Redox potentials Electrochemistry ICS Redox Reactions Oxidation loss











![EXAMPLE: What is the cell potential for the Daniel's cell when the [Zn+2] = EXAMPLE: What is the cell potential for the Daniel's cell when the [Zn+2] =](https://slidetodoc.com/presentation_image_h/12d7301410ae3c5b6393f427f221f116/image-36.jpg)
![Nernst Equation [H + ] acid side ® [H + ] base side RT Nernst Equation [H + ] acid side ® [H + ] base side RT](https://slidetodoc.com/presentation_image_h/12d7301410ae3c5b6393f427f221f116/image-37.jpg)
- Slides: 37

Redox potentials Electrochemistry ICS

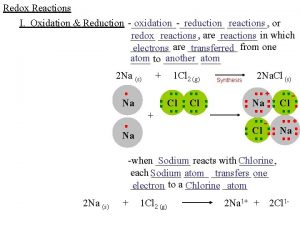
Redox Reactions Oxidation • loss of electrons Reduction • gain of electrons oxidizing agent • substance that cause oxidation by being reduced reducing agent • substance that cause oxidation by being reduced ICS

Electrochemistry In the broadest sense, electrochemistry is the study of chemical reactions that produce electrical effects and of the chemical phenomena that are caused by the action of currents or voltages.

ICS

Voltaic Cells • harnessed chemical reaction which produces an electric current

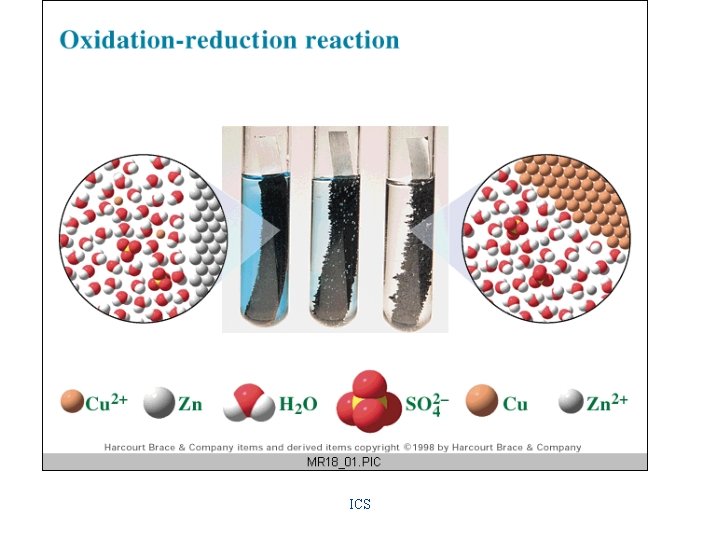
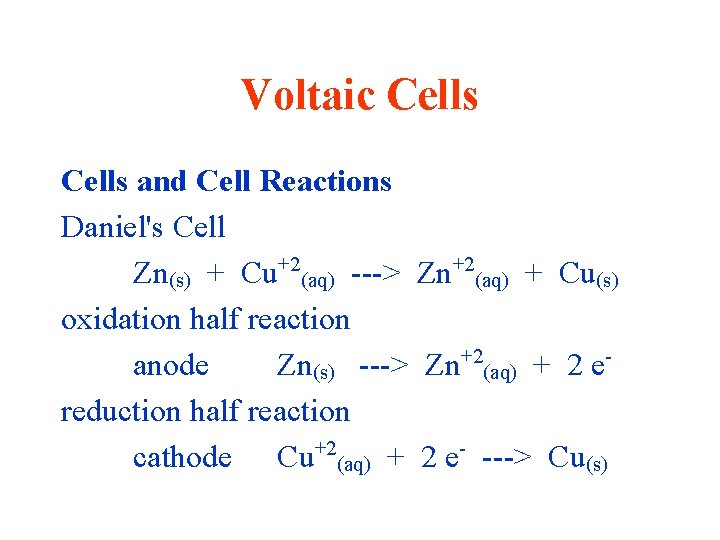
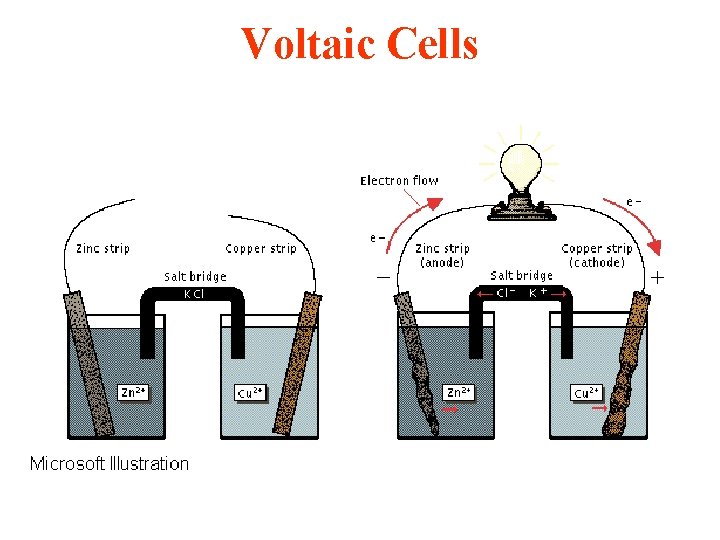
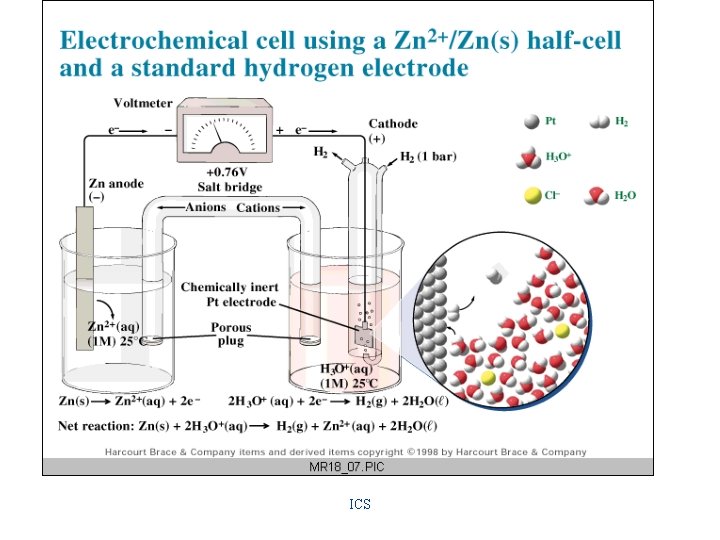
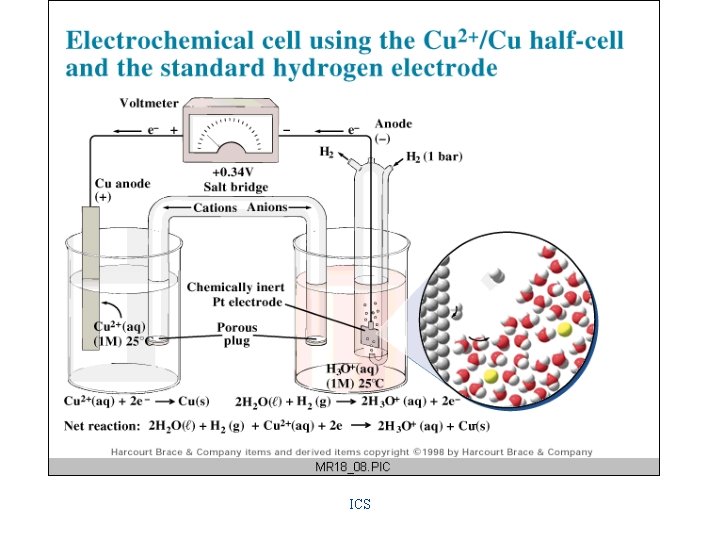
Voltaic Cells and Cell Reactions Daniel's Cell Zn(s) + Cu+2(aq) ---> Zn+2(aq) + Cu(s) oxidation half reaction anode Zn(s) ---> Zn+2(aq) + 2 ereduction half reaction cathode Cu+2(aq) + 2 e- ---> Cu(s)

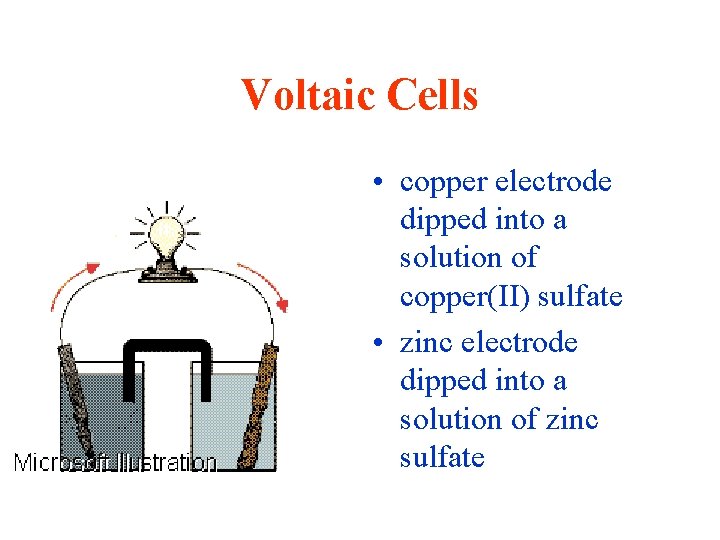
Voltaic Cells • copper electrode dipped into a solution of copper(II) sulfate • zinc electrode dipped into a solution of zinc sulfate

Voltaic Cells

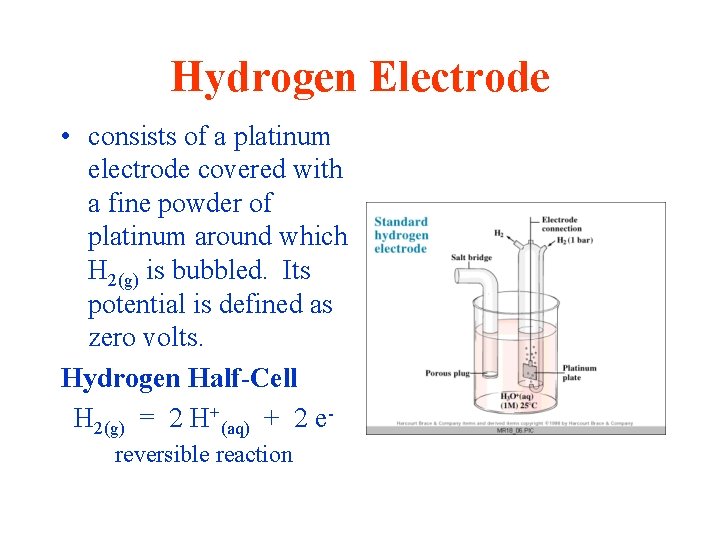
Hydrogen Electrode • consists of a platinum electrode covered with a fine powder of platinum around which H 2(g) is bubbled. Its potential is defined as zero volts. Hydrogen Half-Cell H 2(g) = 2 H+(aq) + 2 ereversible reaction

ICS

ICS

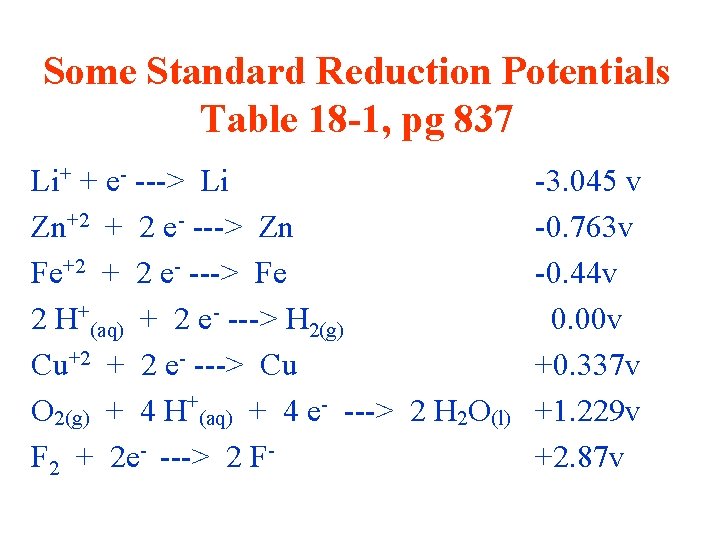
Standard Reduction Potentials • the potential under standard conditions (25 o. C with all ions at 1 M concentrations and all gases at 1 atm pressure) of a halfreaction in which reduction is occurring

Some Standard Reduction Potentials Table 18 -1, pg 837 Li+ + e- ---> Li Zn+2 + 2 e- ---> Zn Fe+2 + 2 e- ---> Fe 2 H+(aq) + 2 e- ---> H 2(g) Cu+2 + 2 e- ---> Cu O 2(g) + 4 H+(aq) + 4 e- ---> 2 H 2 O(l) F 2 + 2 e- ---> 2 F- -3. 045 v -0. 763 v -0. 44 v 0. 00 v +0. 337 v +1. 229 v +2. 87 v

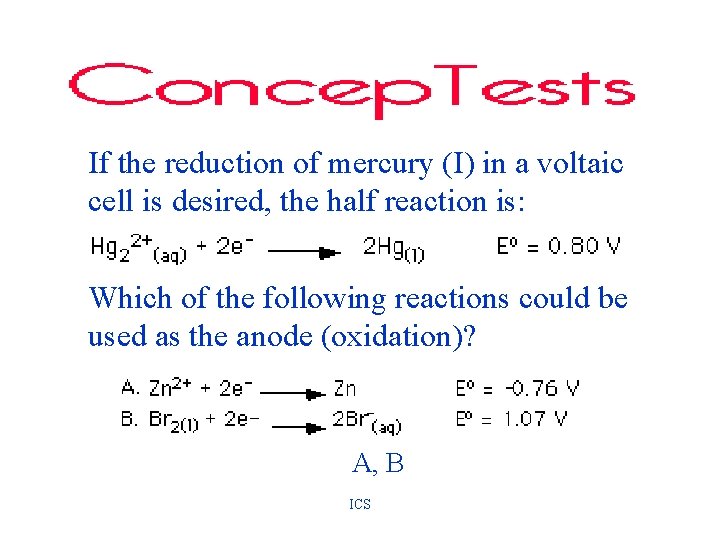
If the reduction of mercury (I) in a voltaic cell is desired, the half reaction is: Which of the following reactions could be used as the anode (oxidation)? A, B ICS

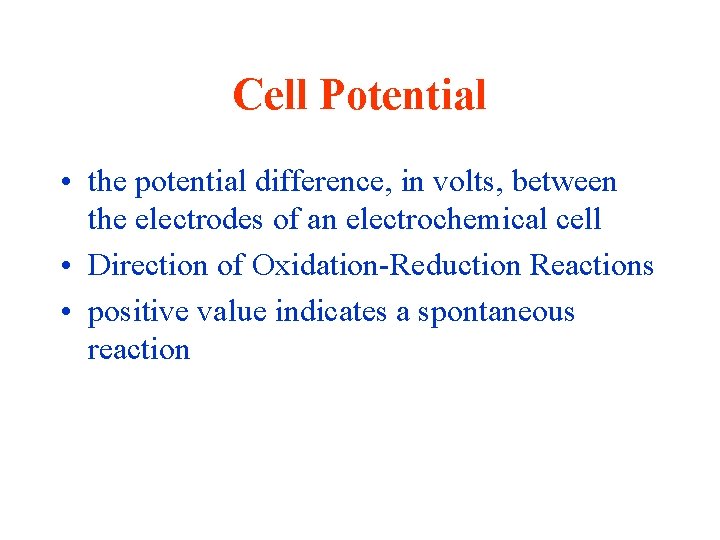
Cell Potential • the potential difference, in volts, between the electrodes of an electrochemical cell • Direction of Oxidation-Reduction Reactions • positive value indicates a spontaneous reaction

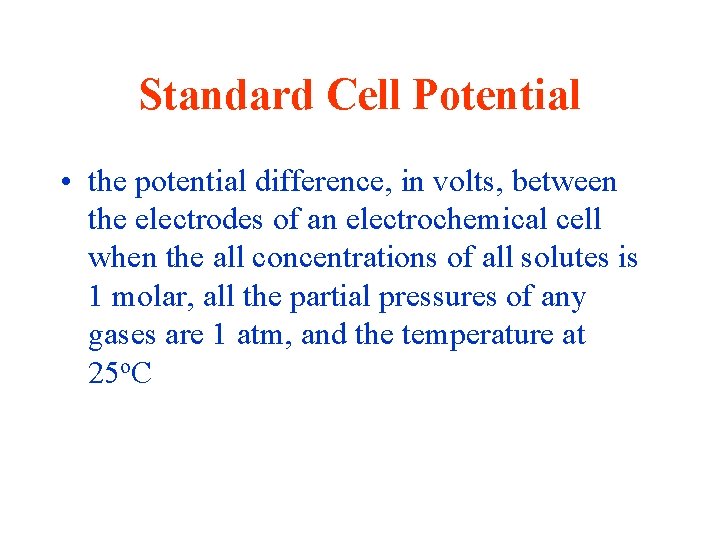
Standard Cell Potential • the potential difference, in volts, between the electrodes of an electrochemical cell when the all concentrations of all solutes is 1 molar, all the partial pressures of any gases are 1 atm, and the temperature at 25 o. C

Cell Diagram • the shorthand representation of an electrochemical cell showing the two halfcells connected by a salt bridge or porous barrier, such as: Zn(s)/Zn. SO 4(aq)//Cu. SO 4(aq)/Cu(s) anode cathode

Metal Displacement Reactions • solid of more reactive metals will displace ions of a less reactive metal from solution • relative reactivity based on potentials of half reactions • metals with very different potentials react most vigorously

Ag+ + e- --->Ag E°= 0. 80 V Cu 2+ + 2 e- ---> Cu E°= 0. 34 V Will Ag react with Cu 2+? yes, no Will Cu react with Ag+? yes, no ICS

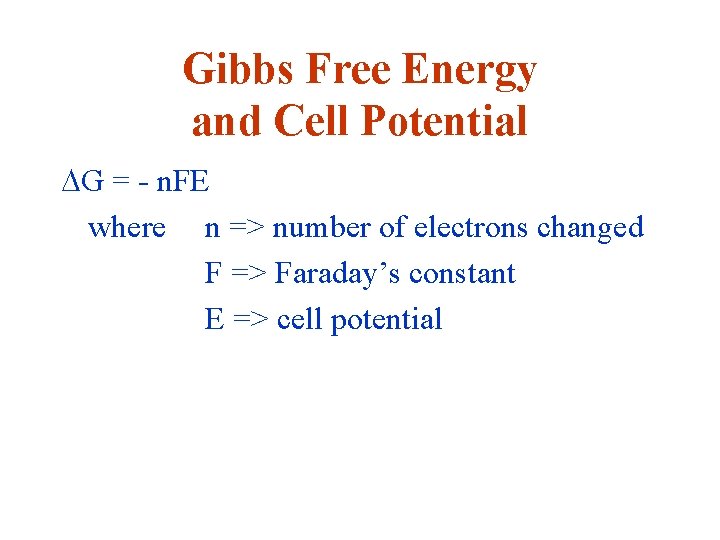
Gibbs Free Energy and Cell Potential DG = - n. FE where n => number of electrons changed F => Faraday’s constant E => cell potential

Applications of Electrochemical Cells Batteries – device that converts chemical energy into electricity Primary Cells – non-reversible electrochemical cell – non-rechargeable cell Secondary Cells – reversible electrochemical cell – rechargeable cell

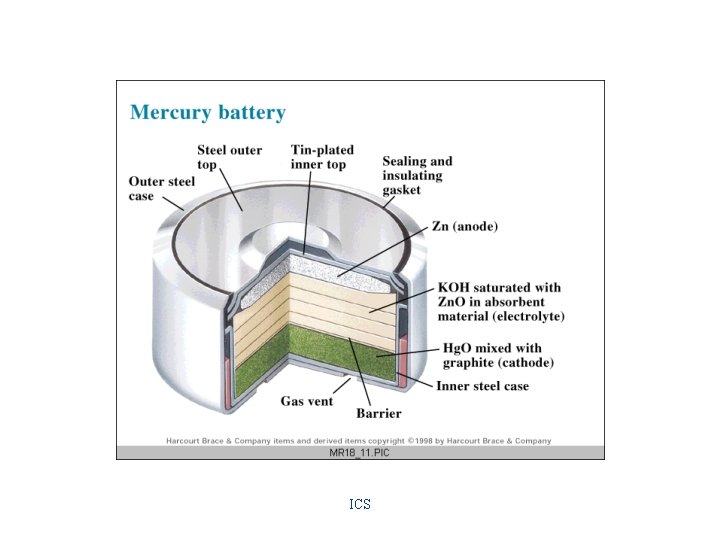
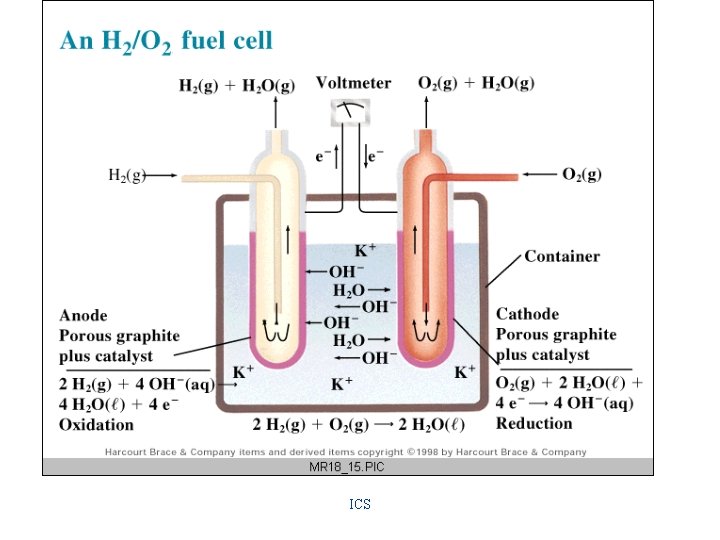
Applications of Electrochemical Cells Batteries Primary Cells "dry" cell & alkaline cell 1. 5 v/cell mercury cell 1. 34 v/cell fuel cell 1. 23 v/cell Secondary Cells lead-acid (automobile battery) 2 v/cell Ni. Cad 1. 25 v/cell

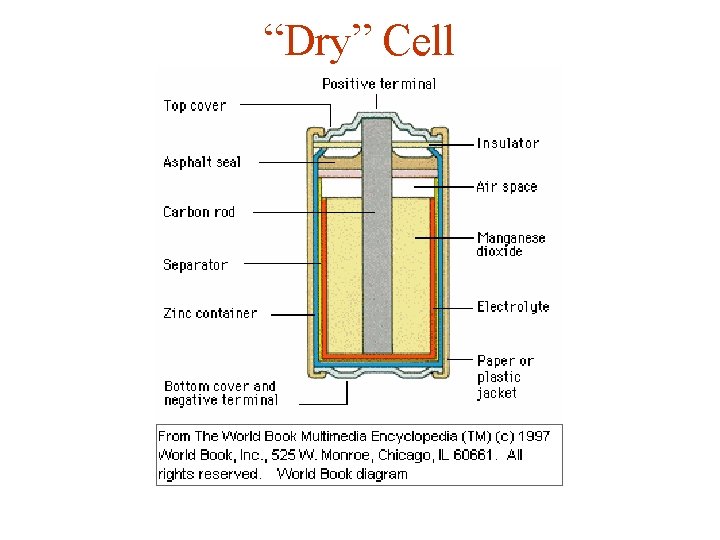
“Dry” Cell

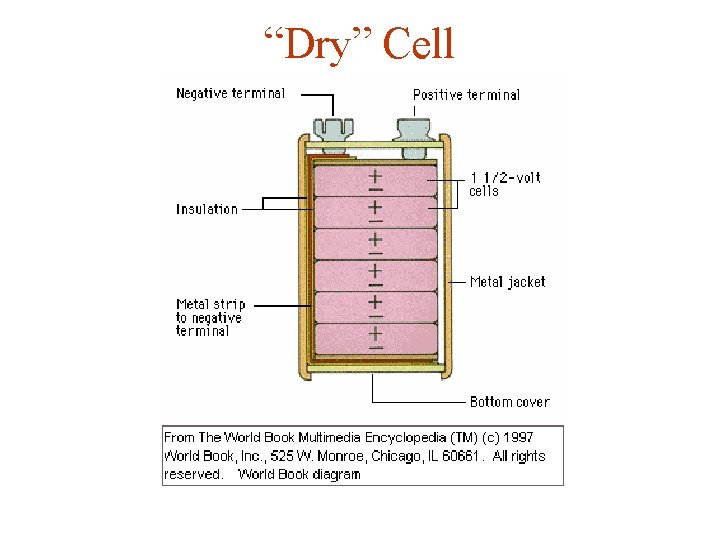
“Dry” Cell

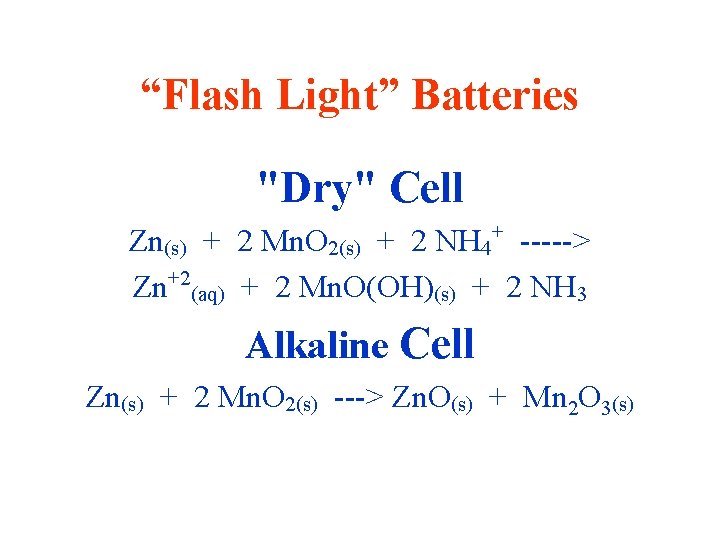
“Flash Light” Batteries "Dry" Cell Zn(s) + 2 Mn. O 2(s) + 2 NH 4+ -----> Zn+2(aq) + 2 Mn. O(OH)(s) + 2 NH 3 Alkaline Cell Zn(s) + 2 Mn. O 2(s) ---> Zn. O(s) + Mn 2 O 3(s)

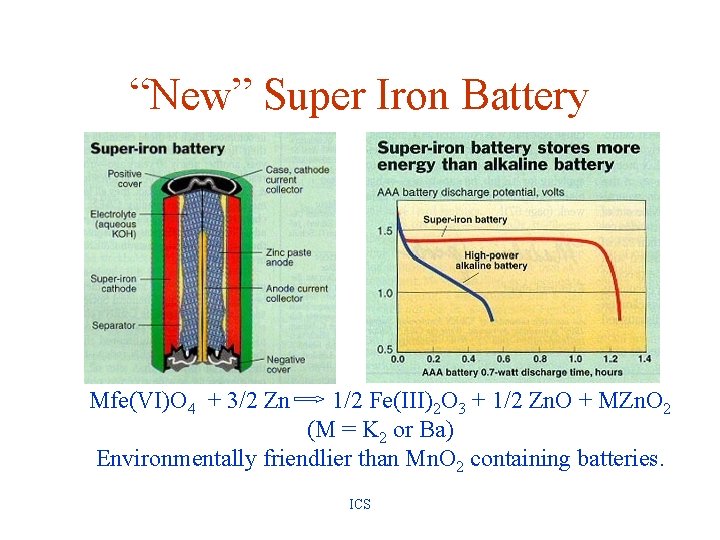
“New” Super Iron Battery Mfe(VI)O 4 + 3/2 Zn 1/2 Fe(III)2 O 3 + 1/2 Zn. O + MZn. O 2 (M = K 2 or Ba) Environmentally friendlier than Mn. O 2 containing batteries. ICS

ICS

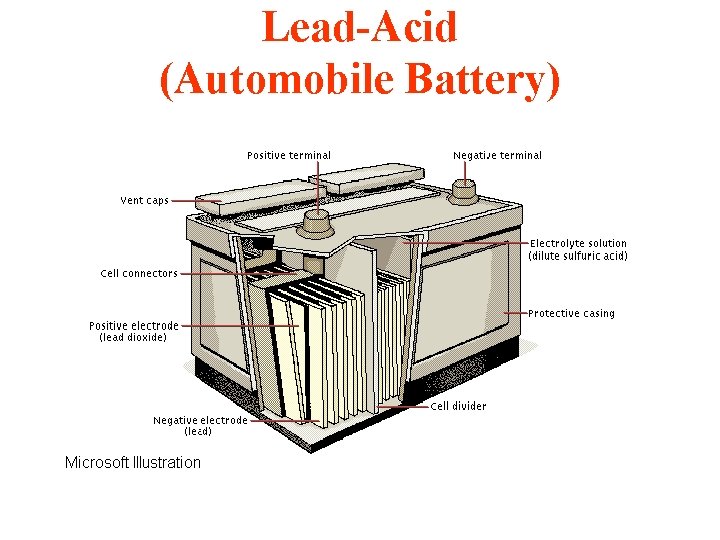
Lead-Acid (Automobile Battery)

Lead-Acid (Automobile Battery) Pb(s) + Pb. O 2(s) + 2 H 2 SO 4 = 2 Pb. SO 4(s) + 2 H 2 O 2 v/cell

Nickel-Cadmium (Ni-Cad) Cd(s) + 2 Ni(OH)3(s) = Cd(OH)2(s) + 2 Ni(OH)2(s) Ni. Cad 1. 25 v/cell

ICS

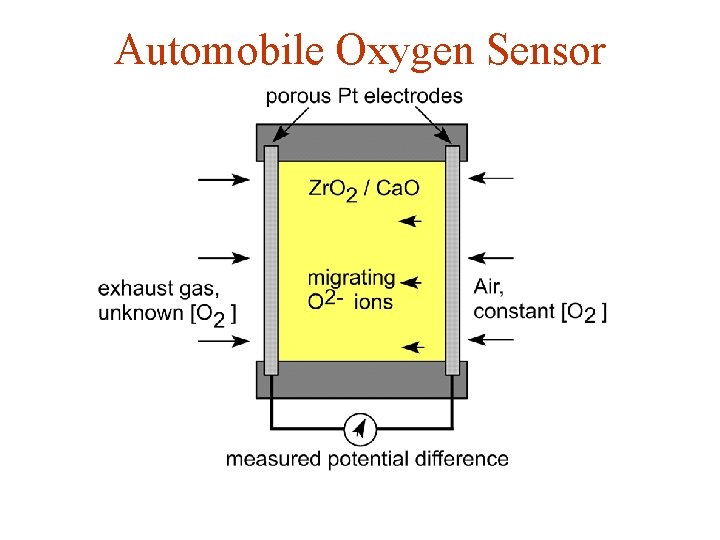
Automobile Oxygen Sensor

Automobile Oxygen Sensor • see Oxygen Sensor Movie from Solid-State Resources CD-ROM

p. H Meter p. H = (Eglass electrode - constant)/0. 0592

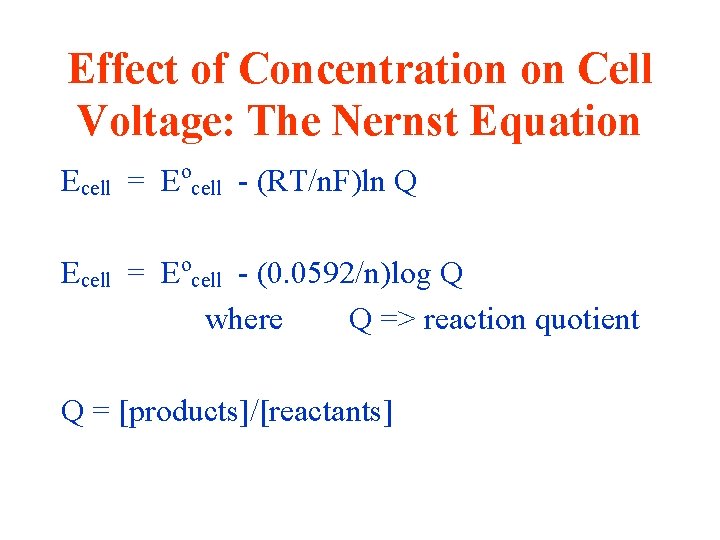
Effect of Concentration on Cell Voltage: The Nernst Equation Ecell = Eocell - (RT/n. F)ln Q Ecell = Eocell - (0. 0592/n)log Q where Q => reaction quotient Q = [products]/[reactants]
![EXAMPLE What is the cell potential for the Daniels cell when the Zn2 EXAMPLE: What is the cell potential for the Daniel's cell when the [Zn+2] =](https://slidetodoc.com/presentation_image_h/12d7301410ae3c5b6393f427f221f116/image-36.jpg)
EXAMPLE: What is the cell potential for the Daniel's cell when the [Zn+2] = 10 [Cu+2] ? Q = ([Zn+2]/[Cu+2] = (10 [Cu+2])/[Cu+2] = 10 Eo = (0. 34 V)Cu couple + (-(-0. 76 V)Zn couple n = 2, 2 electron change Ecell = Eocell - (0. 0257/n)ln Q thus Ecell = (1. 10 - (0. 0257/2)ln 10) V Ecell = (1. 10 - (0. 0257/2)2. 303) V Ecell = (1. 10 - 0. 0296) V = 1. 07 V
![Nernst Equation H acid side H base side RT Nernst Equation [H + ] acid side ® [H + ] base side RT](https://slidetodoc.com/presentation_image_h/12d7301410ae3c5b6393f427f221f116/image-37.jpg)
Nernst Equation [H + ] acid side ® [H + ] base side RT – 2. 3 RT log[H + ] n. F ln Q = F acid side E = E o – [h + ] p-type side ® [h + ] n-type side E (in volts) = [h + ] n-type side – 2. 3 RT log F [h + ] p-type side
 Example of redox reaction
Example of redox reaction Locos bird's beak
Locos bird's beak Complex incident
Complex incident Oxidation–reduction reactions
Oxidation–reduction reactions Redox reactions
Redox reactions Oxidation–reduction reactions
Oxidation–reduction reactions How to do redox reactions
How to do redox reactions Oxidation reduction reactions
Oxidation reduction reactions Chapter 19 redox reactions study guide answers
Chapter 19 redox reactions study guide answers Chapter 19 review oxidation reduction reactions answers
Chapter 19 review oxidation reduction reactions answers Oxidation means
Oxidation means Chapter 20 worksheet redox
Chapter 20 worksheet redox Balancing redox reactions
Balancing redox reactions Concept map about oxidative phosphorylation.
Concept map about oxidative phosphorylation. Balancing redox reactions
Balancing redox reactions Different types of redox reactions
Different types of redox reactions Redox reactions ncea level 2
Redox reactions ncea level 2 Rules for balancing redox reactions
Rules for balancing redox reactions Predicting redox reactions
Predicting redox reactions Cell chap 18
Cell chap 18 Balancing redox reactions
Balancing redox reactions Oil rig chemistry
Oil rig chemistry Half equation worksheet with answers
Half equation worksheet with answers Balancing redox reactions
Balancing redox reactions What are spectator ions
What are spectator ions How to balance equation in acidic solution
How to balance equation in acidic solution Electrode concentration cell
Electrode concentration cell Reactions that produce gas
Reactions that produce gas How redox reactions work
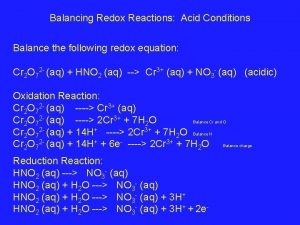
How redox reactions work Balancing redox reactions in acid
Balancing redox reactions in acid How to write half reactions
How to write half reactions Example of redox reaction
Example of redox reaction Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Unit cost meaning
Unit cost meaning Standard cell potentials
Standard cell potentials