Spontaneity Entropy Free Energy and Why All Things

















![ANSWER 58. ΔG° = 3(0) + 2(-229) - [2(-34) + 1(-300. )] = -90. ANSWER 58. ΔG° = 3(0) + 2(-229) - [2(-34) + 1(-300. )] = -90.](https://slidetodoc.com/presentation_image_h/19e6a035a3ea73471fb1605e0724ed32/image-42.jpg)
- Slides: 43

Spontaneity, Entropy, Free Energy, and Why All Things Happen … “The Universe Becomes Less Predictable” ZUMDAHL’S CHAPTER 16

SPONTANEOUS PROCESSES AND ENTROPY One of the main objectives in studying thermodynamics, as far as chemists are concerned, is to be able to predict whether or not a reaction will occur when reactants are brought together under a special set of conditions (for example, at a certain temperature, pressure, and concentration). 2

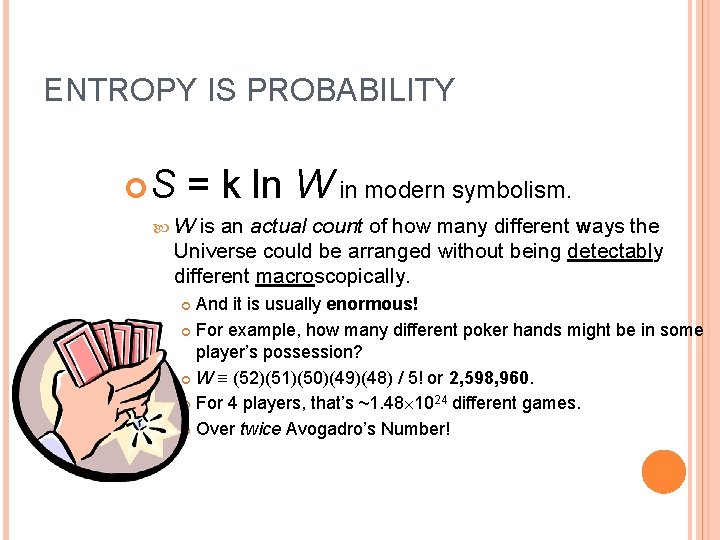
ENTROPY IS PROBABILITY S = k ln W in modern symbolism. W is an actual count of how many different ways the Universe could be arranged without being detectably different macroscopically. And it is usually enormous! For example, how many different poker hands might be in some player’s possession? W (52)(51)(50)(49)(48) / 5! or 2, 598, 960. For 4 players, that’s ~1. 48 1024 different games. Over twice Avogadro’s Number!

POKER MICROSTATES One microstate in poker might be a flush; all cards of the same suit. Wflush = 4(13)(12)(11)(10)(9) / 5! = 5148 as the number of ways to get a flush on the deal. But Wflush / Wtotal gives ~505: 1 odds against. So flushes-on-the-deal are fairly ignorable.

CHEMICAL MICROSTATES Positional In a solid, molecules are frozen in position. But a liquid can swap molecular positions without macroscopic consequence: Sliq > Ssolid A gas is far more chaotic: Sgas >> Sliquid!

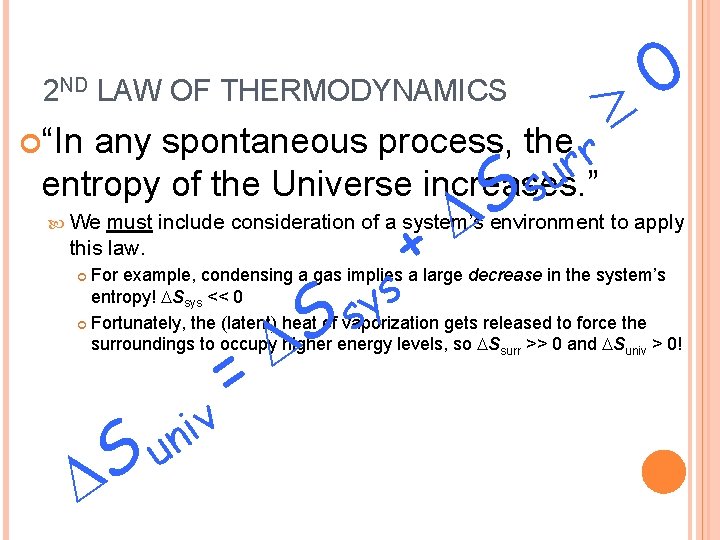
2 ND LAW OF THERMODYNAMICS any spontaneous process, the r r u entropy of the Universe increases. ” s “In We + S 0 must include consideration of a system’s environment to apply this law. s y s For example, condensing a gas implies a large decrease in the system’s entropy! Ssys << 0 Fortunately, the (latent) heat of vaporization gets released to force the surroundings to occupy higher energy levels, so Ssurr >> 0 and Suniv > 0! v i n Su = S

NORSE MYTHOLOGY Valhalla is the abode of the Norse gods. But, contrary to many other mythologies, Norse gods are not immortal. Valhalla is held up by a giant tree, the roots of which are being gnawed by a serpent. The serpent will succeed, and when it does, Valhalla and the Universe will fall. The serpent’s name is haos

UNIVERSAL CHAOS, SUNIV The Norsemen were right! There is Chaos growing in the Universe all the time at the expense of Order. It is now a fundamental principle of Science. It’s called “entropy, ” S, and is a state function that must always increase for the Universe as a whole, but some System’s S may decrease.

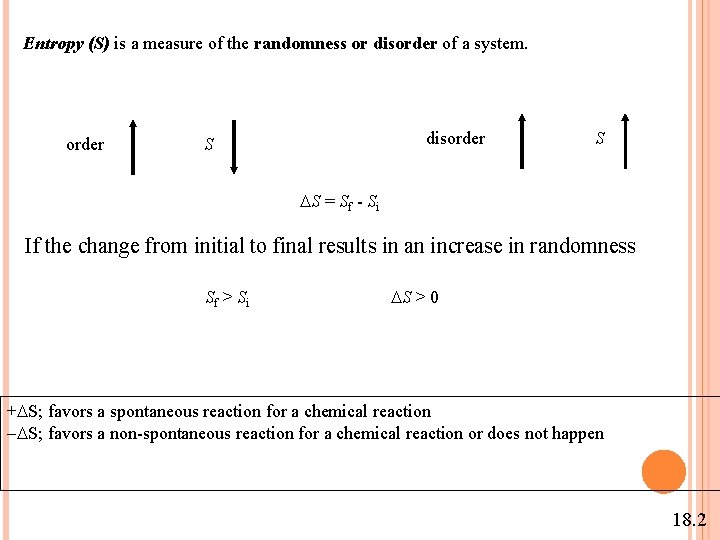
Entropy (S) is a measure of the randomness or disorder of a system. order disorder S S S = Sf - Si If the change from initial to final results in an increase in randomness Sf > Si S > 0 + S; favors a spontaneous reaction for a chemical reaction - S; favors a non-spontaneous reaction for a chemical reaction or does not happen 18. 2

Spontaneous Physical and Chemical Processes • A waterfall runs downhill • A lump of sugar dissolves in a cup of coffee • At 1 atm, water freezes below 0 0 C and ice melts above 0 0 C • Heat flows from a hotter object to a colder object • A gas expands in an evacuated bulb • Iron exposed to oxygen and water forms rust spontaneous nonspontaneous 18. 2

spontaneous nonspontaneous 18. 2

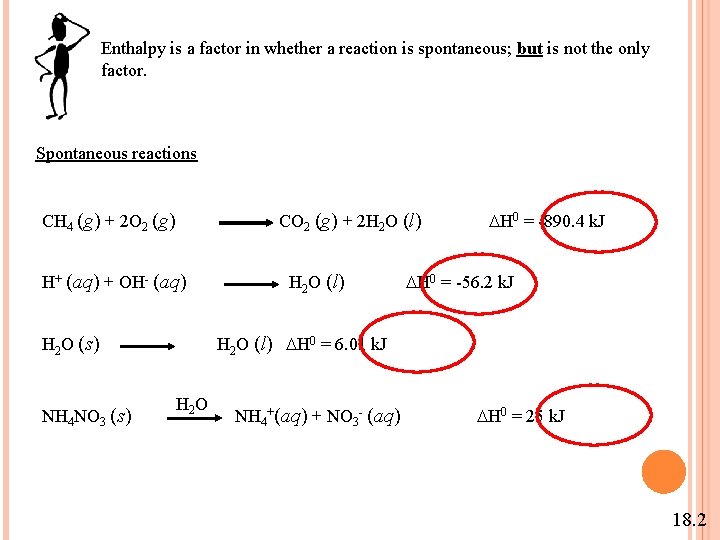
Enthalpy is a factor in whether a reaction is spontaneous; but is not the only factor. Spontaneous reactions CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O (l) H 0 = -890. 4 k. J H+ (aq) + OH- (aq) H 2 O (s) NH 4 NO 3 (s) H 2 O (l) H 0 = -56. 2 k. J H 2 O (l) H 0 = 6. 01 k. J H 2 O NH 4+(aq) + NO 3 - (aq) H 0 = 25 k. J 18. 2

AN EXOTHERMIC REACTION (- H) FAVORS A SPONTANEOUS REACTION SINCE PRODUCTS ARE AT A LOWER POTENTIAL ENERGY BUT DOES NOT GUARANTEE IT. Potential Energy Reactants - H Products

Guidelines for Determining if S is + or -. 1. For any substance, the solid state is more ordered than the liquid state and the liquid state is more ordered than gas state Ssolid < Sliquid << Sgas H 2 O (s) H 2 O (l) S > 0

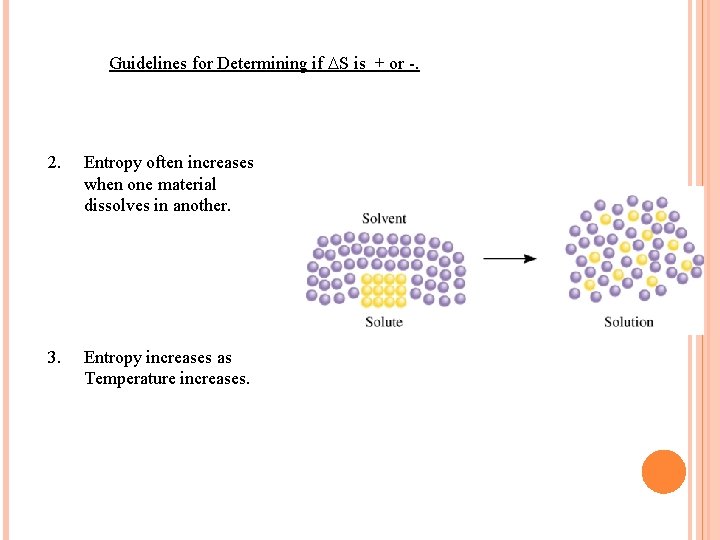
Guidelines for Determining if S is + or -. 2. Entropy often increases when one material dissolves in another. 3. Entropy increases as Temperature increases.

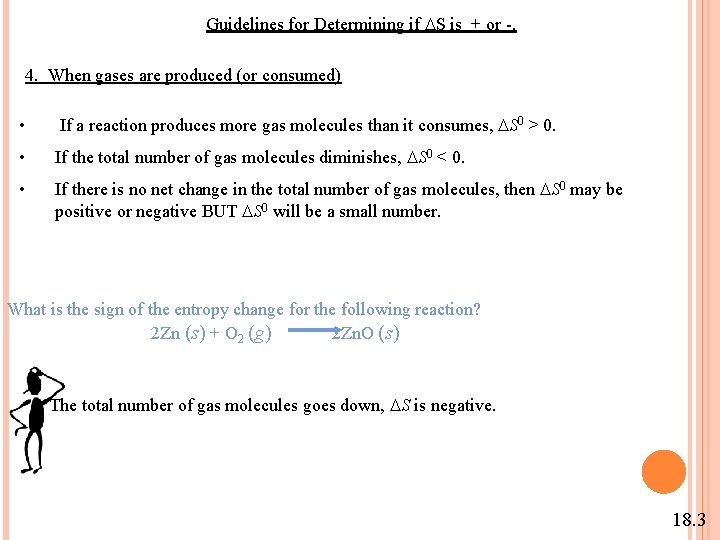
Guidelines for Determining if S is + or -. 4. When gases are produced (or consumed) • If a reaction produces more gas molecules than it consumes, S 0 > 0. • If the total number of gas molecules diminishes, S 0 < 0. • If there is no net change in the total number of gas molecules, then S 0 may be positive or negative BUT S 0 will be a small number. What is the sign of the entropy change for the following reaction? 2 Zn (s) + O 2 (g) 2 Zn. O (s) The total number of gas molecules goes down, S is negative. 18. 3

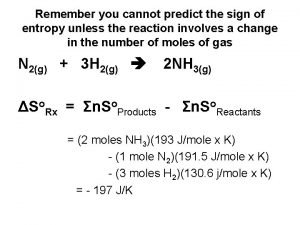
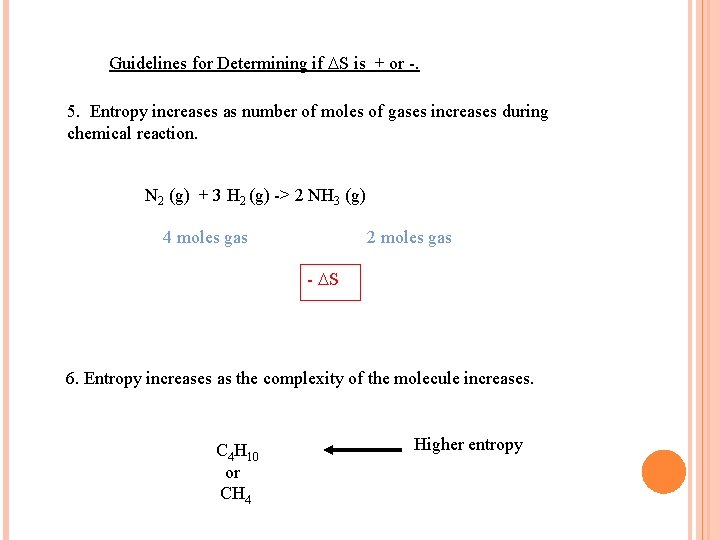
Guidelines for Determining if S is + or -. 5. Entropy increases as number of moles of gases increases during chemical reaction. N 2 (g) + 3 H 2 (g) -> 2 NH 3 (g) 4 moles gas 2 moles gas - S 6. Entropy increases as the complexity of the molecule increases. C 4 H 10 or CH 4 Higher entropy

How does the entropy of a system change for each of the following processes? (a) Condensing water vapor Randomness decreases Entropy decreases ( S < 0) (b) Forming sucrose crystals from a supersaturated solution Randomness decreases Entropy decreases ( S < 0) (c) Heating hydrogen gas from 600 C to 800 C Randomness increases Entropy increases ( S > 0) (d) Subliming dry ice Randomness increases Entropy increases ( S > 0) 18. 2


STANDARD ENTROPY VALUES; S° 240

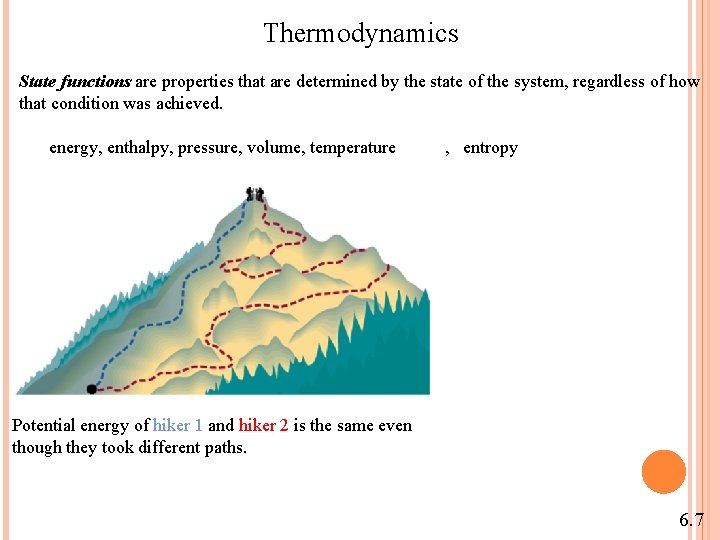
Thermodynamics State functions are properties that are determined by the state of the system, regardless of how that condition was achieved. energy, enthalpy, pressure, volume, temperature , entropy Potential energy of hiker 1 and hiker 2 is the same even though they took different paths. 6. 7

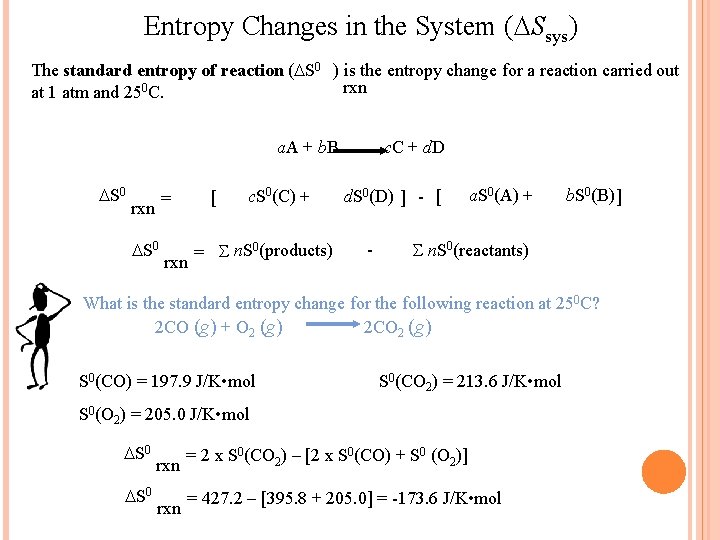
Entropy Changes in the System ( Ssys) The standard entropy of reaction ( S 0 ) is the entropy change for a reaction carried out rxn at 1 atm and 250 C. a. A + b. B c. C + d. D S 0 [ = rxn S 0 rxn c. S 0(C) + = S n. S 0(products) d. S 0(D) ] - [ - a. S 0(A) + b. S 0(B) ] S n. S 0(reactants) What is the standard entropy change for the following reaction at 250 C? 2 CO (g) + O 2 (g) 2 CO 2 (g) S 0(CO) = 197. 9 J/K • mol S 0(CO 2) = 213. 6 J/K • mol S 0(O 2) = 205. 0 J/K • mol S 0 rxn = 2 x S 0(CO 2) – [2 x S 0(CO) + S 0 (O 2)] = 427. 2 – [395. 8 + 205. 0] = -173. 6 J/K • mol

3 FACTORS INVOLVED IN DETERMINING WHETHER A REACTION IS SPONTANEOUS OR NOT § § § H; a negative value (exothermic reactions) favors a spontaneous reaction S; a positive value (disorder increases) favors a spontaneous reaction Temperature; If the above factors conflict; temperature determines if reaction is spontaneous or not

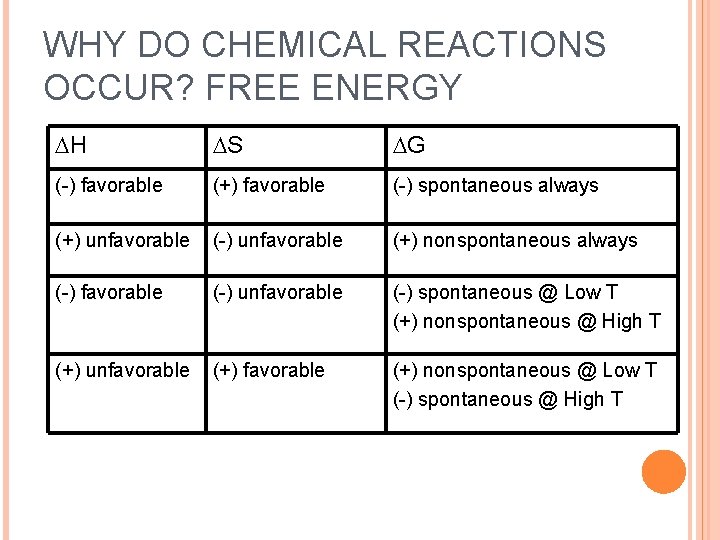
WHY DO CHEMICAL REACTIONS OCCUR? FREE ENERGY Free Energy

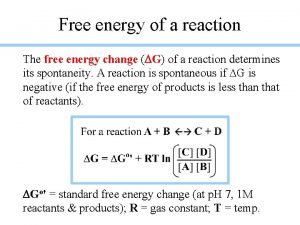
Gibbs Free Energy For a constant-temperature process: Gibbs free energy (G) G = Hsys -T Ssys G < 0 The reaction is spontaneous in the forward direction. G > 0 The reaction is nonspontaneous as written. The reaction is spontaneous in the reverse direction. G = 0 The reaction is at equilibrium.

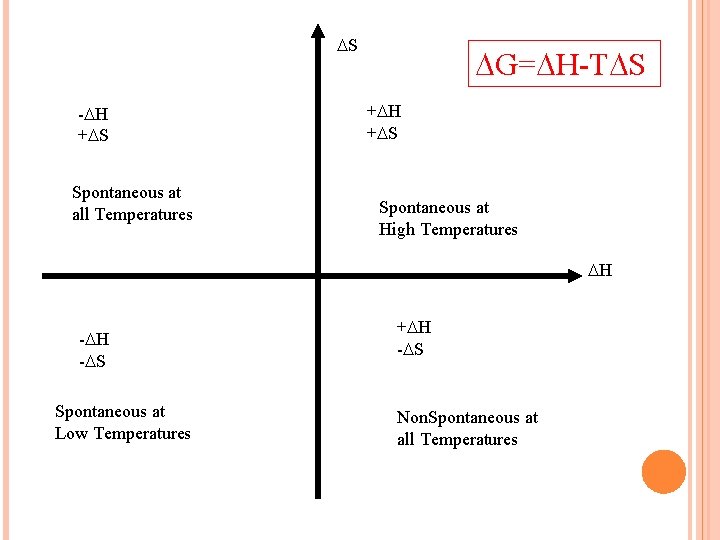
S - H + S Spontaneous at all Temperatures G= H-T S + H + S Spontaneous at High Temperatures H - S Spontaneous at Low Temperatures + H - S Non. Spontaneous at all Temperatures

WHY DO CHEMICAL REACTIONS OCCUR? FREE ENERGY H S G (-) favorable (+) favorable (-) spontaneous always (+) unfavorable (-) unfavorable (+) nonspontaneous always (-) favorable (-) unfavorable (-) spontaneous @ Low T (+) nonspontaneous @ High T (+) unfavorable (+) nonspontaneous @ Low T (-) spontaneous @ High T

G; CHANGE IN GIBB’S FREE ENERGY G = Hsys -T Ssys 1. 2. Determine if reaction is spontaneous or not. wmax; free energy available to do useful work. G = wmaximum Hsys; represents energy available for useful work -T Ssys; represents energy that can’t Be harnessed to do useful work

WAYS TO OBTAIN GREACTION 1. 2. From Gfo Values From Gibb’s Free Energy Equation G = Hsys -T Ssys

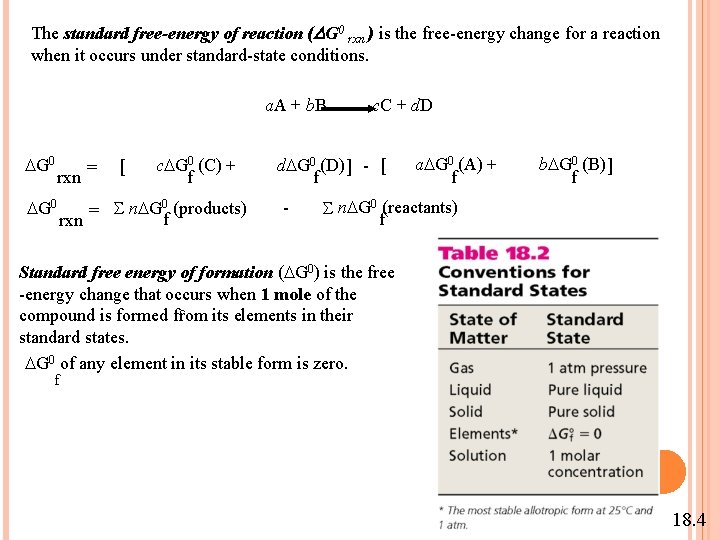
The standard free-energy of reaction (DG 0 rxn ) is the free-energy change for a reaction when it occurs under standard-state conditions. a. A + b. B c. C + d. D G 0 rxn G 0 = [ c G 0 (C) + f = S n G 0 (products) f rxn d G 0 (D) ] - [ f - a G 0 (A) + f b G 0 (B) ] f S n G 0 (reactants) f Standard free energy of formation ( G 0) is the free -energy change that occurs when 1 mole of the f compound is formed from its elements in their standard states. G 0 of any element in its stable form is zero. f 18. 4

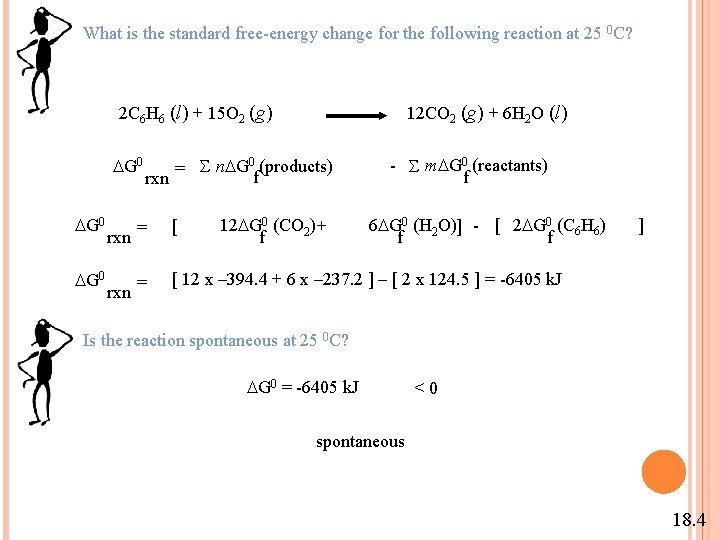
What is the standard free-energy change for the following reaction at 25 0 C? 2 C 6 H 6 (l) + 15 O 2 (g) G 0 G 0 rxn = S n G 0 (products) f rxn 12 G 0 (CO 2) + f 12 CO 2 (g) + 6 H 2 O (l) - S m G 0 (reactants) f 6 G 0 (H 2 O)] - [ 2 G 0 (C 6 H 6) f f = [ 12 x – 394. 4 + 6 x – 237. 2 ] – [ 2 x 124. 5 ] = -6405 k. J ] Is the reaction spontaneous at 25 0 C? G 0 = -6405 k. J < 0 spontaneous 18. 4

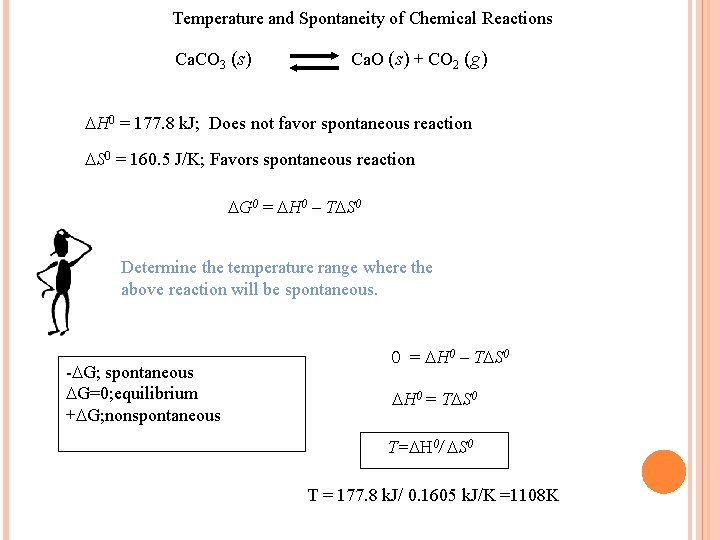
Temperature and Spontaneity of Chemical Reactions Ca. CO 3 (s) Ca. O (s) + CO 2 (g) H 0 = 177. 8 k. J; Does not favor spontaneous reaction S 0 = 160. 5 J/K; Favors spontaneous reaction G 0 = H 0 – T S 0 Determine the temperature range where the above reaction will be spontaneous. - G; spontaneous G=0; equilibrium + G; nonspontaneous 0 = H 0 – T S 0 H 0 = T S 0 T= H 0/ S 0 T = 177. 8 k. J/ 0. 1605 k. J/K =1108 K

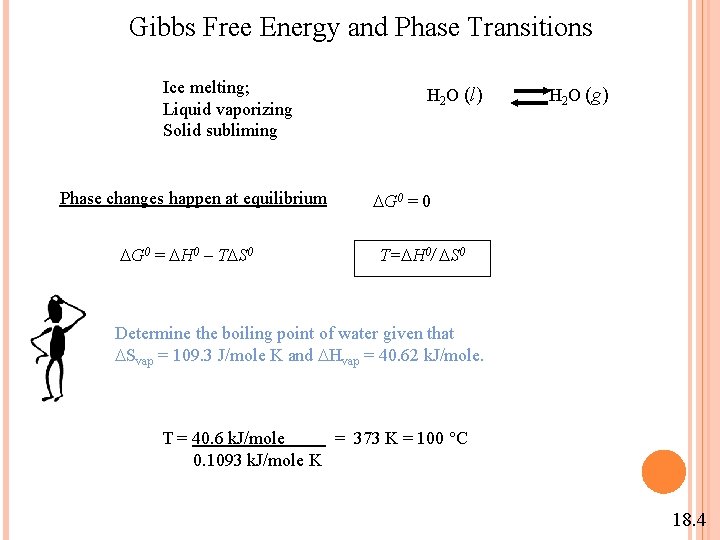
Gibbs Free Energy and Phase Transitions Ice melting; Liquid vaporizing Solid subliming Phase changes happen at equilibrium G 0 = H 0 – T S 0 H 2 O (l) H 2 O (g) G 0 = 0 T= H 0/ S 0 Determine the boiling point of water given that Svap = 109. 3 J/mole K and Hvap = 40. 62 k. J/mole. T = 40. 6 k. J/mole = 373 K = 100 °C 0. 1093 k. J/mole K 18. 4

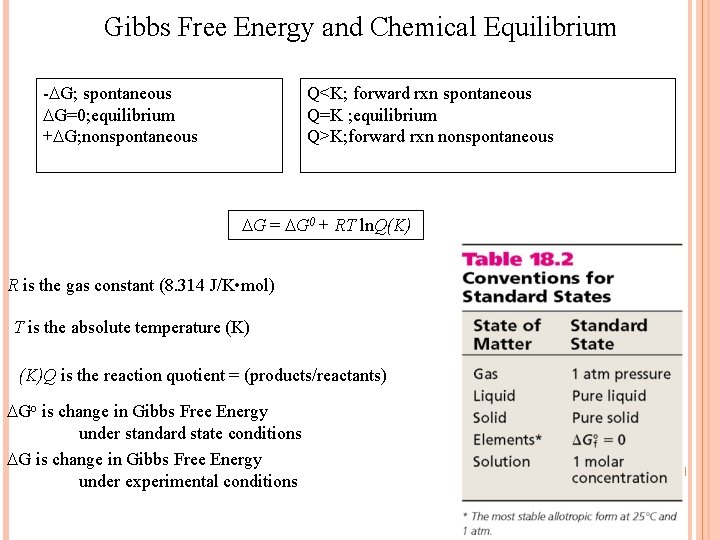
Gibbs Free Energy and Chemical Equilibrium - G; spontaneous G=0; equilibrium + G; nonspontaneous Q<K; forward rxn spontaneous Q=K ; equilibrium Q>K; forward rxn nonspontaneous G = G 0 + RT ln. Q(K) R is the gas constant (8. 314 J/K • mol) T is the absolute temperature (K)Q is the reaction quotient = (products/reactants) Go is change in Gibbs Free Energy under standard state conditions G is change in Gibbs Free Energy under experimental conditions

CHEMICAL EQUILIBRIUM Chemical Equilibrium predicts how reaction will proceed and how far can it go. Chemical equilibrium does not indicate rates of reactions, just extent of reaction at equilibrium and how far the reaction is from equilibrium Dynamic nature of chemical reaction a. A + b. B <------> c. C + d. D A, B decrease with time, C, D increase with time until no change. Equilibrium: rxn proceeds to a point where reactant to product balanced the reverse rxn, product to reactant.

EQUILIBRIUM EQUATIONS AND EQUILIBRIUM CONSTANTS Consider reaction: the following general equilibrium a. A + b. B + … m. M + n. N + … Where A, B, … are the reactants; M, N, …. are the products; a, b, …. m, n, …. are coefficients in the balanced equation. At equilibrium, the composition of the reaction mixture obeys an equilibrium equation.

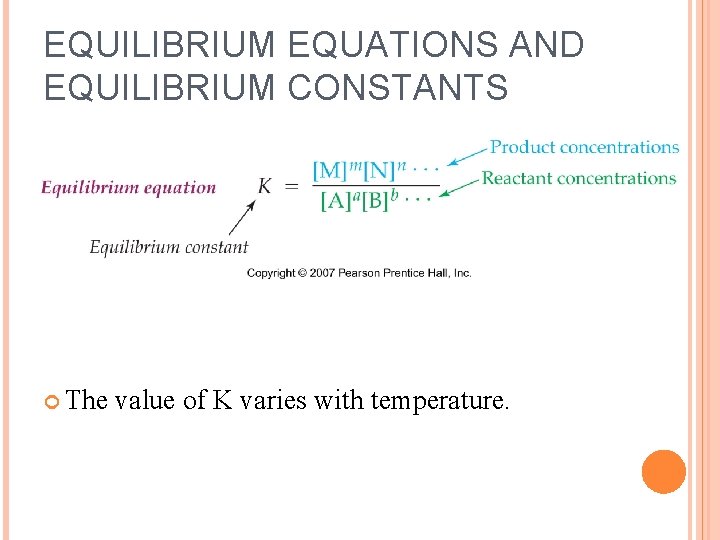
EQUILIBRIUM EQUATIONS AND EQUILIBRIUM CONSTANTS The value of K varies with temperature.

THE LAW OF MASS ACTION K = equilibrium constant = kforward/kreverse for single-step reactions §K depends on temperature ONLY §Don’t use solids or pure liquids (i. e. water) for the Law of Mass Action only gases or aqueous solutions §Gases have partial pressure equilibrium constants

WHAT ABOUT NON-EQUILIBRIUM REACTIONS? Use the reaction quotient equation! Can use Q to figure out which direction the reaction will go: Q = K reaction is at equilibrium Q > K reverse reaction rate is greater than forward reaction rate (leftward shift) Q < K forward reaction rate is greater than reverse reaction rate (rightward shift)

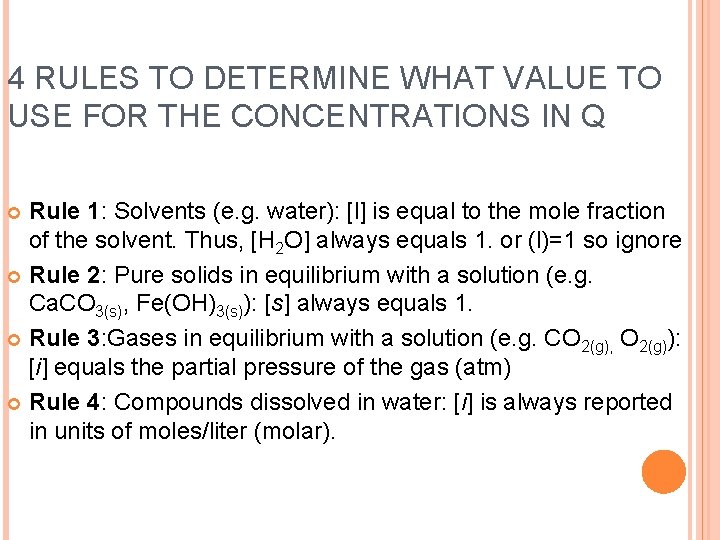
4 RULES TO DETERMINE WHAT VALUE TO USE FOR THE CONCENTRATIONS IN Q Rule 1: Solvents (e. g. water): [l] is equal to the mole fraction of the solvent. Thus, [H 2 O] always equals 1. or (l)=1 so ignore Rule 2: Pure solids in equilibrium with a solution (e. g. Ca. CO 3(s), Fe(OH)3(s)): [s] always equals 1. Rule 3: Gases in equilibrium with a solution (e. g. CO 2(g), O 2(g)): [i] equals the partial pressure of the gas (atm) Rule 4: Compounds dissolved in water: [i] is always reported in units of moles/liter (molar).

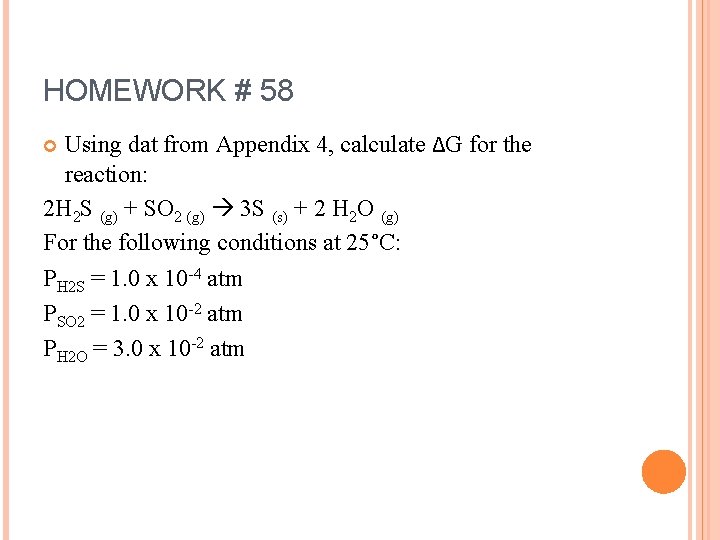
HOMEWORK # 58 Using dat from Appendix 4, calculate ∆G for the reaction: 2 H 2 S (g) + SO 2 (g) 3 S (s) + 2 H 2 O (g) For the following conditions at 25°C: PH 2 S = 1. 0 x 10 -4 atm PSO 2 = 1. 0 x 10 -2 atm PH 2 O = 3. 0 x 10 -2 atm
![ANSWER 58 ΔG 30 2229 234 1300 90 ANSWER 58. ΔG° = 3(0) + 2(-229) - [2(-34) + 1(-300. )] = -90.](https://slidetodoc.com/presentation_image_h/19e6a035a3ea73471fb1605e0724ed32/image-42.jpg)
ANSWER 58. ΔG° = 3(0) + 2(-229) - [2(-34) + 1(-300. )] = -90. k. J ΔG = ΔG° + RT ln = -90. k. J + ΔG = -90. k. J + 39. 7 k. J = -50. k. J

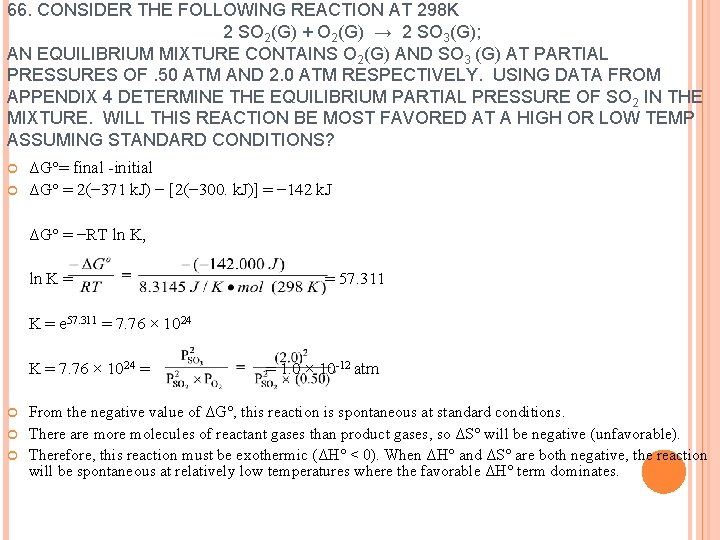
66. CONSIDER THE FOLLOWING REACTION AT 298 K 2 SO 2(G) + O 2(G) → 2 SO 3(G); AN EQUILIBRIUM MIXTURE CONTAINS O 2(G) AND SO 3 (G) AT PARTIAL PRESSURES OF. 50 ATM AND 2. 0 ATM RESPECTIVELY. USING DATA FROM APPENDIX 4 DETERMINE THE EQUILIBRIUM PARTIAL PRESSURE OF SO 2 IN THE MIXTURE. WILL THIS REACTION BE MOST FAVORED AT A HIGH OR LOW TEMP ASSUMING STANDARD CONDITIONS? ΔG°= final -initial ΔG° = 2(− 371 k. J) − [2(− 300. k. J)] = − 142 k. J ΔG° = −RT ln K, ln K = = 57. 311 K = e 57. 311 = 7. 76 × 1024 K = 7. 76 × 1024 = = 1. 0 × 10 -12 atm From the negative value of ΔG°, this reaction is spontaneous at standard conditions. There are molecules of reactant gases than product gases, so ΔS° will be negative (unfavorable). Therefore, this reaction must be exothermic (ΔH° < 0). When ΔH° and ΔS° are both negative, the reaction will be spontaneous at relatively low temperatures where the favorable ΔH° term dominates.
 Entropy ap chem
Entropy ap chem What is spontaneity in chemistry
What is spontaneity in chemistry Bpch21
Bpch21 Gibbs free energy of formation
Gibbs free energy of formation Enthalpy vs entropy
Enthalpy vs entropy Entropy and gibbs free energy
Entropy and gibbs free energy Helmholtz free energy
Helmholtz free energy Delta g positive or negative
Delta g positive or negative Enthalpy entropy free energy
Enthalpy entropy free energy Boltzmann entropy equation
Boltzmann entropy equation Gibbs energy and equilibrium
Gibbs energy and equilibrium Hey hey bye bye
Hey hey bye bye The 7 life processes of all living things
The 7 life processes of all living things Thermodynamics ppt
Thermodynamics ppt Predicting spontaneity
Predicting spontaneity Spontaneity of redox reaction
Spontaneity of redox reaction Predicting spontaneity
Predicting spontaneity Complete the following table on reaction spontaneity
Complete the following table on reaction spontaneity Is the redox spontaneity rule empirical
Is the redox spontaneity rule empirical Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Name
Name Dont ask
Dont ask Venn diagram of living and non living things
Venn diagram of living and non living things The story of an hour summary
The story of an hour summary Happy isles ulysses
Happy isles ulysses Entropy and heat transfer
Entropy and heat transfer What is enthalpy and entropy
What is enthalpy and entropy Minimum enthalpy and maximum entropy
Minimum enthalpy and maximum entropy Entropy change formula
Entropy change formula Entropy order parameters and complexity
Entropy order parameters and complexity All things are yours and you are christ's
All things are yours and you are christ's What is the poem the bright lights of sarajevo about
What is the poem the bright lights of sarajevo about Allocation map
Allocation map Free-free absorption
Free-free absorption Phosphoanhydride group
Phosphoanhydride group Internal energy formula thermodynamics
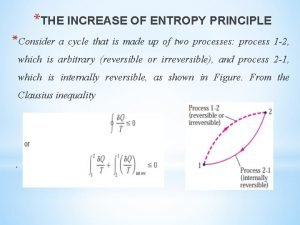
Internal energy formula thermodynamics The increase of entropy principle
The increase of entropy principle Reverse entropy
Reverse entropy Reversible process
Reversible process Second law of thermodynamics
Second law of thermodynamics Entropy is scalar or vector
Entropy is scalar or vector Predict the sign of the entropy change
Predict the sign of the entropy change Thermodynamics
Thermodynamics