e. , it gives electrons to something else). Fe 2+ + 2 e-

2. Reduction - The gain of electron(s). Reduction Reaction X + n e - X n. While X is reduced, it acts as an oxidizing agent because it causes something else to be oxidized (i. e. , it takes electrons from something else). Cl 2 + 2 e- 2 Cl. Ag+ + e- Ag

L E O loss of electrons (products) oxidation the lion says G gain of E electrons (reactants) R!!! reduction

C. Properties of Oxidation-Reduction Rxns 1. “Redox reaction” always consists of the two processes (oxidation and reduction) which occurs simultaneously. One species must lose electrons so that another species can gain those same # of electrons. 2. A redox reaction may be recognized if a metal is being oxidized or reduced. Metal gains or loses electrons as it goes from reactants to products. How can we recognize this in a reaction?

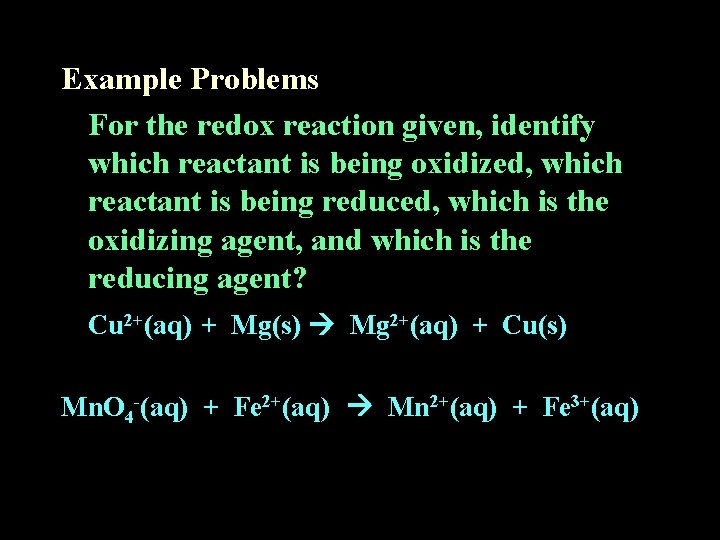
Example Problems For the redox reaction given, identify which reactant is being oxidized, which reactant is being reduced, which is the oxidizing agent, and which is the reducing agent? Cu 2+(aq) + Mg(s) Mg 2+(aq) + Cu(s) Mn. O 4 -(aq) + Fe 2+(aq) Mn 2+(aq) + Fe 3+(aq)

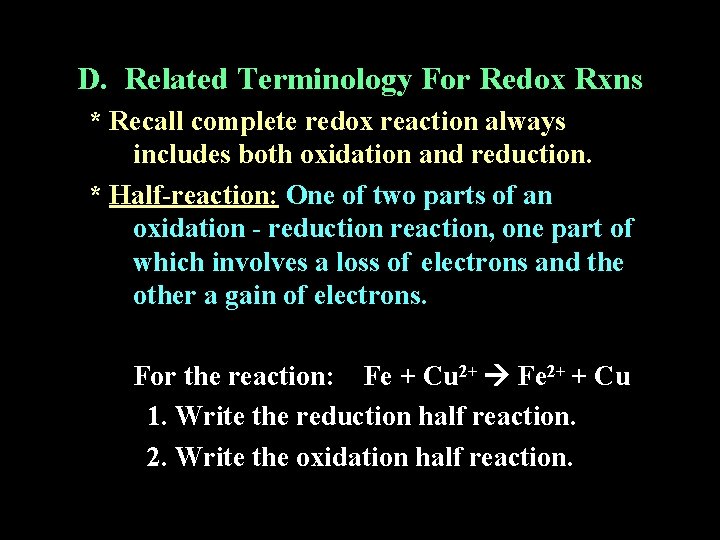
D. Related Terminology For Redox Rxns * Recall complete redox reaction always includes both oxidation and reduction. * Half-reaction: One of two parts of an oxidation - reduction reaction, one part of which involves a loss of electrons and the other a gain of electrons. For the reaction: Fe + Cu 2+ Fe 2+ + Cu 1. Write the reduction half reaction. 2. Write the oxidation half reaction.

For the reaction: Zn(s) + Cu 2+(aq) → Zn 2+(aq) + Cu(s) d. What is the reducing agent? Reducing agent: The reactant that is oxidized. It loses electrons so that some other reactant can be reduced. e. What is the oxidizing agent? Oxidizing Agent: The reactant that is reduced. It gains electrons so that some other reactant can be oxidized.

E. Balancing Redox Reactions 1. Introduction Oxidation Number Method Arbitrary book-keeping system for electrons which can be used to identify what is being oxidized or reduced. Half-Reaction Method *** You will be responsible for a simplified version of this.

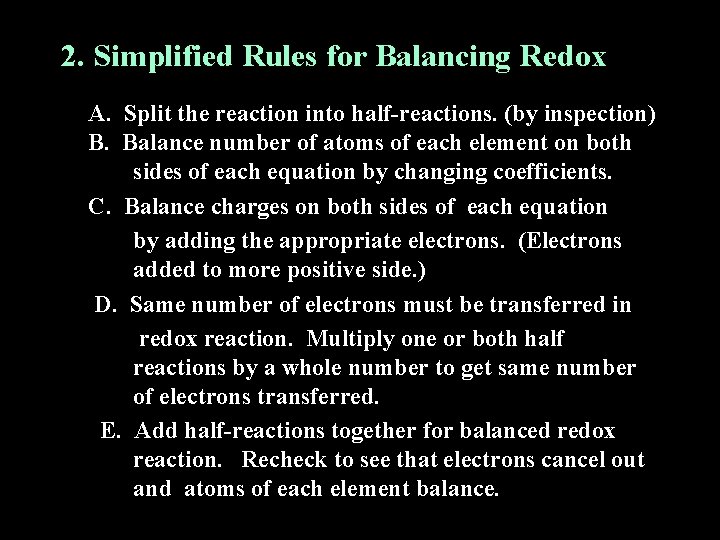
2. Simplified Rules for Balancing Redox A. Split the reaction into half-reactions. (by inspection) B. Balance number of atoms of each element on both sides of each equation by changing coefficients. C. Balance charges on both sides of each equation by adding the appropriate electrons. (Electrons added to more positive side. ) D. Same number of electrons must be transferred in redox reaction. Multiply one or both half reactions by a whole number to get same number of electrons transferred. E. Add half-reactions together for balanced redox reaction. Recheck to see that electrons cancel out and atoms of each element balance.

Example Problems 1. Balance the following redox reactions: Sn 2+(aq) + Fe 3+(aq) → Sn 4+(aq) + Fe 2+(aq) Sb 3+(aq) + Sr(s) → Sb(s) + Sr 2+(aq) 2. How many mol es of electrons are being transferred in the following balanced redox reaction? 2 Al(s) + 3 Cl 2(g) → 2 Al. Cl 3(aq)

II. Voltaic (Galvanic) Cells A. Introduction 1. An electrochemical cell in which a product-favored (spontaneous) redox reaction generates an electric current. 2. The reaction produces an electron flow through an outside conductor (wire). 3. Examples: batteries

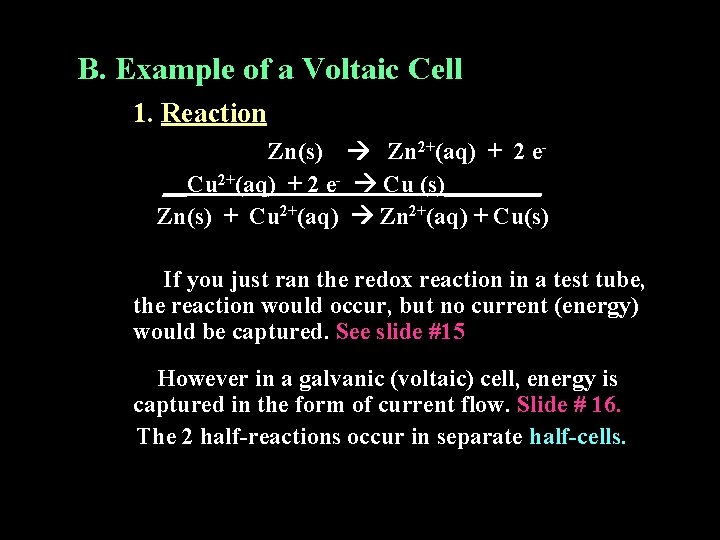
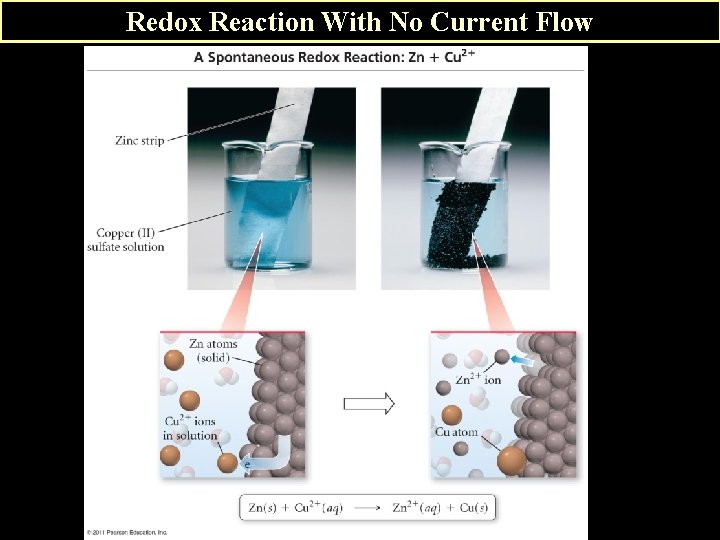
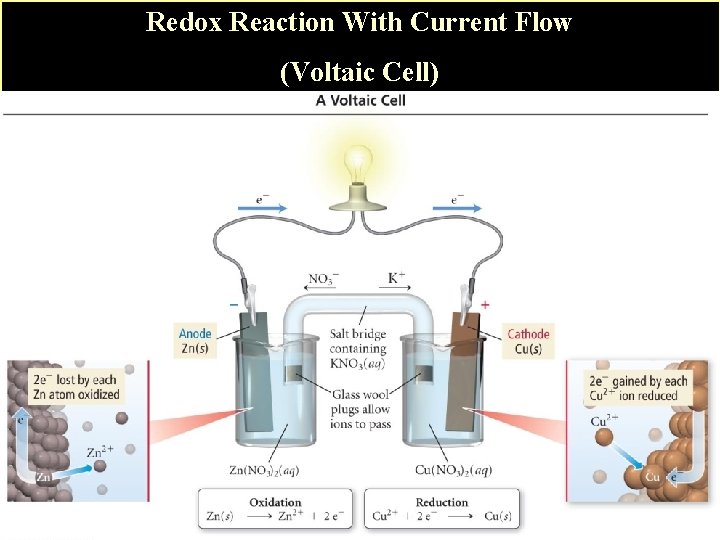
B. Example of a Voltaic Cell 1. Reaction Zn(s) Zn 2+(aq) + 2 e__Cu 2+(aq) + 2 e- Cu (s)____ Zn(s) + Cu 2+(aq) Zn 2+(aq) + Cu(s) If you just ran the redox reaction in a test tube, the reaction would occur, but no current (energy) would be captured. See slide #15 However in a galvanic (voltaic) cell, energy is captured in the form of current flow. Slide # 16. The 2 half-reactions occur in separate half-cells.

Redox Reaction With No Current Flow

Redox Reaction With Current Flow (Voltaic Cell)

C. Requirements of Galvanic Cell 1. Anode: an electrode (conductor such as metal strip or graphite) where oxidation occurs. 2. Cathode: an electrode (conductor such as metal strip or graphite) where reduction occurs. 3. Salt Bridge: A tube of an electrolyte (sometimes in a gel) that is connected to the two half-cells of a voltaic cell: the salt bridge allows the flow of ions but prevents the mixing of the different solutions that would allow direct reaction of the cell reactants. Charge does not build up in half cells. Electrical neutrality must be maintained.

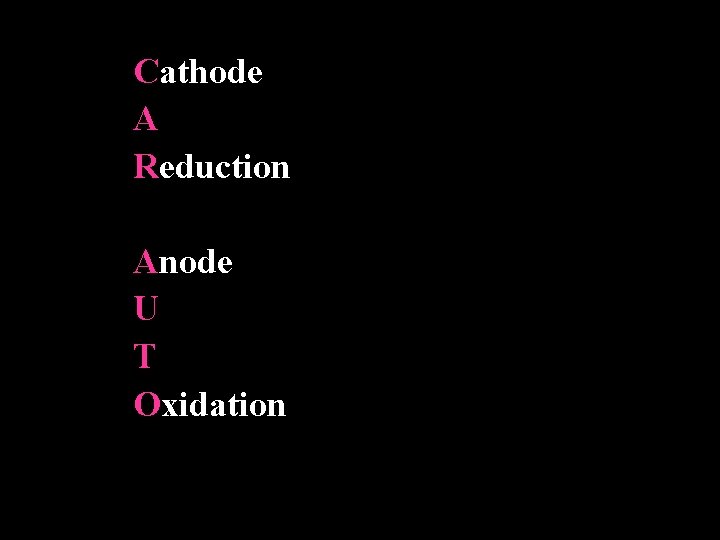
Cathode A Reduction Anode U T Oxidation

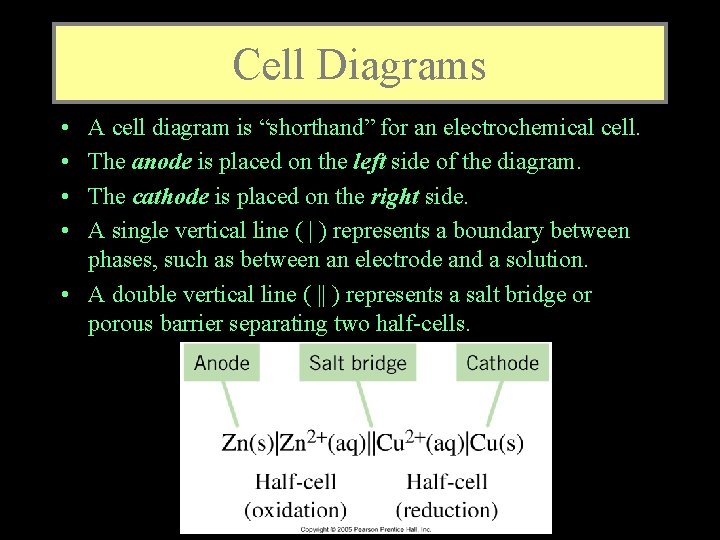
Cell Diagrams • • A cell diagram is “shorthand” for an electrochemical cell. The anode is placed on the left side of the diagram. The cathode is placed on the right side. A single vertical line ( | ) represents a boundary between phases, such as between an electrode and a solution. • A double vertical line ( || ) represents a salt bridge or porous barrier separating two half-cells.

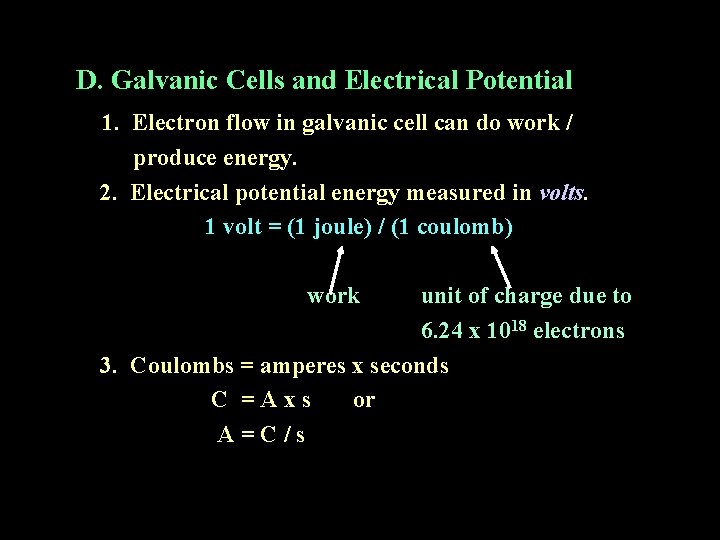
D. Galvanic Cells and Electrical Potential 1. Electron flow in galvanic cell can do work / produce energy. 2. Electrical potential energy measured in volts. 1 volt = (1 joule) / (1 coulomb) work unit of charge due to 6. 24 x 1018 electrons 3. Coulombs = amperes x seconds C =Axs or A=C/s

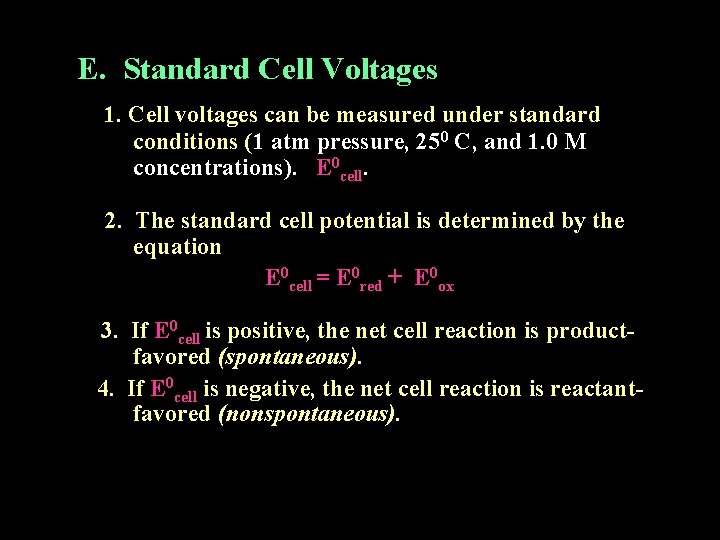
E. Standard Cell Voltages 1. Cell voltages can be measured under standard conditions (1 atm pressure, 250 C, and 1. 0 M concentrations). E 0 cell. 2. The standard cell potential is determined by the equation E 0 cell = E 0 red + E 0 ox 3. If E 0 cell is positive, the net cell reaction is productfavored (spontaneous). 4. If E 0 cell is negative, the net cell reaction is reactantfavored (nonspontaneous).

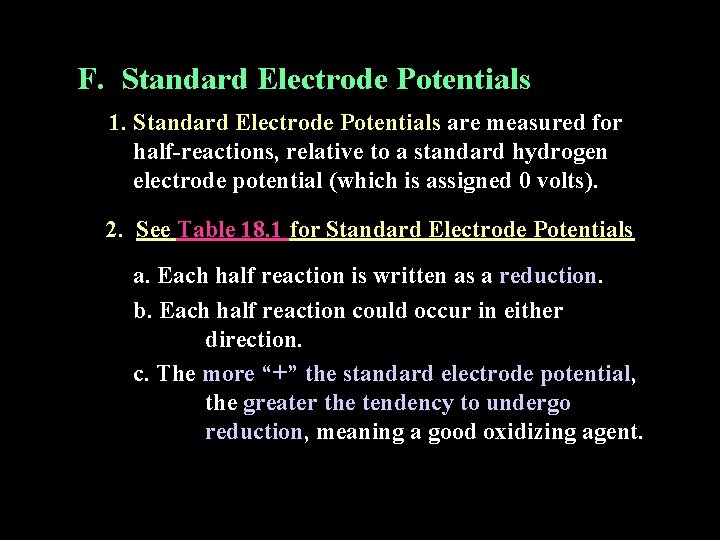
F. Standard Electrode Potentials 1. Standard Electrode Potentials are measured for half-reactions, relative to a standard hydrogen electrode potential (which is assigned 0 volts). 2. See Table 18. 1 for Standard Electrode Potentials a. Each half reaction is written as a reduction. b. Each half reaction could occur in either direction. c. The more “+” the standard electrode potential, the greater the tendency to undergo reduction, meaning a good oxidizing agent.

d. The more “-” the standard electrode potential, the greater the tendency to undergo oxidation, meaning a good reducing agent. e. If a half-reaction is written in the reverse direction, the sign of the corresponding standard electrode potential must change. (Same magnitude, opposite in sign) f. If a half-reaction is multiplied by a factor (coefficient), the standard electrode potential is not multiplied by that factor.

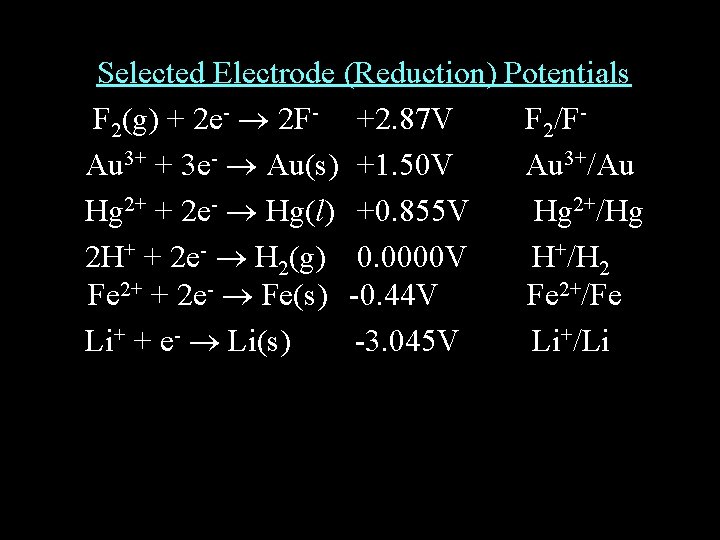
Selected Electrode (Reduction) Potentials F 2(g) + 2 e- 2 F- +2. 87 V F 2/FAu 3+ + 3 e- Au(s) +1. 50 V Au 3+/Au Hg 2+ + 2 e- Hg(l) +0. 855 V Hg 2+/Hg 2 H+ + 2 e- H 2(g) 0. 0000 V H+/H 2 Fe 2+ + 2 e- Fe(s) -0. 44 V Fe 2+/Fe Li+ + e- Li(s) -3. 045 V Li+/Li

G. Problem Solving and Voltaic Cells 1. A voltaic (galvanic) cell is constructed using a piece of Sn in 1. 00 M Sn 2+ for one half-cell and a piece of Au in 1. 00 M Au 3+ for the other half-cell. The reaction is run under standard conditions of pressure and temperature. a. b. c. d. e. Determine the net cell potential (voltage). Determine the net cell reaction. What is the oxidizing agent for the net reaction? What is the reaction occurring at the anode? Sketch the cell information in the following diagram.


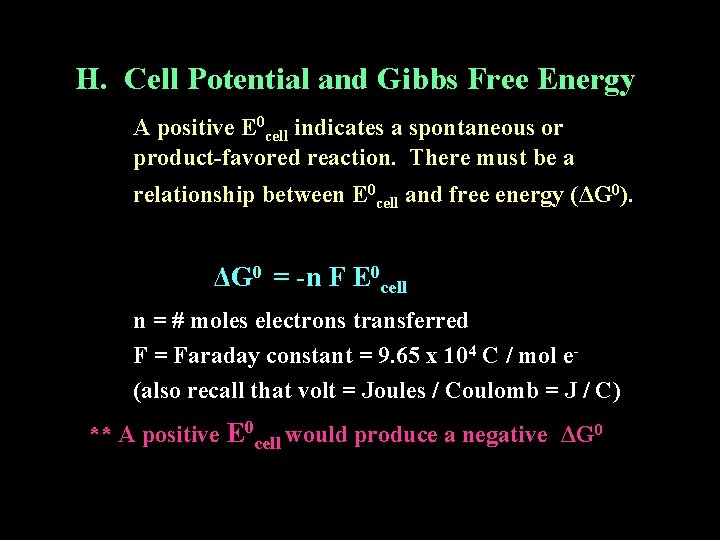
H. Cell Potential and Gibbs Free Energy A positive E 0 cell indicates a spontaneous or product-favored reaction. There must be a relationship between E 0 cell and free energy (ΔG 0). ΔG 0 = -n F E 0 cell n = # moles electrons transferred F = Faraday constant = 9. 65 x 104 C / mol e(also recall that volt = Joules / Coulomb = J / C) ** A positive E 0 cell would produce a negative ΔG 0

1. Calculate the standard free energy change (ΔG 0) for the reaction below under standard conditions. Ni. O 2 + 2 Cl- + 4 H+ Cl 2 + Ni 2+ + 2 H 2 O E 0 cell = 0. 320 v

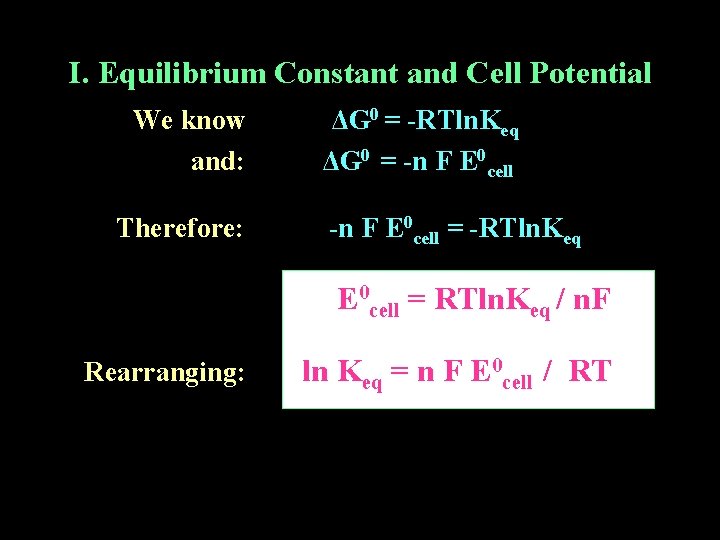
I. Equilibrium Constant and Cell Potential We know and: Therefore: ΔG 0 = -RTln. Keq ΔG 0 = -n F E 0 cell = -RTln. Keq E 0 cell = RTln. Keq / n. F Rearranging: ln Keq = n F E 0 cell / RT

1. Calculate Kc for the following reaction under standard conditions: Cu 2+(aq) + 2 Ag(s) Cu(s) + 2 Ag+(aq) Is the reaction product-favored? Do you expect a large amount of product formed at equilibrium?

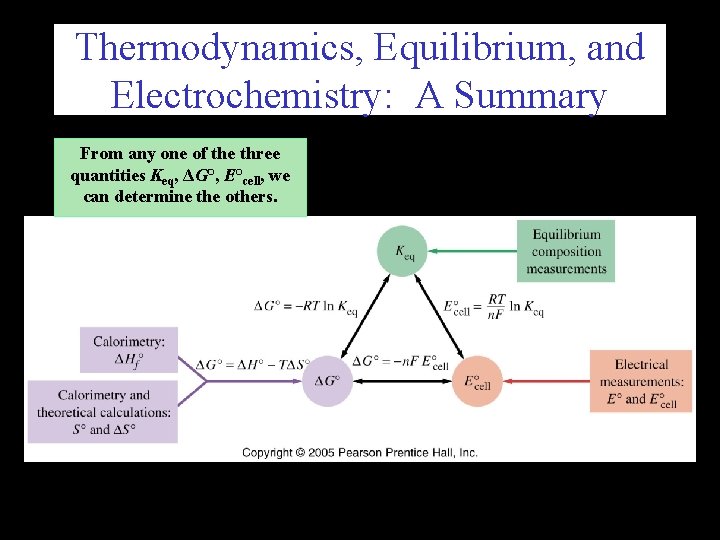
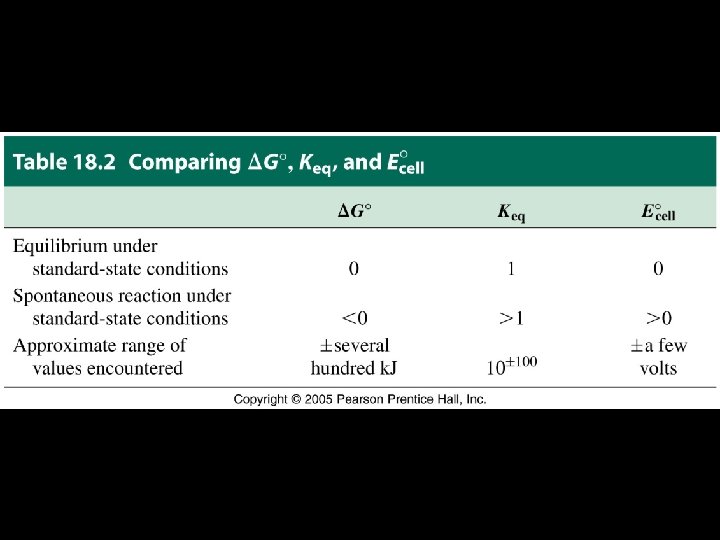
Thermodynamics, Equilibrium, and Electrochemistry: A Summary From any one of the three quantities Keq, ΔG°, E°cell, we can determine the others.


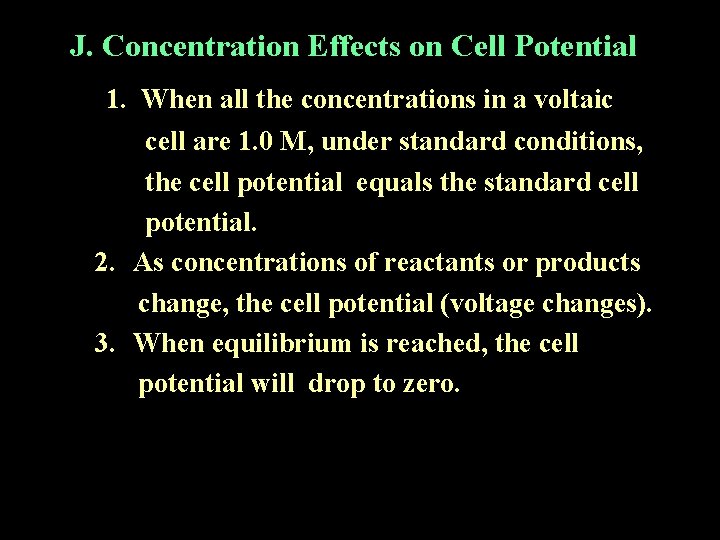
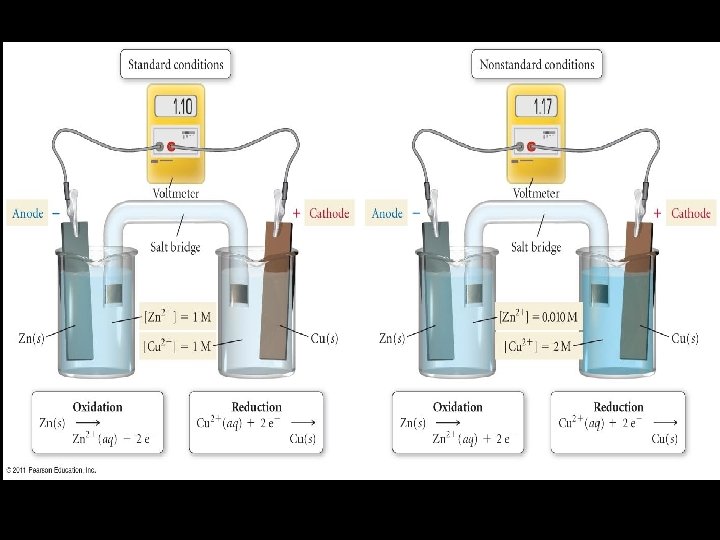
J. Concentration Effects on Cell Potential 1. When all the concentrations in a voltaic cell are 1. 0 M, under standard conditions, the cell potential equals the standard cell potential. 2. As concentrations of reactants or products change, the cell potential (voltage changes). 3. When equilibrium is reached, the cell potential will drop to zero.


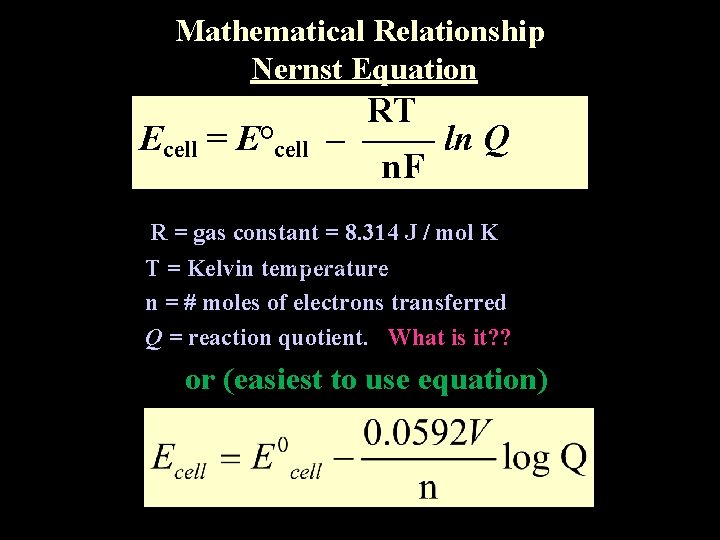
Mathematical Relationship Nernst Equation Ecell = E°cell RT – –––– ln Q n. F R = gas constant = 8. 314 J / mol K T = Kelvin temperature n = # moles of electrons transferred Q = reaction quotient. What is it? ? or (easiest to use equation)

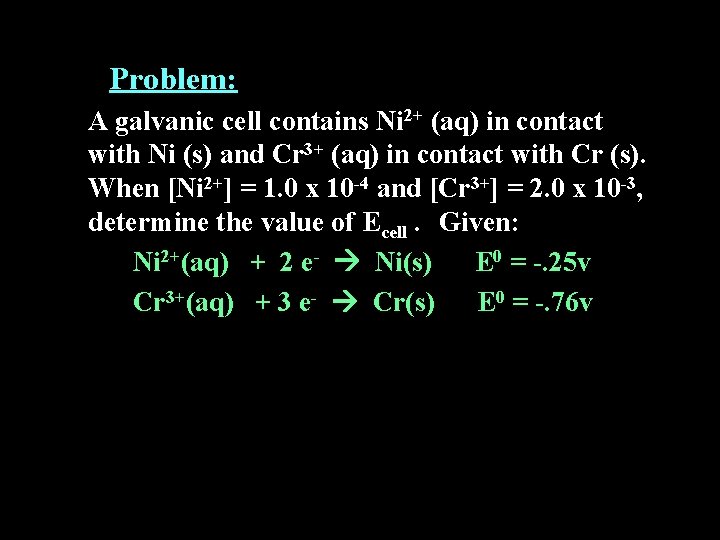
Problem: A galvanic cell contains Ni 2+ (aq) in contact with Ni (s) and Cr 3+ (aq) in contact with Cr (s). When [Ni 2+] = 1. 0 x 10 -4 and [Cr 3+] = 2. 0 x 10 -3, determine the value of Ecell. Given: Ni 2+(aq) + 2 e- Ni(s) E 0 = -. 25 v Cr 3+(aq) + 3 e- Cr(s) E 0 = -. 76 v

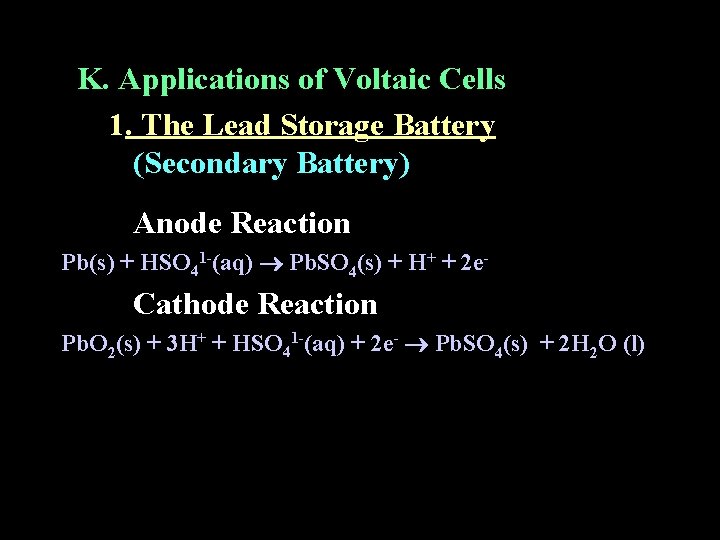
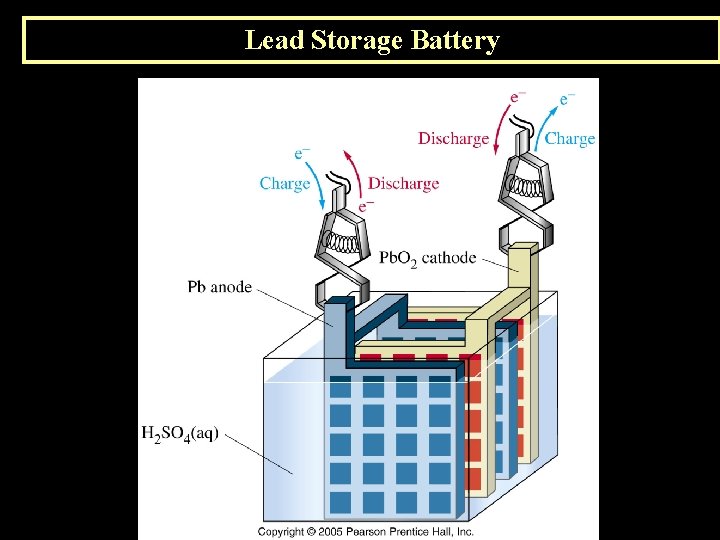
K. Applications of Voltaic Cells 1. The Lead Storage Battery (Secondary Battery) Anode Reaction Pb(s) + HSO 41 -(aq) Pb. SO 4(s) + H+ + 2 e- Cathode Reaction Pb. O 2(s) + 3 H+ + HSO 41 -(aq) + 2 e- Pb. SO 4(s) + 2 H 2 O (l)

Lead Storage Battery

2. Dry Cell Batteries 3. Fuel Cells What are they? Where are they used? How do they differ from batteries?

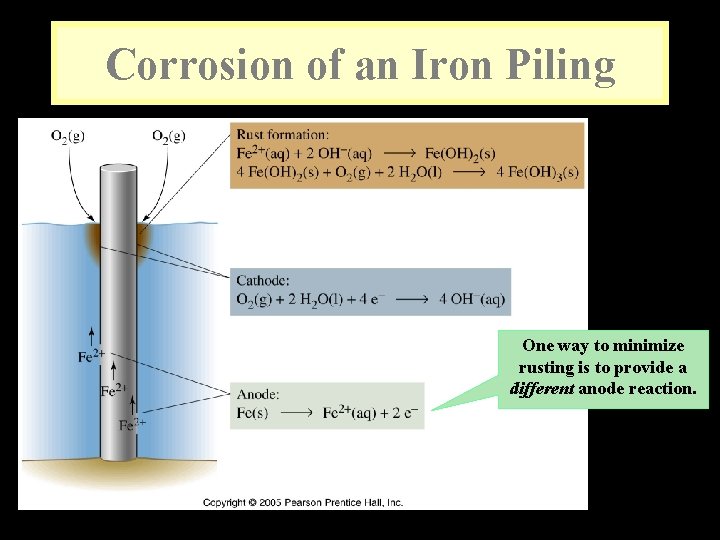
Corrosion of an Iron Piling One way to minimize rusting is to provide a different anode reaction.

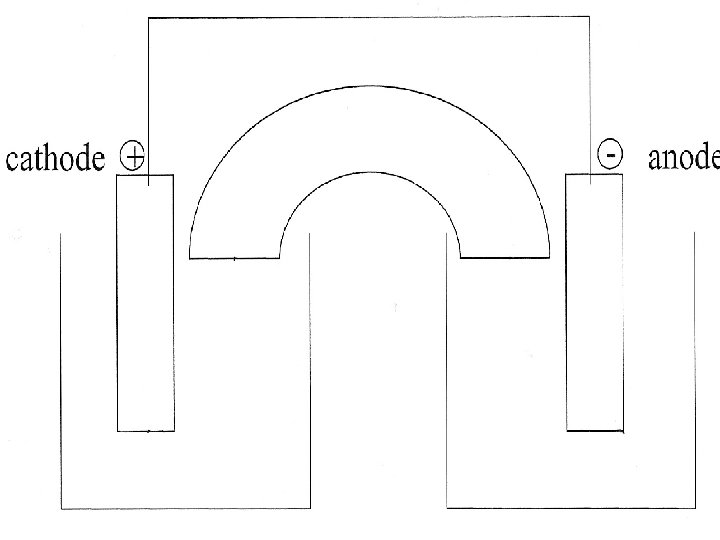
IV. Electrolytic Cells A. Electrolytic vs. Voltaic (Galvanic) Cells Redox reaction which proceeds spontaneously in a product favored direction, generating electricity. Electrolytic Cell Redox reaction in which an electrical current is supplied to drive a nonspontaneous, reactionfavored reaction.

B. Properties of Electrolytic Cell 1. Energy requiring (in form of electric current). 2. No physical separation needed for the two electrode reactions. 3. Usually no salt bridge required. 4. Conducting medium is molten salt or aqueous solution. 5. For electrolytic redox reaction: E 0 cell is negative. ΔG 0 is positive. Kc is small (<1).

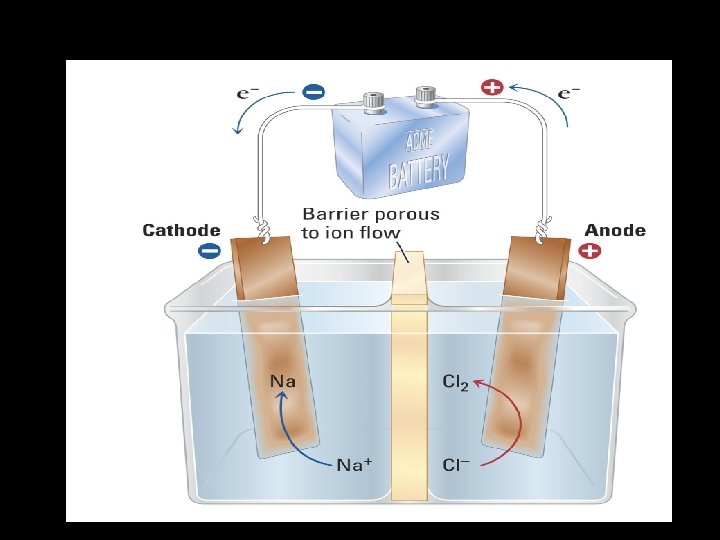
C. Electrolysis of Molten Sodium Chloride 1. Redox Reaction: 2 Na+ + 2 e- 2 Na(l) cathode (reduction) 2 Cl Cl 2(g) + 2 e- anode (oxidation) 2 Na+ + 2 Cl- 2 Na(l) + Cl 2(g) net cell rxn 2. Electrolytic Cell: See next slide



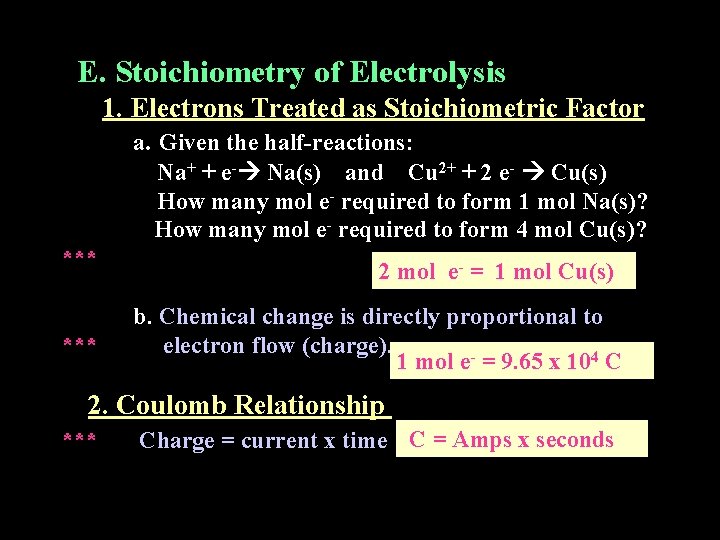
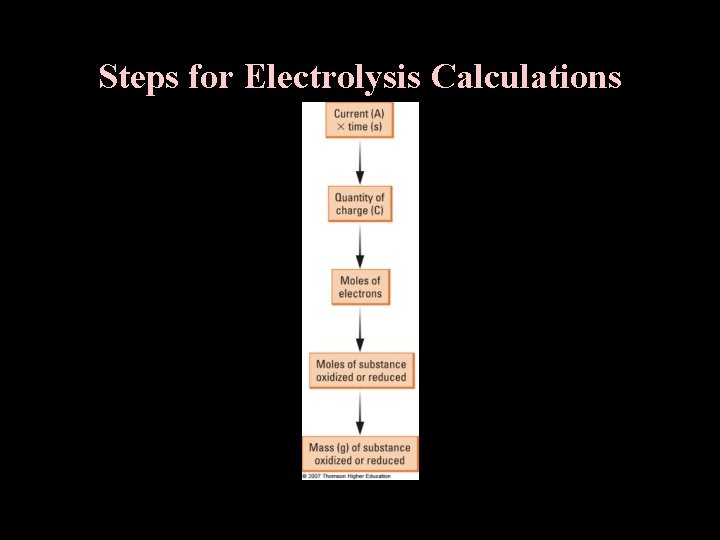
E. Stoichiometry of Electrolysis 1. Electrons Treated as Stoichiometric Factor a. Given the half-reactions: Na+ + e- Na(s) and Cu 2+ + 2 e- Cu(s) How many mol e- required to form 1 mol Na(s)? How many mol e- required to form 4 mol Cu(s)? *** 2 mol e- = 1 mol Cu(s) b. Chemical change is directly proportional to electron flow (charge). 1 mol e- = 9. 65 x 104 C 2. Coulomb Relationship *** Charge = current x time C = Amps x seconds

Steps for Electrolysis Calculations

3. Problem Solving a. How many moles of electrons are required in an electrolytic cell to deposit 2. 00 grams of chromium, Cr(s), from a solution of Cr. Cl 3?

b. What mass of aluminum metal can be produced per hour in the electrolysis of a molten aluminum salt by a current of 26 A?

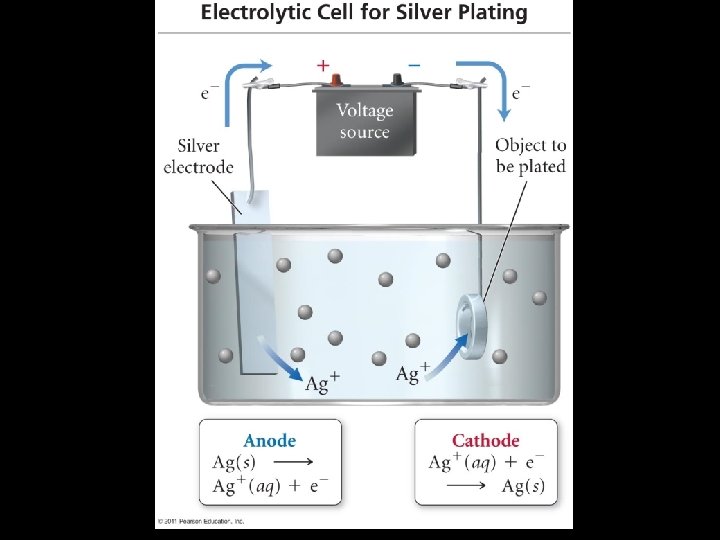
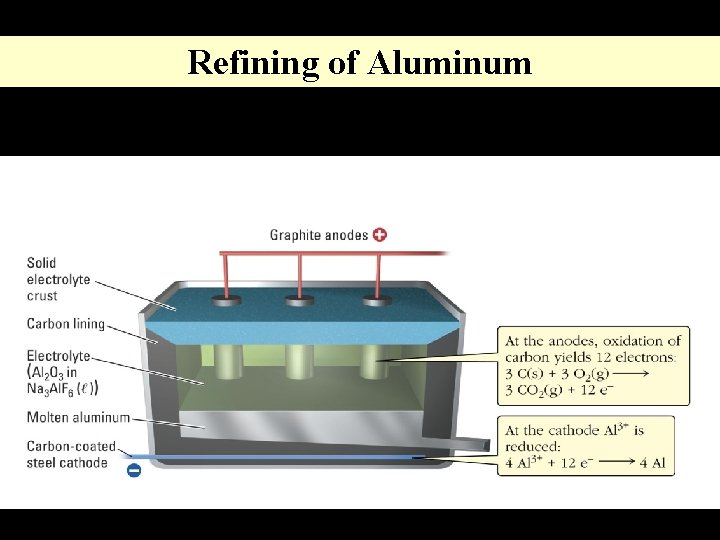
F. Applications of Electrolysis Electroplating Refining of Metals Copper Aluminum

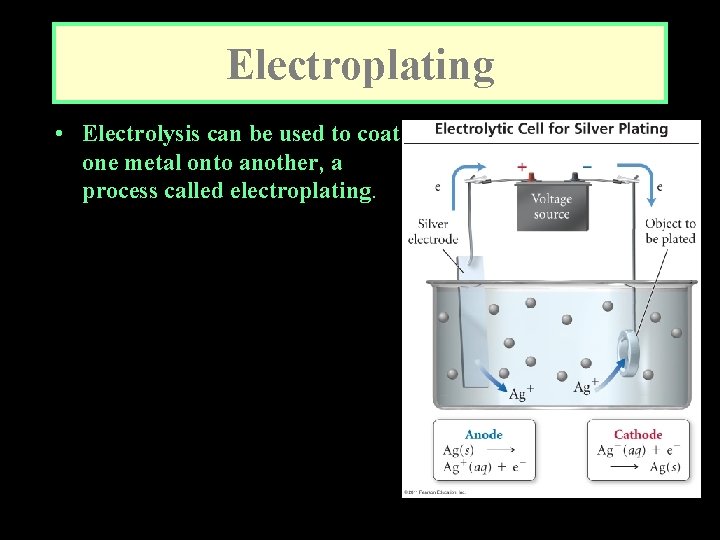
Electroplating • Electrolysis can be used to coat one metal onto another, a process called electroplating. • Usually, the object to be electroplated, such as a spoon, is cast of an inexpensive metal. It is then coated with a thin layer of a more attractive, corrosionresistant, and expensive metal, such as silver or gold.

Refining of Aluminum