ELECTROCHEMISTRY Chapter 18 1 Why Study Electrochemistry 2












- Slides: 54

ELECTROCHEMISTRY Chapter 18 1

Why Study Electrochemistry? 2 • It’s important because it involves many of the devices that we use every day that are battery powered. • Corrosion • Industrial production of chemicals such as Cl 2, Na. OH, F 2 and Al.

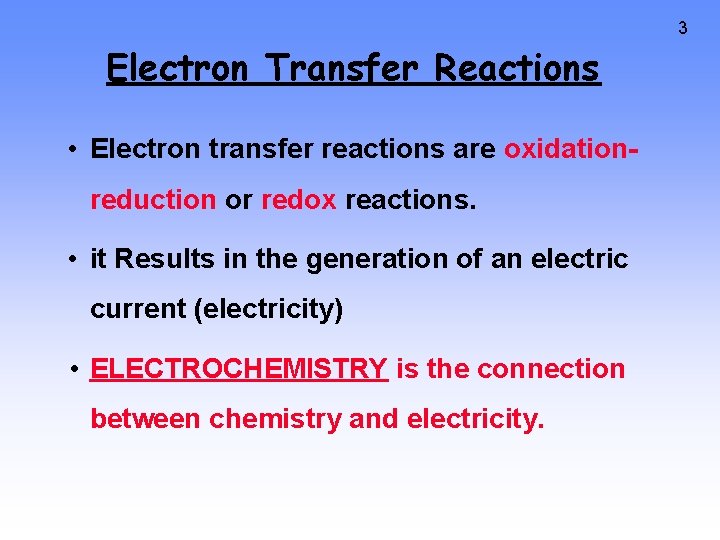
3 Electron Transfer Reactions • Electron transfer reactions are oxidationreduction or redox reactions. • it Results in the generation of an electric current (electricity) • ELECTROCHEMISTRY is the connection between chemistry and electricity.

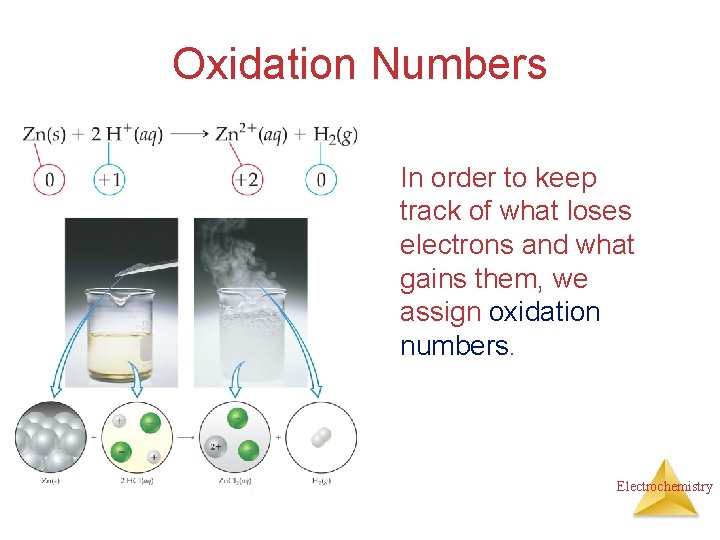
Oxidation Numbers In order to keep track of what loses electrons and what gains them, we assign oxidation numbers. Electrochemistry

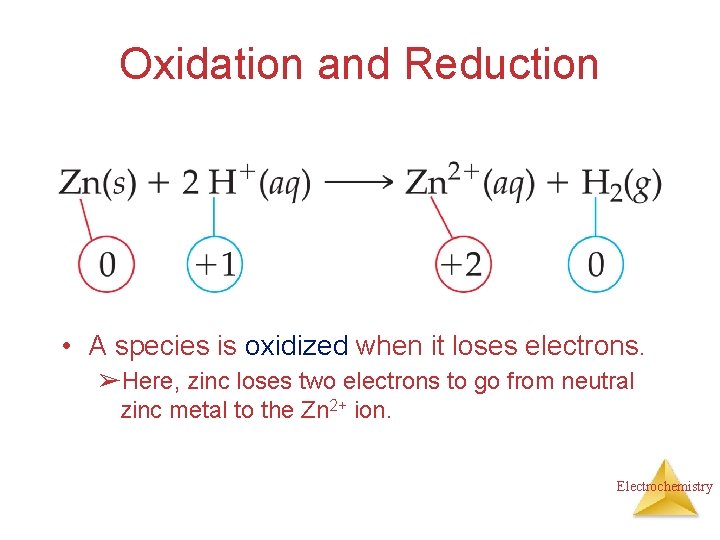
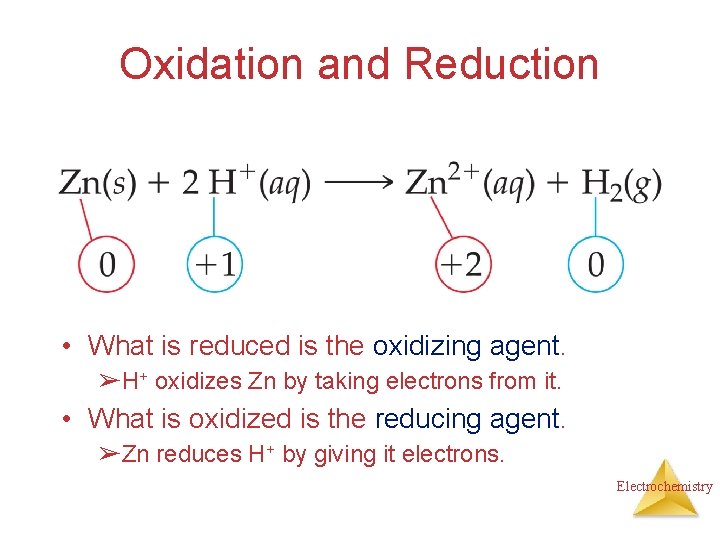
Oxidation and Reduction • A species is oxidized when it loses electrons. ➢Here, zinc loses two electrons to go from neutral zinc metal to the Zn 2+ ion. Electrochemistry

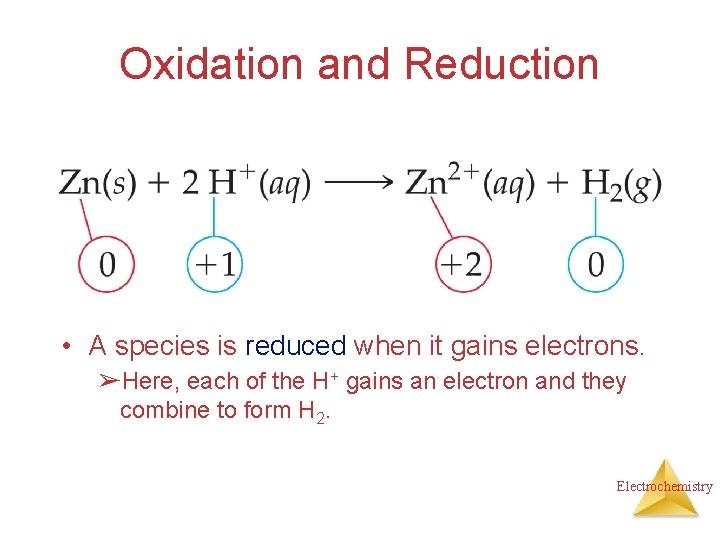
Oxidation and Reduction • A species is reduced when it gains electrons. ➢Here, each of the H+ gains an electron and they combine to form H 2. Electrochemistry

Oxidation and Reduction • What is reduced is the oxidizing agent. ➢H+ oxidizes Zn by taking electrons from it. • What is oxidized is the reducing agent. ➢Zn reduces H+ by giving it electrons. Electrochemistry

8 You can’t have one… without the other! • Reduction (gaining electrons) can’t happen without an oxidation to provide the electrons. • You can’t have 2 oxidations or 2 reductions in the same equation. Reduction has to occur at the cost of oxidation LEO the lion says GER!

9 Another way to remember • OIL RIG Oxidation is loss of electrons and Reduction is gain of electrons.

The Big Idea! In spontaneous oxidation-reduction (redox) reactions, electrons are transferred and energy is released. Electrochemistry

Voltaic Cells • We can use the energy from the Redox Rxn to do work if we make the electrons flow through an external device. • We call such a setup a voltaic cell or a Galvanic cell or even an electrochemical cell. . Electrochemistry

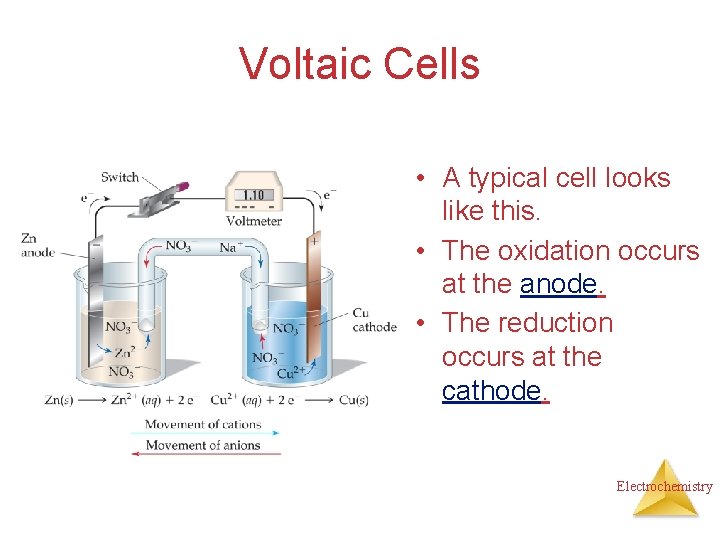
Voltaic Cells • A typical cell looks like this. • The oxidation occurs at the anode. • The reduction occurs at the cathode. Electrochemistry

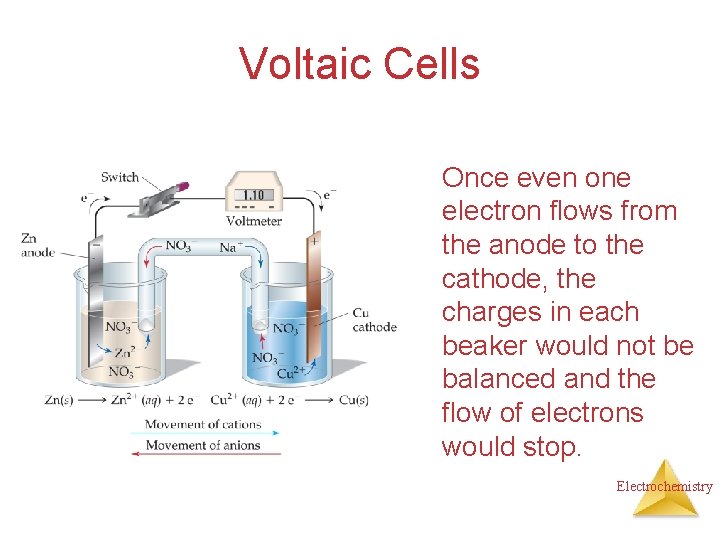
Voltaic Cells Once even one electron flows from the anode to the cathode, the charges in each beaker would not be balanced and the flow of electrons would stop. Electrochemistry

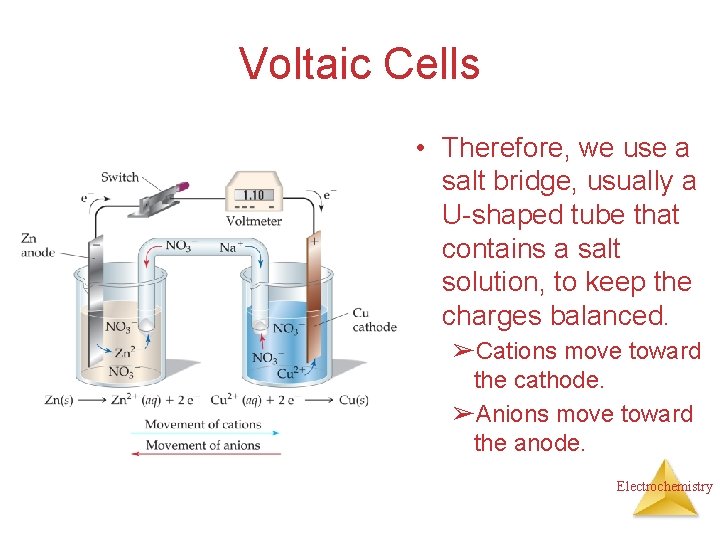
Voltaic Cells • Therefore, we use a salt bridge, usually a U-shaped tube that contains a salt solution, to keep the charges balanced. ➢Cations move toward the cathode. ➢Anions move toward the anode. Electrochemistry

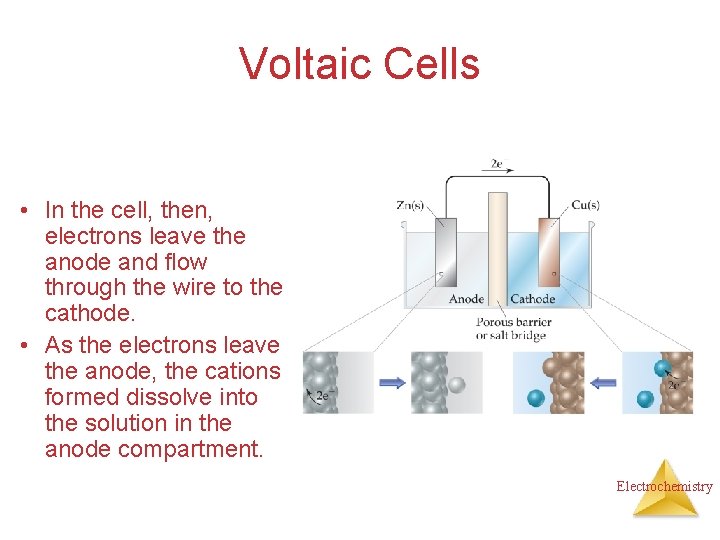
Voltaic Cells • In the cell, then, electrons leave the anode and flow through the wire to the cathode. • As the electrons leave the anode, the cations formed dissolve into the solution in the anode compartment. Electrochemistry

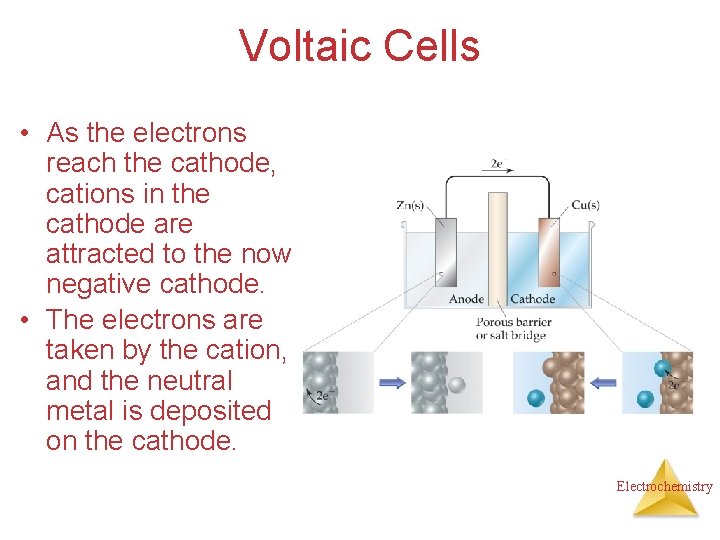
Voltaic Cells • As the electrons reach the cathode, cations in the cathode are attracted to the now negative cathode. • The electrons are taken by the cation, and the neutral metal is deposited on the cathode. Electrochemistry

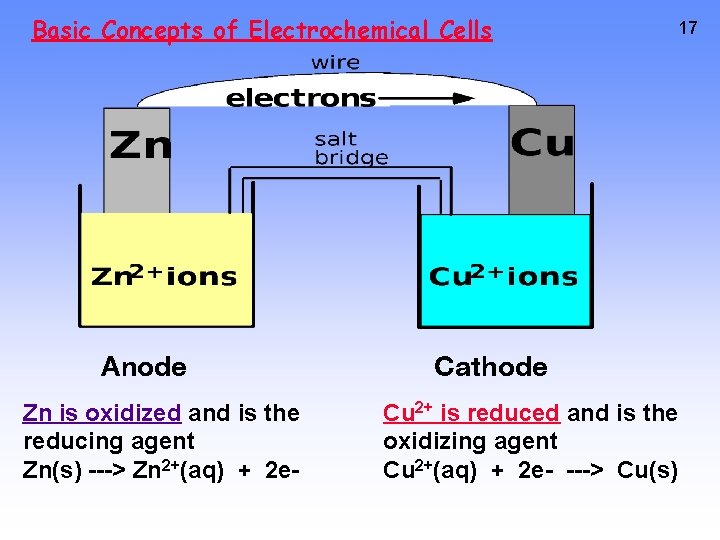
Basic Concepts of Electrochemical Cells Anode Zn is oxidized and is the reducing agent Zn(s) ---> Zn 2+(aq) + 2 e- 17 Cathode Cu 2+ is reduced and is the oxidizing agent Cu 2+(aq) + 2 e- ---> Cu(s)

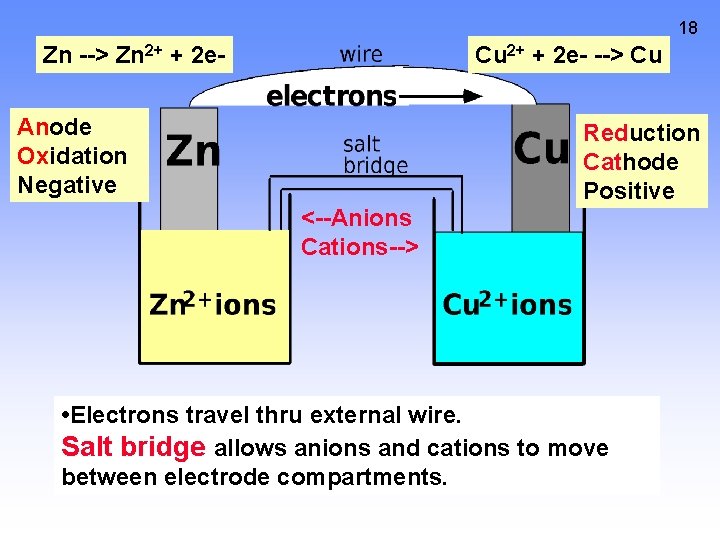
18 Zn --> Zn 2+ + 2 e- Cu 2+ + 2 e- --> Cu Anode Oxidation Negative Reduction Cathode Positive <--Anions Cations--> • Electrons travel thru external wire. Salt bridge allows anions and cations to move between electrode compartments.

Cell Potential • Water only spontaneously flows one way in a waterfall. • Likewise, electrons only spontaneously flow one way in a redox reaction— from higher to lower potential energy. Electrochemistry

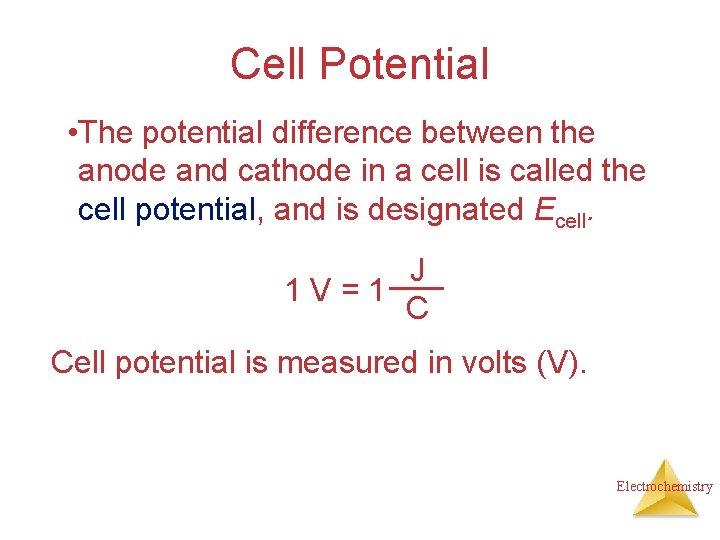
Cell Potential • The potential difference between the anode and cathode in a cell is called the cell potential, and is designated Ecell. J 1 V=1 C Cell potential is measured in volts (V). Electrochemistry

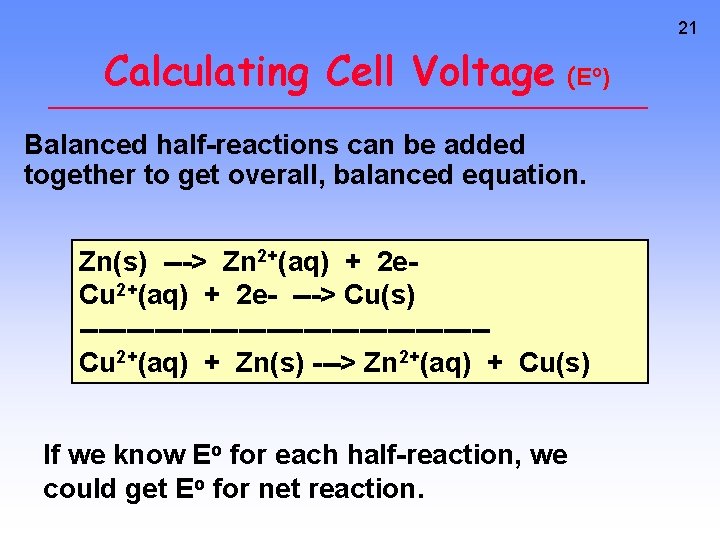
21 Calculating Cell Voltage (E ) o Balanced half-reactions can be added together to get overall, balanced equation. Zn(s) ---> Zn 2+(aq) + 2 e. Cu 2+(aq) + 2 e- ---> Cu(s) ----------------------Cu 2+(aq) + Zn(s) ---> Zn 2+(aq) + Cu(s) If we know Eo for each half-reaction, we could get Eo for net reaction.

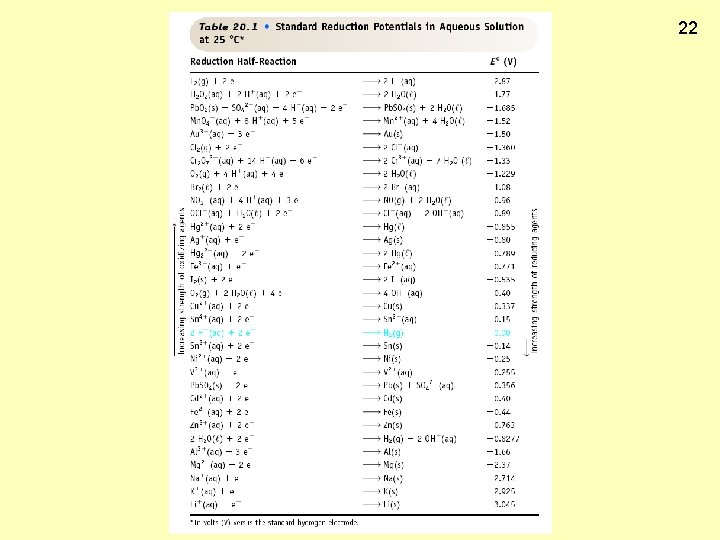
22

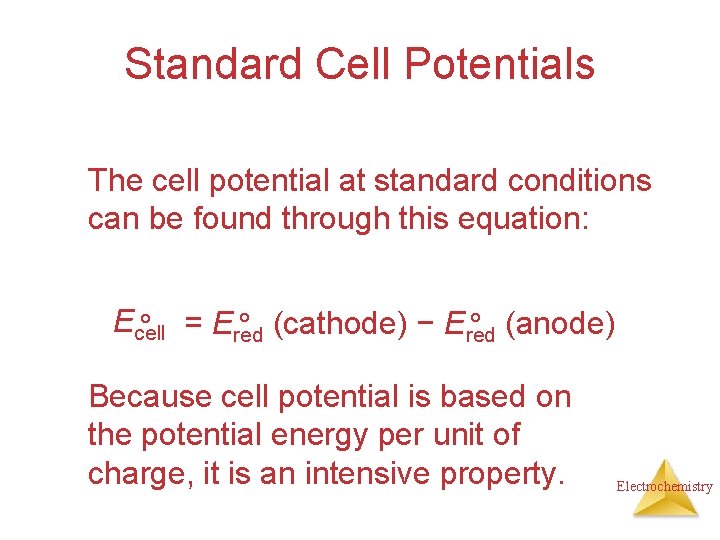
Standard Cell Potentials The cell potential at standard conditions can be found through this equation: Ecell ° (cathode) − Ered ° (anode) ° = Ered Because cell potential is based on the potential energy per unit of charge, it is an intensive property. Electrochemistry

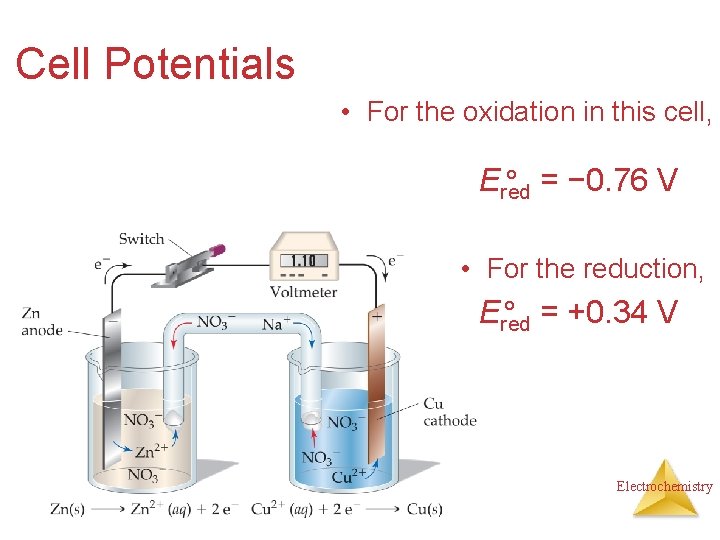
Cell Potentials • For the oxidation in this cell, Ered ° = − 0. 76 V • For the reduction, Ered ° = +0. 34 V Electrochemistry

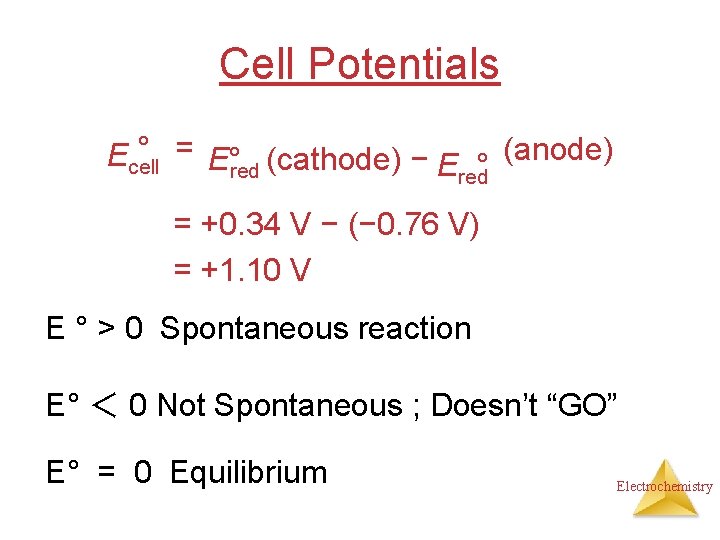
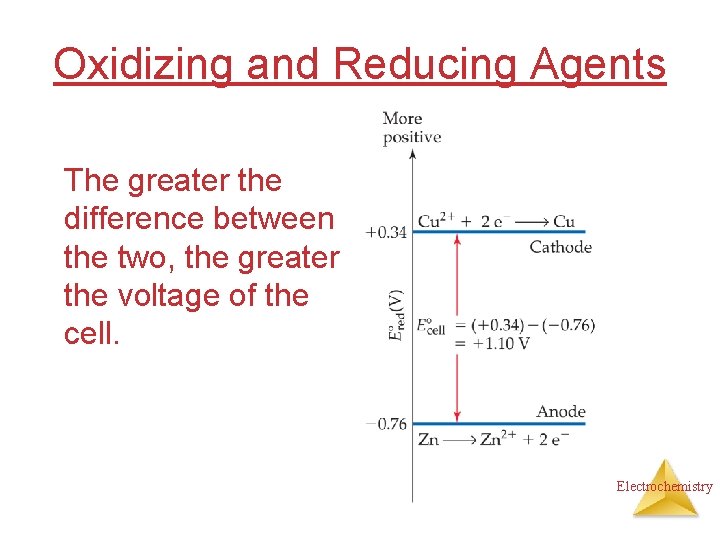
Cell Potentials ° = E° (cathode) − (anode) Ecell Ered° red = +0. 34 V − (− 0. 76 V) = +1. 10 V E ° > 0 Spontaneous reaction E° < 0 Not Spontaneous ; Doesn’t “GO” E° = 0 Equilibrium Electrochemistry

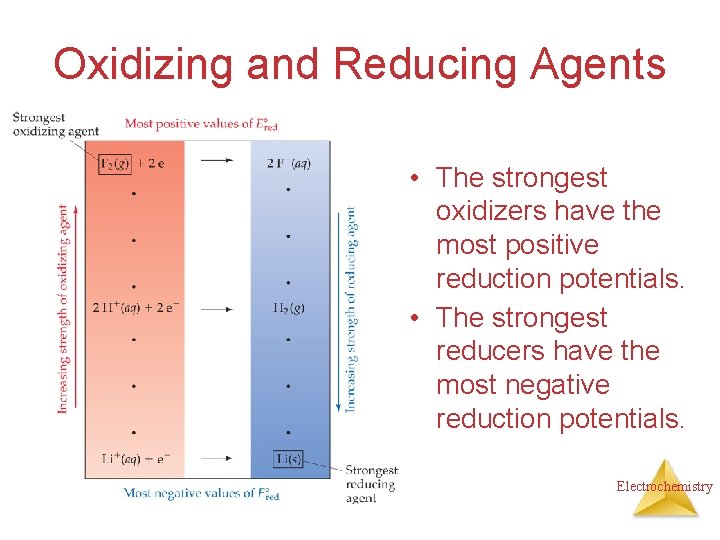
Oxidizing and Reducing Agents • The strongest oxidizers have the most positive reduction potentials. • The strongest reducers have the most negative reduction potentials. Electrochemistry

Oxidizing and Reducing Agents The greater the difference between the two, the greater the voltage of the cell. Electrochemistry

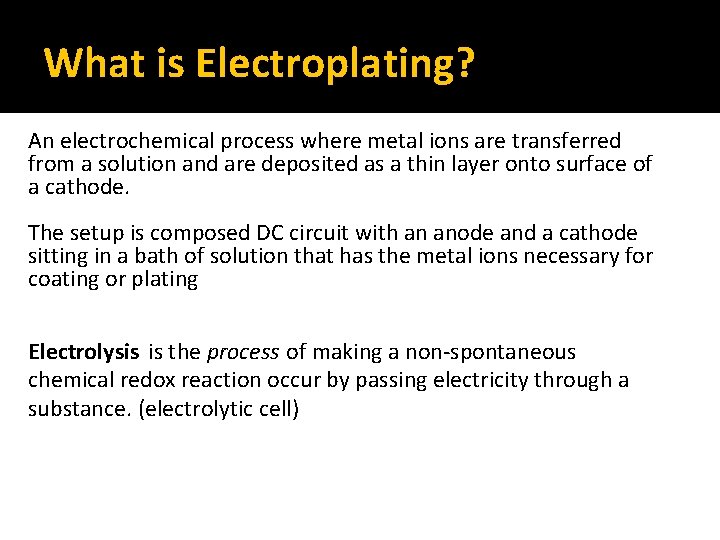
What is Electroplating? An electrochemical process where metal ions are transferred from a solution and are deposited as a thin layer onto surface of a cathode. The setup is composed DC circuit with an anode and a cathode sitting in a bath of solution that has the metal ions necessary for coating or plating Electrolysis is the process of making a non-spontaneous chemical redox reaction occur by passing electricity through a substance. (electrolytic cell)

History � In 1805, Italian chemist Luigi Brugnatelli, successfully electroplated silver medals with gold � 1940 first patent for electroplating awarded, and soon factories in England were mass producing silver plated items, including teapots, brushes and utensils Wikienergia. com

Benefits of Electroplating can enhance: ▪ Chemical properties---increase corrosion resistance ▪ Physical properties---increase thickness of part ▪ Mechanical properties---increase tensile strength & hardness Commercial Examples: �Coating jewelry with thin layer of expensive metal. �Coating chromium over steel to make rust resistant.

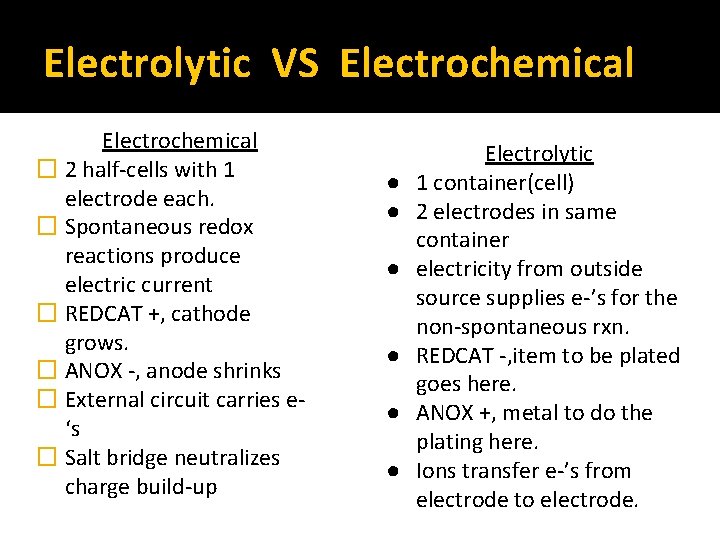
Electrolytic VS Electrochemical � 2 half-cells with 1 electrode each. � Spontaneous redox reactions produce electric current � REDCAT +, cathode grows. � ANOX -, anode shrinks � External circuit carries e‘s � Salt bridge neutralizes charge build-up ● ● ● Electrolytic 1 container(cell) 2 electrodes in same container electricity from outside source supplies e-’s for the non-spontaneous rxn. REDCAT -, item to be plated goes here. ANOX +, metal to do the plating here. Ions transfer e-’s from electrode to electrode.

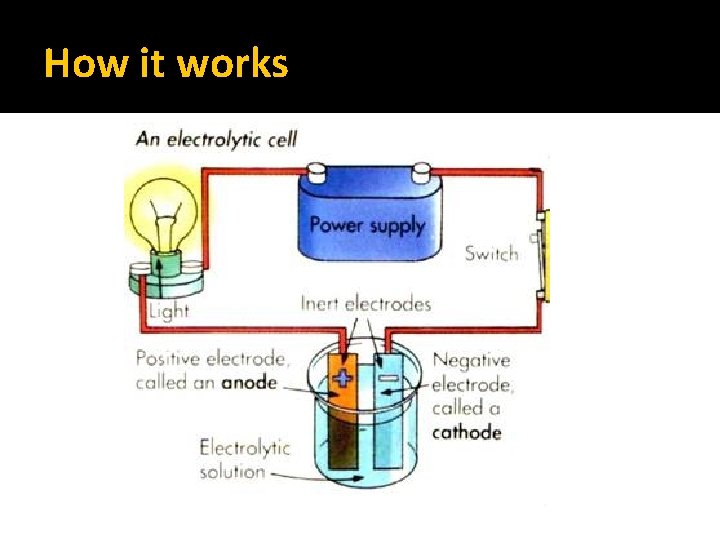
How it Works �In electroplating, the anode is made up of the metal you want to coat the surface of another metal with. �There is also a salt solution present of the anode metal. �While electrolysis is taking place, the anode metal is oxidized and goes into solution as positive ions. �These positive ions are then reduced on the surface of the cathode (the metal you wish to coat).

How it works

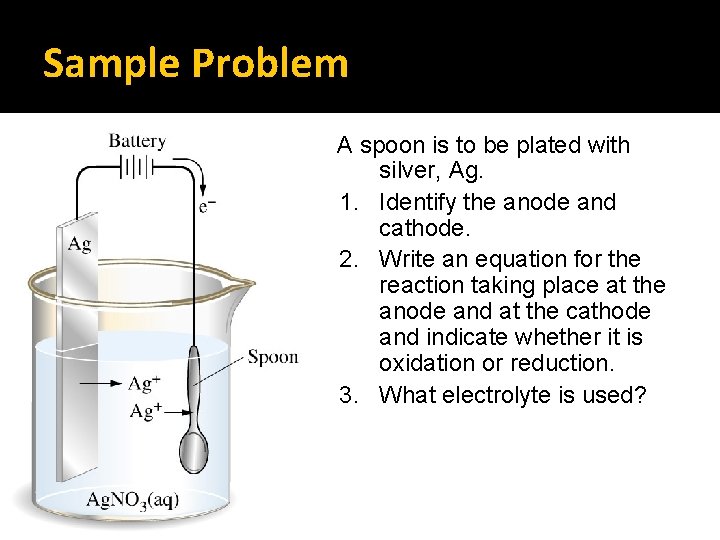
Sample Problem A spoon is to be plated with silver, Ag. 1. Identify the anode and cathode. 2. Write an equation for the reaction taking place at the anode and at the cathode and indicate whether it is oxidation or reduction. 3. What electrolyte is used?

Answers 1. Cathode = spoon (metal object to be coated), Anode = silver electrode 2. As electrolysis takes place, the silver anode is oxidized, Ag(s) → Ag+(aq) + 1 e�The Ag+ (aq) ions in solution travel to the spoon cathode and are reduced to form neutral Ag(s) on the surface of the spoon (cathode): Ag+ (aq) + 1 e- → Ag(s) 3. Electrolyte solution is made of Ag. NO 3

electric Current An ampere is a unit of measure of the rate of electron flow or current in an electrical conductor. One ampere of current represents one coulomb of electrical charge (6. 24 x 1018 charge carriers) moving past a specific point in one second. 1 mole = 96 500 Coulombs The coulomb (symbolized C) is the standard unit of 23 International electric the 1 mole = charge 6. 02 xin 10 electrons System of Units (SI). It is a dimensionless quantity, sharing this aspect with the mole.

Stoichiometry of Electrolysis problems To determine the quantity of substance either produced or consumed during electrolysis given the time a known current flowed: Write the balanced half-reactions involved. Calculate the number of moles of electrons that were transferred. Calculate the number of moles of substance that was produced/consumed at the electrode. Convert the moles of substance to desired units of measure.

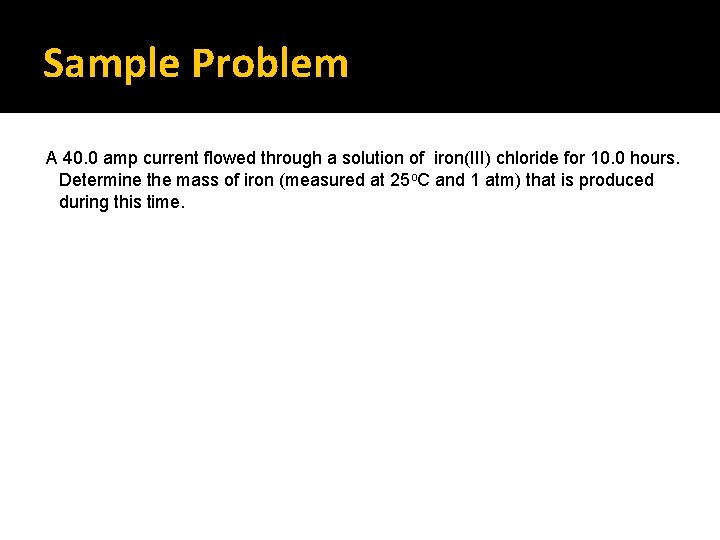
Sample Problem A 40. 0 amp current flowed through a solution of iron(III) chloride for 10. 0 hours. Determine the mass of iron (measured at 25 o. C and 1 atm) that is produced during this time.

Calculating the time required To determine the quantity of time required to produce a known quantity of a substance given the amount of current that flowed: ● Find the quantity of substance produced/consumed in moles. ● Write the balanced half-reaction involved. ● Calculate the number of moles of electrons required. ● Convert the moles of electrons into coulombs. ● Calculate the time required.

Sample Problem How long must a 20. 0 amp current flow through a solution of Zn. SO 4 in order to produce 25. 00 g of Zn metal.

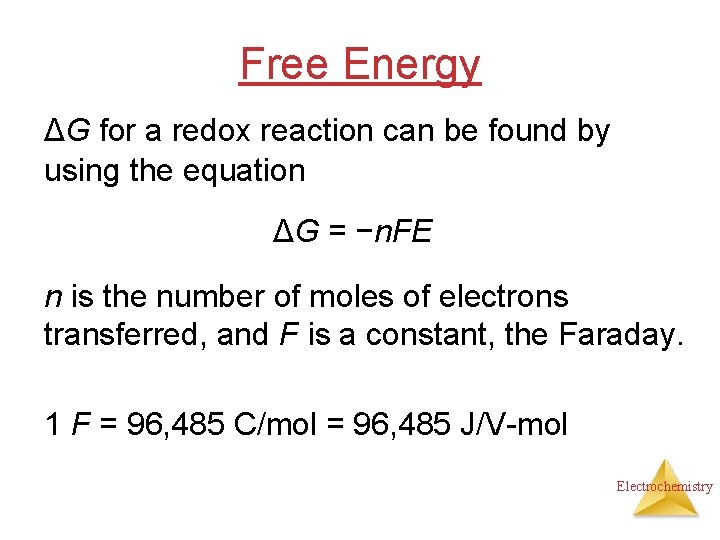
Free Energy ΔG for a redox reaction can be found by using the equation ΔG = −n. FE n is the number of moles of electrons transferred, and F is a constant, the Faraday. 1 F = 96, 485 C/mol = 96, 485 J/V-mol Electrochemistry

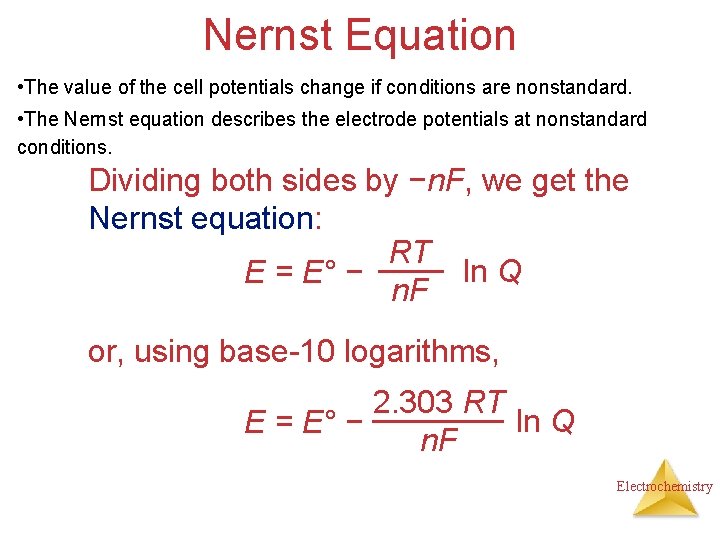
Nernst Equation • The value of the cell potentials change if conditions are nonstandard. • The Nernst equation describes the electrode potentials at nonstandard conditions. Dividing both sides by −n. F, we get the Nernst equation: RT ln Q E = E° − n. F or, using base-10 logarithms, 2. 303 RT ln Q E = E° − n. F Electrochemistry

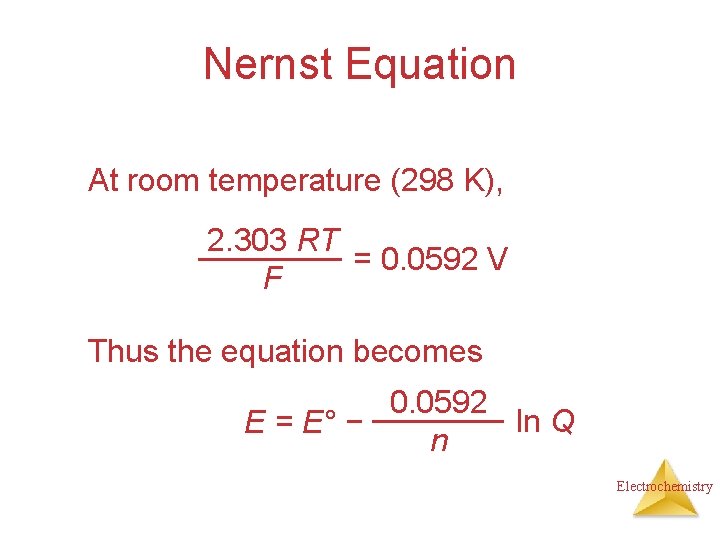
Nernst Equation At room temperature (298 K), 2. 303 RT = 0. 0592 V F Thus the equation becomes 0. 0592 ln Q E = E° − n Electrochemistry

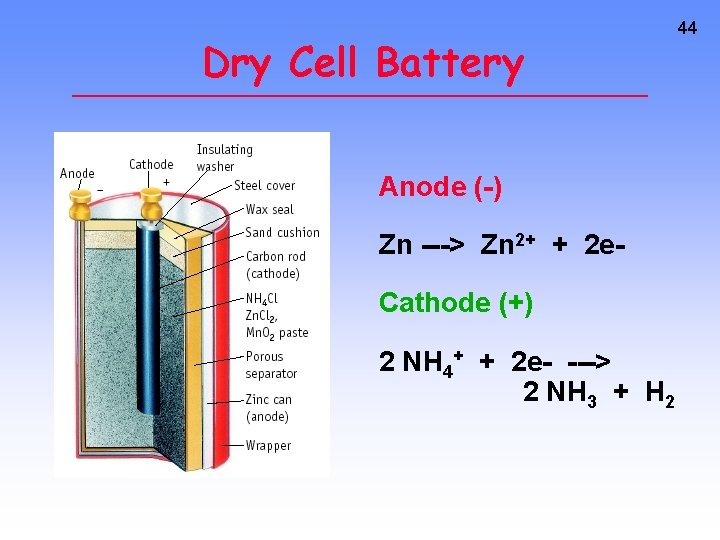
Dry Cell Battery Anode (-) Zn ---> Zn 2+ + 2 e. Cathode (+) 2 NH 4+ + 2 e- ---> 2 NH 3 + H 2 44

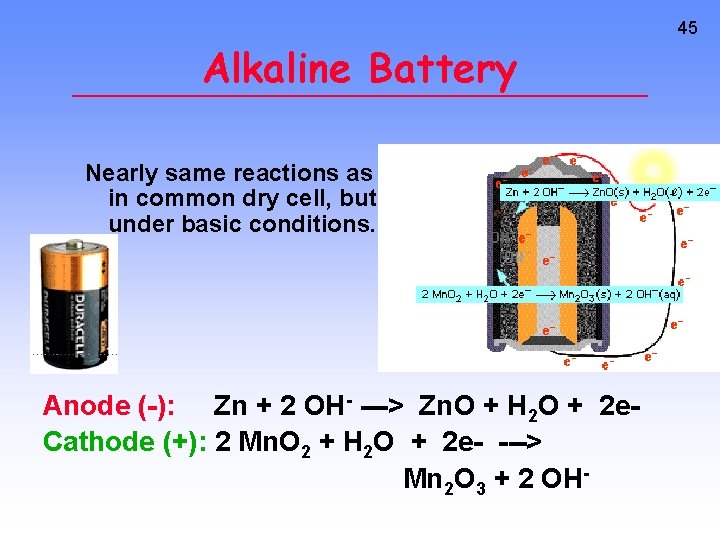
45 Alkaline Battery Nearly same reactions as in common dry cell, but under basic conditions. Anode (-): Zn + 2 OH- ---> Zn. O + H 2 O + 2 e. Cathode (+): 2 Mn. O 2 + H 2 O + 2 e- ---> Mn 2 O 3 + 2 OH-

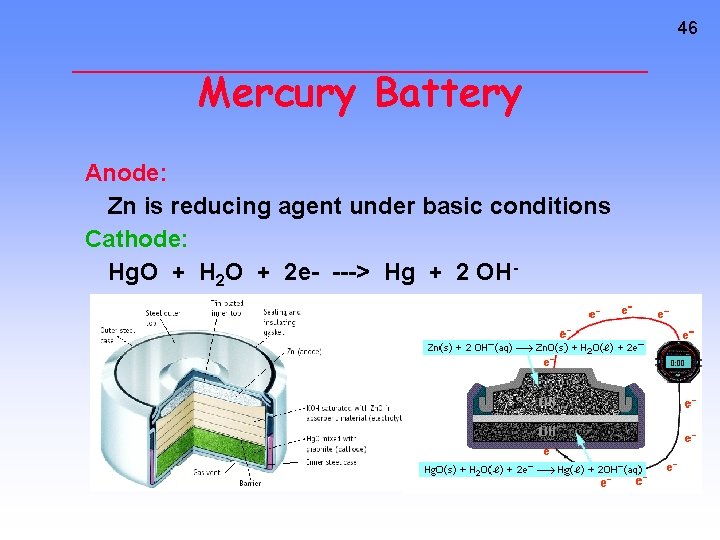
46 Mercury Battery Anode: Zn is reducing agent under basic conditions Cathode: Hg. O + H 2 O + 2 e- ---> Hg + 2 OH-

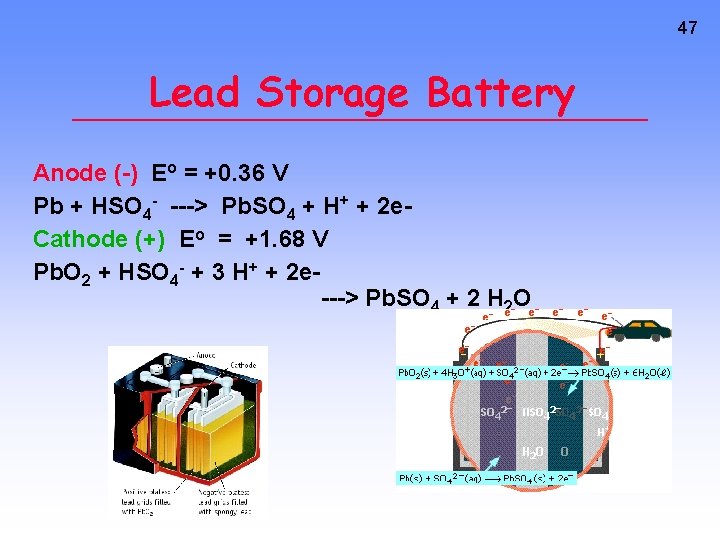
47 Lead Storage Battery Anode (-) Eo = +0. 36 V Pb + HSO 4 - ---> Pb. SO 4 + H+ + 2 e. Cathode (+) Eo = +1. 68 V Pb. O 2 + HSO 4 - + 3 H+ + 2 e---> Pb. SO 4 + 2 H 2 O

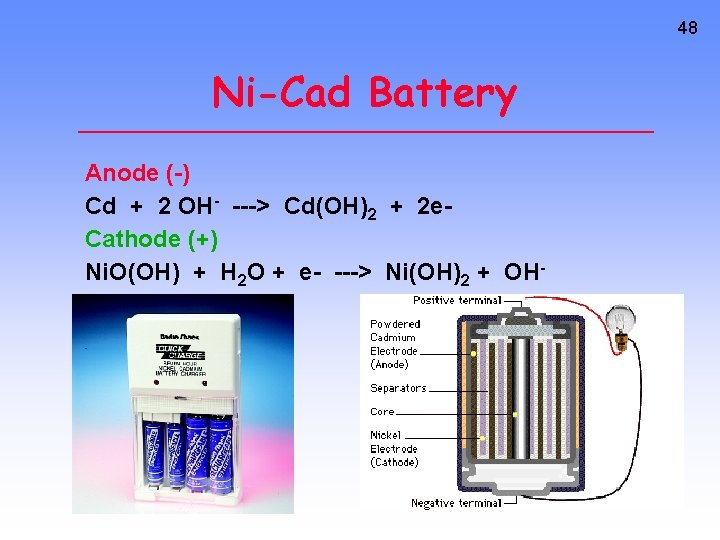
48 Ni-Cad Battery Anode (-) Cd + 2 OH- ---> Cd(OH)2 + 2 e. Cathode (+) Ni. O(OH) + H 2 O + e- ---> Ni(OH)2 + OH-

H 2 as a Fuel Cars can use electricity generated by H 2/O 2 fuel cells. H 2 carried in tanks or generated from hydrocarbons 49

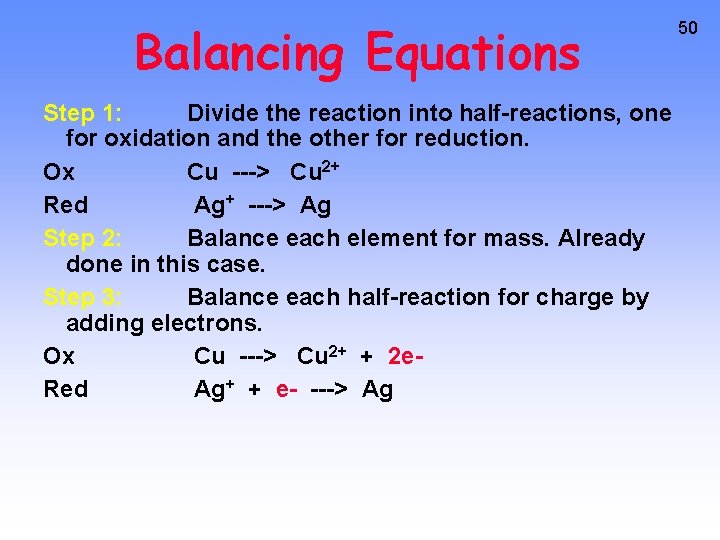
Balancing Equations Step 1: Divide the reaction into half-reactions, one for oxidation and the other for reduction. Ox Cu ---> Cu 2+ Red Ag+ ---> Ag Step 2: Balance each element for mass. Already done in this case. Step 3: Balance each half-reaction for charge by adding electrons. Ox Cu ---> Cu 2+ + 2 e. Red Ag+ + e- ---> Ag 50

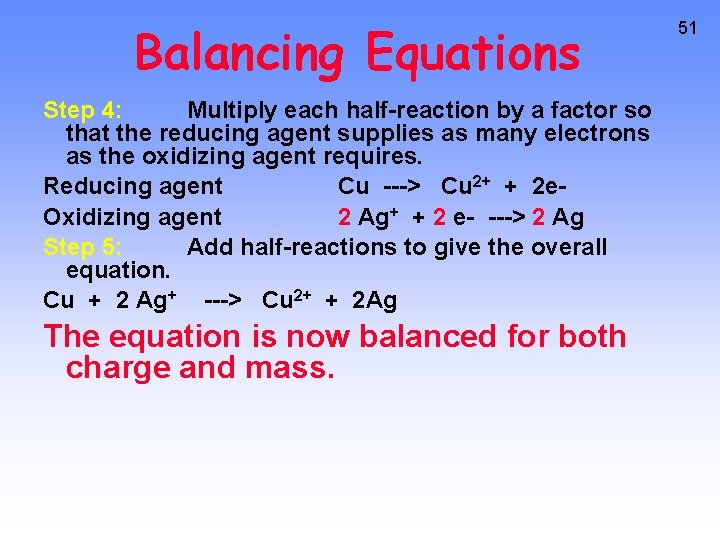
Balancing Equations Step 4: Multiply each half-reaction by a factor so that the reducing agent supplies as many electrons as the oxidizing agent requires. Reducing agent Cu ---> Cu 2+ + 2 e. Oxidizing agent 2 Ag+ + 2 e- ---> 2 Ag Step 5: Add half-reactions to give the overall equation. Cu + 2 Ag+ ---> Cu 2+ + 2 Ag The equation is now balanced for both charge and mass. 51

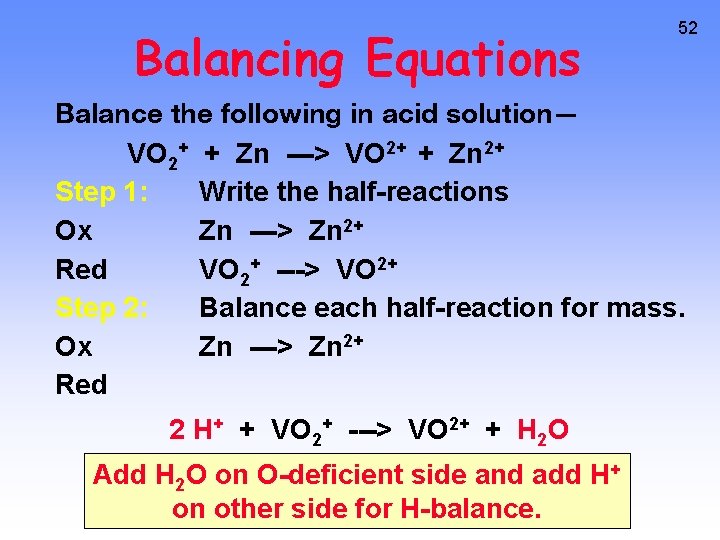
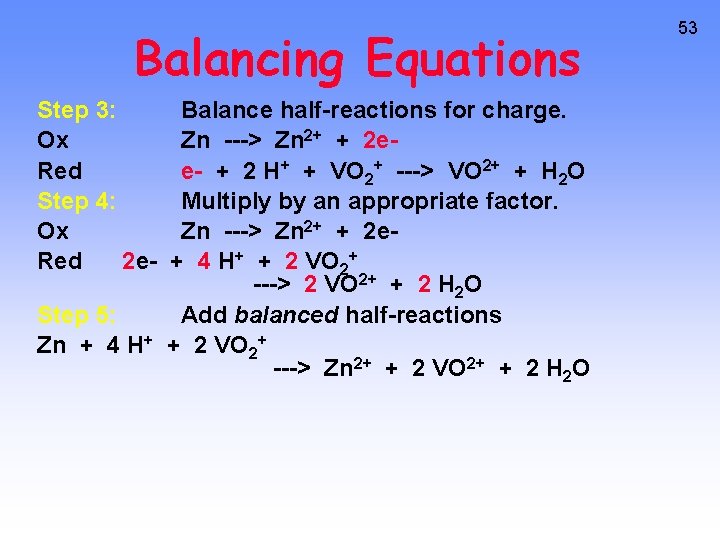
Balancing Equations 52 Balance the following in acid solution— VO 2+ + Zn ---> VO 2+ + Zn 2+ Step 1: Write the half-reactions Ox Zn ---> Zn 2+ Red VO 2+ ---> VO 2+ Step 2: Balance each half-reaction for mass. Ox Zn ---> Zn 2+ Red 2 H+ + VO 2+ ---> VO 2+ + H 2 O Add H 2 O on O-deficient side and add H+ on other side for H-balance.

Balancing Equations Step 3: Ox Red Step 4: Ox Red 2 e- Balance half-reactions for charge. Zn ---> Zn 2+ + 2 ee- + 2 H+ + VO 2+ ---> VO 2+ + H 2 O Multiply by an appropriate factor. Zn ---> Zn 2+ + 2 e+ 4 H+ + 2 VO 2+ ---> 2 VO 2+ + 2 H 2 O Step 5: Add balanced half-reactions Zn + 4 H+ + 2 VO 2+ ---> Zn 2+ + 2 VO 2+ + 2 H 2 O 53

54 Tips on Balancing Equations • Never add O 2, O atoms, or O 2 - to balance oxygen. • Never add H 2 or H atoms to balance hydrogen. • Be sure to write the correct charges on all the ions. • Check your work at the end to make sure mass and charge are balanced. • PRACTICE!