5 Electrochemistry 5 3 Standard reduction potentials Determine
- Slides: 11

5. Electrochemistry 5. 3: Standard reduction potentials • Determine standard cell potentials for redox reactions • Use standard reduction potentials to determine the better oxidizing or reducing agent from among choices.

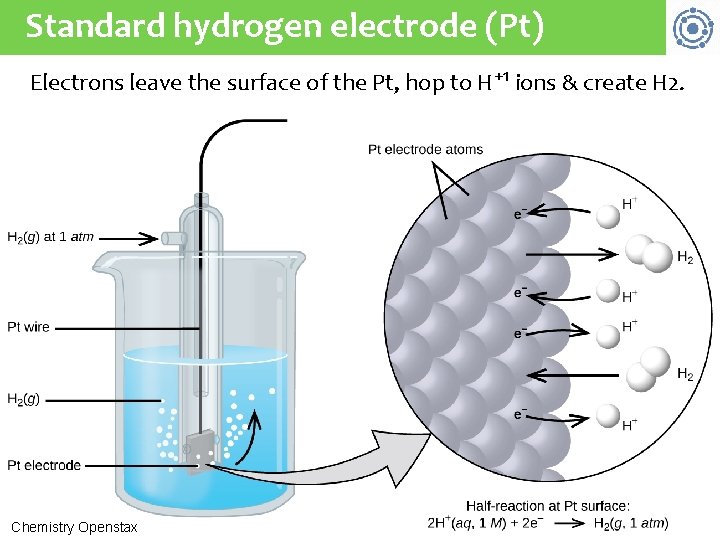
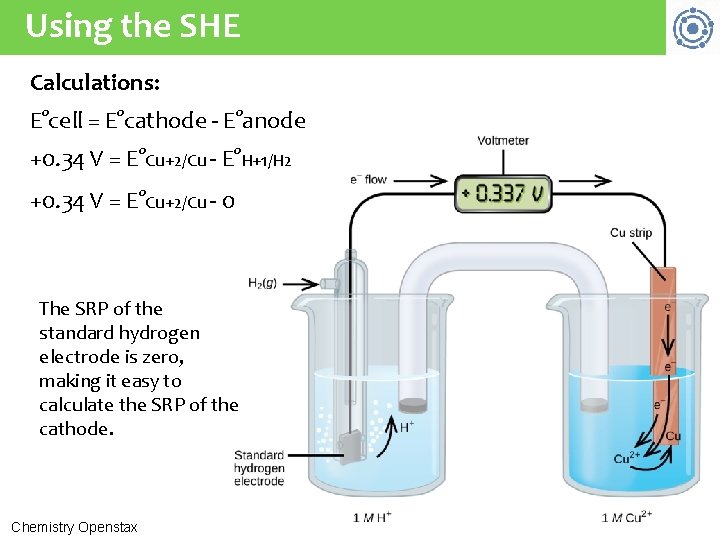
Determining standard reduction pot’l The electrical potential (or cell potential) of a galvanic cell (the voltage produced) is determined by the difference in electrical potentials between the two half-cells. • This is a relative, rather than absolute, value. The electrical potential of a single electrode or half-cell can’t be measured in isolation. Instead, the relative value of half-cells is determined in comparison to an agreed upon reference electrode called the 3 standard hydrogen electrode (SHE): 1 atm of H 2 gas bubbled through 1 M HCl at room temperature & Pt electrode Chemistry Openstax

Standard hydrogen electrode (Pt) Electrons leave the surface of the Pt, hop to H+1 ions & create H 2. Instead, the relative value of half-cells is determined in comparison to an agreed upon reference electrode called the standard 3 hydrogen electrode (SHE): 1 atm of H 2 gas bubbled through 1 M HCl at room temperature & Pt electrode Chemistry Openstax

Using the SHE A galvanic cell is constructed with a SHE and a Cu/Cu +2 half-cell to determine the standard reduction potential of Cu+2. 6 (a) Write the two half-reactions. (b) Label each as oxidation & reduction. (c) Diagram out the galvanic cell using shorthand. (a) H 2(q) 2 H+1 + 2 e- Cu+2(aq) + 2 e- Cu(s) (b) oxidation, anode (Pt) reduction, cathode (c) Pt(s) | H 2(g, 1 atm | H+1 (1 M) || Cu+2 (1 M) | Cu(s) 3

Using the SHE Calculations: E°cell = E°cathode - E°anode +0. 34 V = E°Cu+2/Cu - E°H+1/H 2 +0. 34 V = E°Cu+2/Cu - 0 The SRP of the standard hydrogen electrode is zero, making it easy to calculate the SRP of the cathode. Chemistry Openstax

Try this This galvanic cell is constructed to calculate the standard reduction potential of silver: Pt(s) | H 2(g, 1 atm) | H+1 (1 M) || Ag+1 (1 M) | Ag(s) The cell potential is +0. 80 V. (a) Write the two half-reactions. (b) Label them as oxidation & reduction. (c) Determine the standard reduction potential of the silver electrode. (a) H 2 2 H+1 + 2 e 3 (a) oxidation 2 Ag+1 + 2 e- 2 Ag reduction (c) E°cell = E°cathode - E°anode +0. 80 V = E°Ag+1/Ag - E°H+1/H 2 +0. 34 V = E°Ag+1/Ag - 0 7

Using standard reduction potentials When standard reduction potentials of both half-reactions are known, the cell potential of a galvanic cell can be calculated rather than experimentally determined. A positive cell potential indicates that a galvanic cell reacts spontaneously and will produce an electric current. Calculate the cell potential of this galvanic cell: 8 Cu(s) | Cu+2(1 M) || Ag+1 (1 M) | Ag(s) 3 anode E°cell = E°cathode - E°anode E°cell = E°Ag+1/Ag – E°Cu+2/Cu = (+0. 80 V) – (+ 0. 34 V) = +0. 46 V cathode

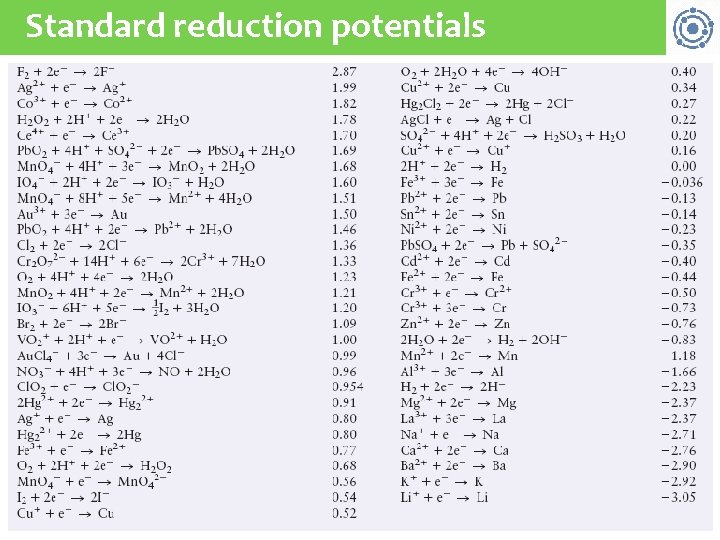
Standard reduction potentials

Try this A galvanic cell is made with Au+3/Au and Ni+2/Ni half-cells. (a) Identify the oxidizing & reducing agents. (b) Calculate the cell potential. (a) Au+3 + 3 e- Au(s) Ni+2 + 2 e- Ni(s) 9 E° = + 1. 498 V cathode E° = - 0. 257 V anode (smaller SRP) So, Ni is oxidized; the reducing agent. And Au is reduced; the oxidizing agent. 3 (b) E°cell = E°cathode - E°anode E°cell = E°Au+3/Au – E°Ni+2/Ni = (+1. 498) – (- 0. 257 V) = +1. 755 V

One more A galvanic cell is made with Mg electrode in a 1 M solution of Mg(NO 3)2 and a Ag electrode and a 1 M Ag(NO 3) solution. 10 (a) Identify the oxidizing & reducing agents. (b) Calculate the cell potential. (a) Mg+2 + 2 e- Mg(s) Ag+1 + e- Ag(s) E° = - 2. 37 V E° = +0. 80 V So, Mg is oxidized; the reducing agent. And Ag is reduced; the oxidizing agent. 3 (b) E°cell = E°cathode - E°anode E°cell = E°Ag+1/Ag – E°Mg+2/Mg = (+0. 80) – (- 2. 37 V) = +3. 17 V anode cathode

Can you? (1) Explain why the standard reduction potential of a half-cell cannot be determined in isolation (without another half-cell)? (2) Describe a standard hydrogen electrode, its construction and what it is used for? (3) Write the calculation used to determine cell potential? (4) Find both the appropriate SRP for both anode and cathode using a table of SRP values? (5) Calculate cell potential given a galvanic cell diagram? (6) Determine if a redox reaction is spontaneous?