Gibbs Free Energy and Spontaneity and the meaning
Gibbs Free Energy and Spontaneity and the meaning of the universe… 1

Relationship Between DS surr & H Water DS Surr (system) q DS surr affected by heat transfer into or out of closed system Entropy of the surroundings will be affected only by the heat transferred into or out of any closed system. Heat added to surroundings: • K. E. surr increases; Molecules are moving faster. • Disorder increases since there is more randomness • Entropy increases. Note: 2 qp = - DH sys µ DS surr

Heat flow; Temperature Dependent Remember that heat transfer is temperature dependent. Heat will transfer more efficiently with changes at low temperature than at high temperature. i. e. , 100€ to an International College student is worth more than to Raul. @ High temperature, molecules are already moving fast, an extra 10°C will not increase their velocities as much as molecules at very low temperature. -D H sys = D Ssurr units: T mol K Therefore since: J DS univ = DS sys + DS surr DS univ = DS sys - DH sys T 3 .

DS univ and Spontaneity Criteria for Spontaneity in terms of the system: DS univ = DS sys + DS surr DS univ = DS sys - DH sys T DS univ DH sys (-) (+) Spontaneous DS sys (+) Spontaneou s 4
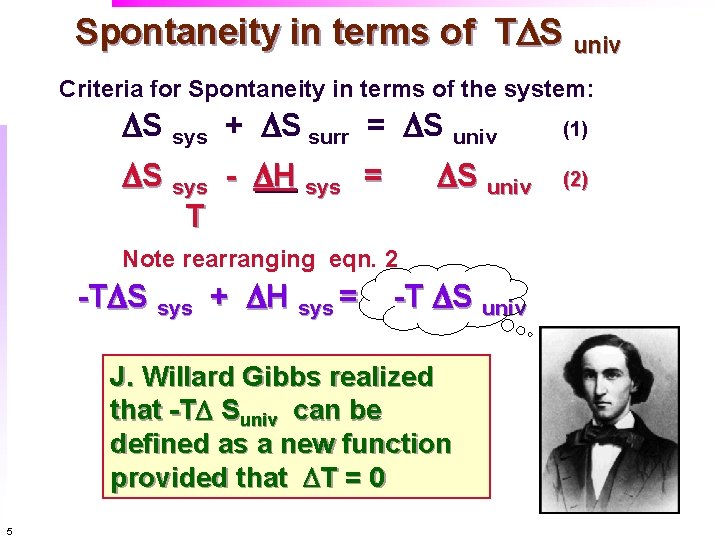
Spontaneity in terms of TDS univ Criteria for Spontaneity in terms of the system: DS sys + DS surr = DS univ DS sys - DH sys = T DS univ Note rearranging eqn. 2 -TDS sys + DH sys = -T DS univ J. Willard Gibbs realized that -TD Suniv can be defined as a new function provided that DT = 0 5 (1) (2)

J. Willard Gibbs (1839 -1903) was not particularly well known in his day, nor is his name widely recognized today, yet he is considered by some to be among the greatest scientists ever born in America. He was awarded th first doctorate in engineering granted in the United States, by Yale University. Gibbs became a professor of mathematical physics at Yale when he was 32 years old and began to publish a series of papers related to thermodynamics and equilibrium. Perhaps because his work was so theoretical, it was largely unappreciated at the time, though its great value was recognized by James Clerk Maxwell. Gibb’s work, if not his name, remains current and vital to this day. 6 The Free Energy change (DG) is a measure of spontaneity of a process and of the useful energy available from such a process.

DG and Spontaneity Defining a new State function DG: -T DS univ = DG @ DT, DP= 0 Consider DG = - T DS univ DG < 0 Spontaneous DS univ > 0 DG = 0 @ equilibrium DS univ = 0 DG > 0 nonspontaneous (rev is spontaneous) 7 DS univ < 0

DG: Pictorial View DG < 0 Forward reaction occur Spontaneous DG = 0 DG > 0 Reverse reaction occur nonspontaneou s in forward @ equilb in forward direction See later that : DG g Keq or Q DG < 0 DG > 0 Gibbs’ Free Energy can be defined in terms of the enthalpy of the system (DH sys) and the entropy of the system (DSsys) -T DS univ = DH sys - TDS sys = DG DG = DH - T DS 8
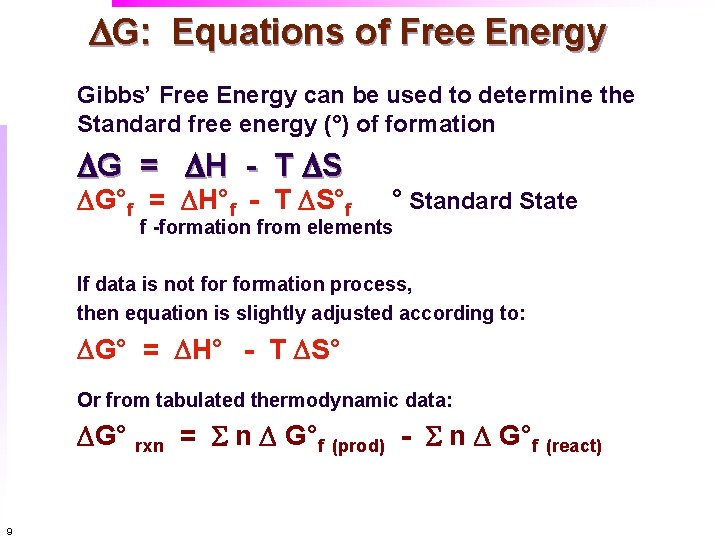
DG: Equations of Free Energy Gibbs’ Free Energy can be used to determine the Standard free energy (°) of formation DG = DH - T DS DG°f = DH°f - T DS°f ° Standard State f -formation from elements If data is not formation process, then equation is slightly adjusted according to: DG° = DH° - T DS° Or from tabulated thermodynamic data: DG° rxn = S n D G°f (prod) - S n D G°f (react) 9

DG: Evaluation of Free Energy Consider the calculation for the following reaction: CH 3 OH (g) + O 2 (g) g CO 2 (g) + H 2 O (g) Determine DG°rxn 2 CH 3 OH (g) + 3 O 2 (g) g 2 CO 2 (g) + 4 H 2 O (g) DH°rxn DS°rxn DG°rxn - 201. 2 + 237. 6 - 161. 9 0 205. 0 0 -393. 5 -213. 6 -394. 4 -241. 82 c -188. 83 c -228. 57 c Evaluate by: DG°rxn = DH° rxn - T DS° rxn DX° rxn = S n D X°f (prod) - S n D X°f (react) Or 1 DG°rxn = S n D G°f (prod) - S n D G°f (react)

Effect of temperature on Free Energy Temperature influence on Free Energy and Spontaneity DG = DH - T DS both DH, DS (+) (1000) What is the lg. # (1) sm. # sign of DG ? Temperature will dictate outcome of DG. Tlow: Temperature small DH - dominates TDS negligible Thigh: Temperature large DH negligible 1 - TDS dominates g DG (+) nonspontaneous g DG (-) spontaneous
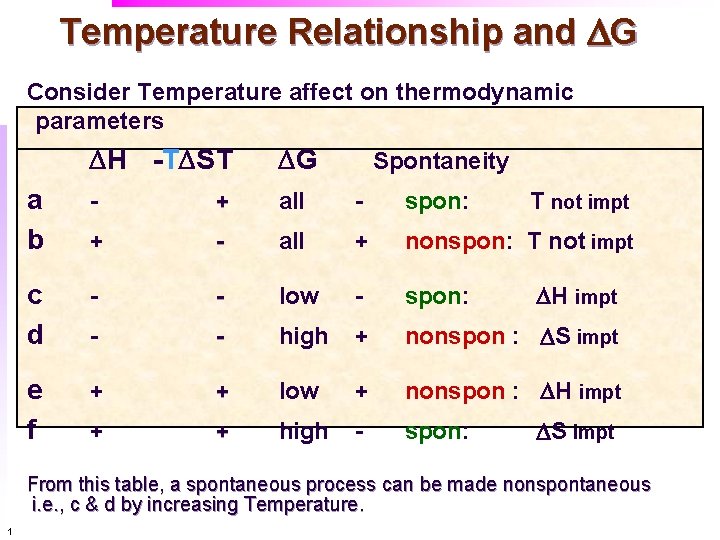
Temperature Relationship and DG Consider Temperature affect on thermodynamic parameters DH -TDST DG a b - + all - spon: + - all + nonspon: T not impt c d - - low - spon: - - high + nonspon : DS impt e f + + low + nonspon : DH impt + + high - spon: Spontaneity T not impt DH impt DS impt From this table, a spontaneous process can be made nonspontaneous i. e. , c & d by increasing Temperature. 1

Spontaneity: Example : (c) N 2 F 4(g) g @ T Low @ T High 2 NF 2 (g) DH° - T DS° DG° 85 k. J 198 J/K DH° dominates DG° (+) Nonspontaneous -TDS° dominates DG° (-) Spontaneous Example : (c) What temp will spontaneity switch for the reaction: N 2 (g) + 3 H 2(g) D 2 NH 3 (g) DH° - T DS°DG° @ T Low @ T High - 92 k. J -198. 5 J/K DH° dominates DG° (-) Spontaneous -TDS° dominates DG° (+) Nonspontaneous To go from spontaneous to nonspontaneous, DG° = 0 … T = - 92 k. J = 463. 5 K below spontaneous -0. 198 k. J / K above, nonspontaneous 1
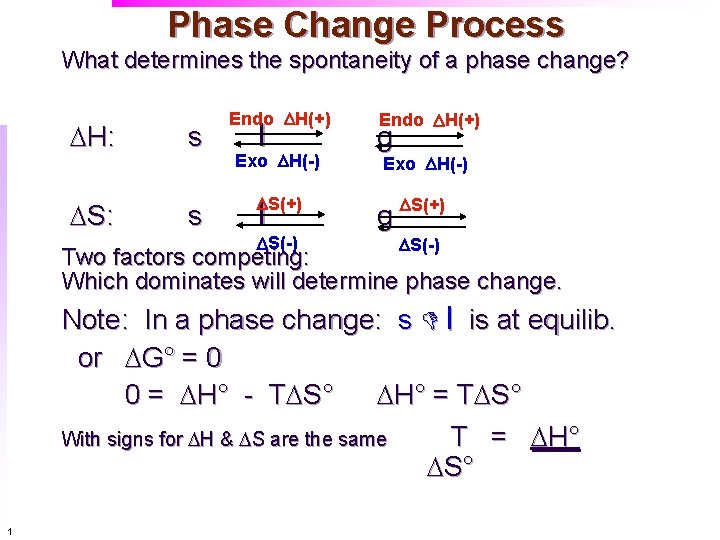
Phase Change Process What determines the spontaneity of a phase change? DH: DS: s s Endo DH(+) l Exo DH(-) DS(+) l DS(-) Endo DH(+) g Exo DH(-) g DS(+) DS(-) Two factors competing: Which dominates will determine phase change. Note: In a phase change: s D l is at equilib. or DG° = 0 0 = DH° - TDS° DH° = TDS° With signs for DH & DS are the same T = DH° DS° 1

Free Energy and work Science and Technology use physical and or chemical processes because these can do work. Economics: To make money €, the work to be preformed must be a possibility and efficient. DG provides information on spontaneity: DG (+) or (-) provides information on the spontaneity of the process @ DP, DT = 0 Wasting time: DG is useful because it prevents the wasted effort on process with no inherent tendency to occur. DG isn’t whole story, Kinetics also important: Note that thermodynamically favorable process may still not occur to any appreciable extent because of the Kinetics. - It makes sense to find a catalyst to speed up the reaction. - Prevents wasting time and resource of seeking a catalyst on a reaction that won’t even work. 1
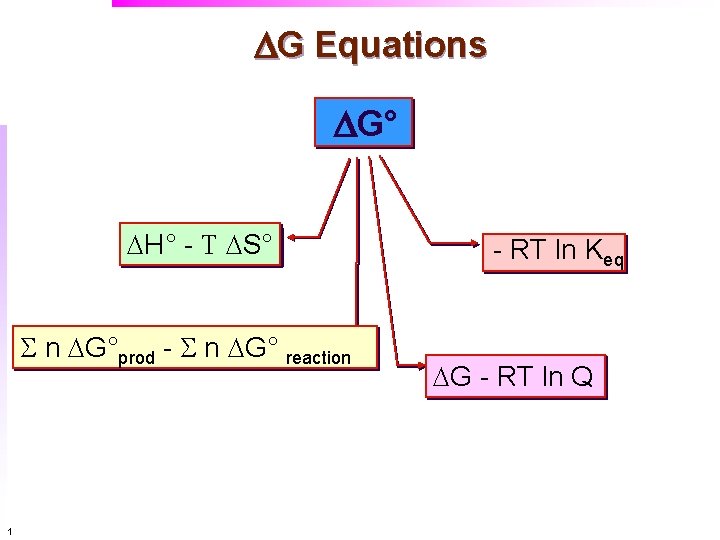
DG Equations DG° DH° - T DS° S n DG°prod - S n DG° reaction 1 - RT ln Keq DG - RT ln Q
- Slides: 16