17 2 17 3 Spontaneous and Nonspontaneous Processes










- Slides: 27

17. 2 -17. 3 Spontaneous and Nonspontaneous Processes- Entropy and Second Law of Thermodynamics Presented by Katie Hall

Nature’s Heat Tax ● First Law of Thermodynamics: Energy cannot be created or destroyed SO energy cannot be greater at the end of an energy transfer ● During every transfer of energy, heat is lost to the surroundings ○ 5 Watts emitted as light 95 watts dissipated as heat Impossibility of perpetual motion machines ● Most energy transfers don’t even run to maximum efficiency 100 Watts of electrical energy

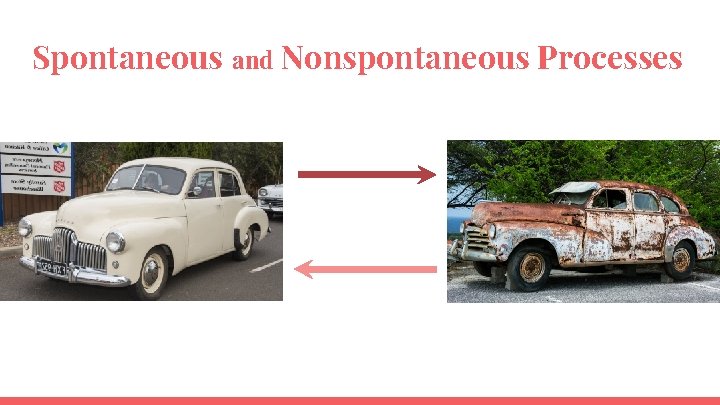
Spontaneous and Nonspontaneous Processes

Spontaneous and Nonspontaneous Processes ● A spontaneous process is one that occurs without ongoing outside intervention. Spontaneity of a chemical reaction ≠ Speed of a reaction ● Spontaneity=the direction in which and extent to which a chemical reaction proceeds ● Speed=how fast a reaction takes place ● Catalysts can speed up spontaneous reactions but can not make nonspontaneous reactions spontaneous

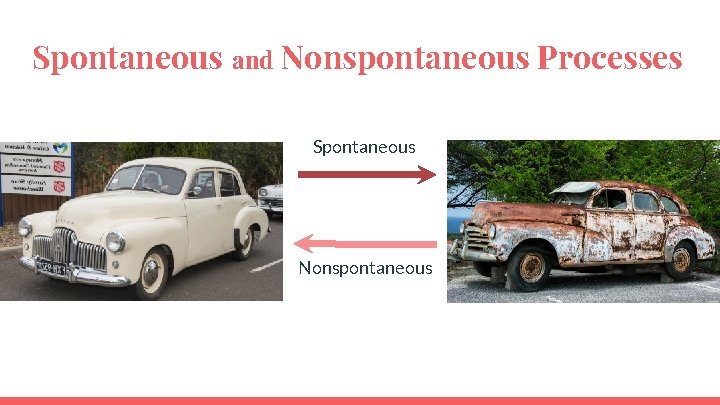
Spontaneous and Nonspontaneous Processes

Spontaneous and Nonspontaneous Processes Spontaneous Nonspontaneous

Spontaneous and Nonspontaneous Processes A nonspontaneous process is not impossible. It can be made spontaneous by coupling it to another process that is spontaneous OR by supplying energy from an external source.

Entropy and the Second Law of Thermodynamics

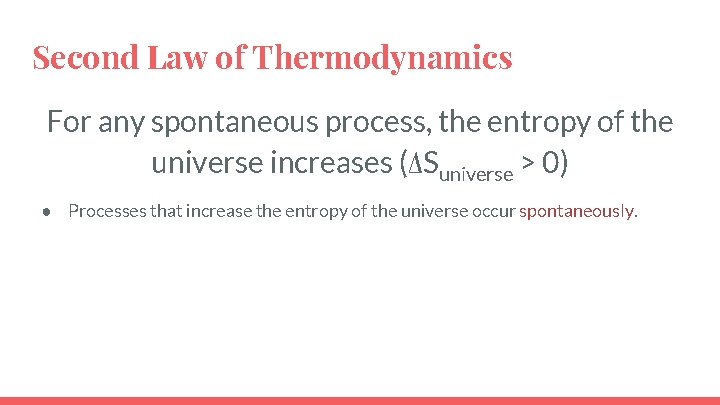
Second Law of Thermodynamics For any spontaneous process, the entropy of the universe increases (ΔSuniverse > 0) ● Processes that increase the entropy of the universe occur spontaneously.

Enthalpy and Spontaneity ● Reactions that start with more potential energy than they end with (exothermic reactions) are often spontaneous ● Endothermic reactions can also be spontaneous due to entropy.

What is Entropy?

What is Entropy? A measure of the energy randomization or energy dispersal in a system.

What is Entropy? A measure of the energy randomization or energy dispersal in a system. But more specifically. . .

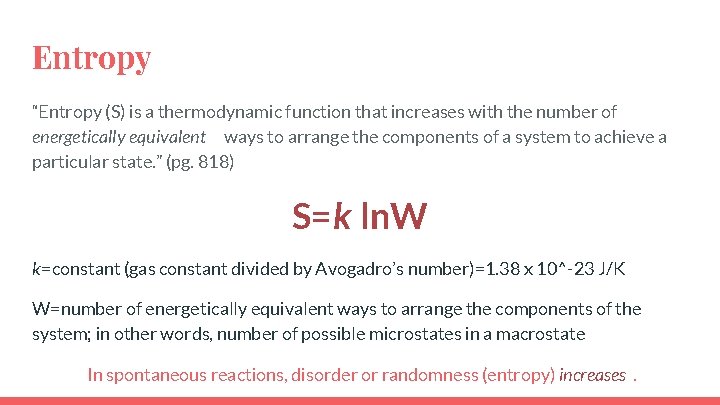
Entropy “Entropy (S) is a thermodynamic function that increases with the number of energetically equivalent ways to arrange the components of a system to achieve a particular state. ” (pg. 818) S=k ln. W k=constant (gas constant divided by Avogadro’s number)=1. 38 x 10^-23 J/K W=number of energetically equivalent ways to arrange the components of the system; in other words, number of possible microstates in a macrostate In spontaneous reactions, disorder or randomness (entropy) increases.

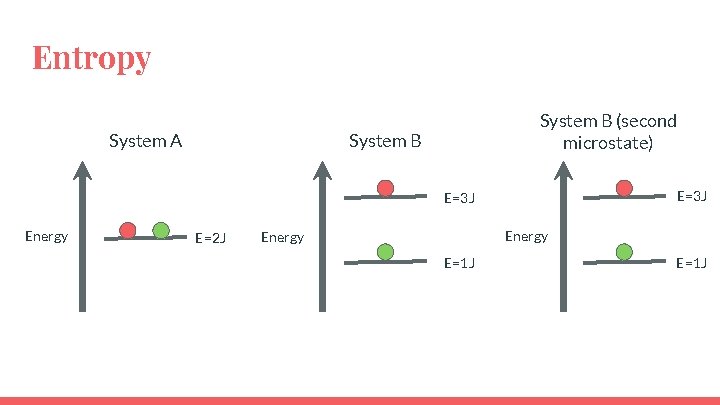
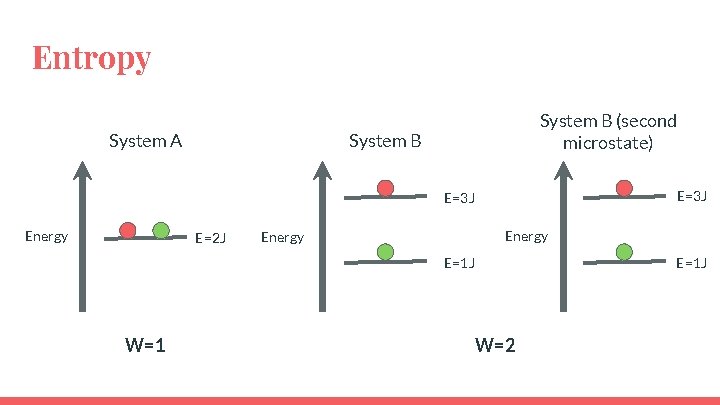
Entropy System A System B (second microstate) System B E=3 J Energy E=2 J Energy E=1 J

Entropy System A System B (second microstate) System B E=3 J Energy E=2 J Energy E=1 J W=1 E=1 J W=2

Entropy ● System B has more energetically equivalent ways to arrange the system so it has greater entropy. ● So the state with the highest entropy also has the highest dispersal of energy. ● “A state in which a given amount of energy is more highly dispersed (or more highly randomized) has more entropy than a state in which the same energy is more concentrated” (pg. 820)

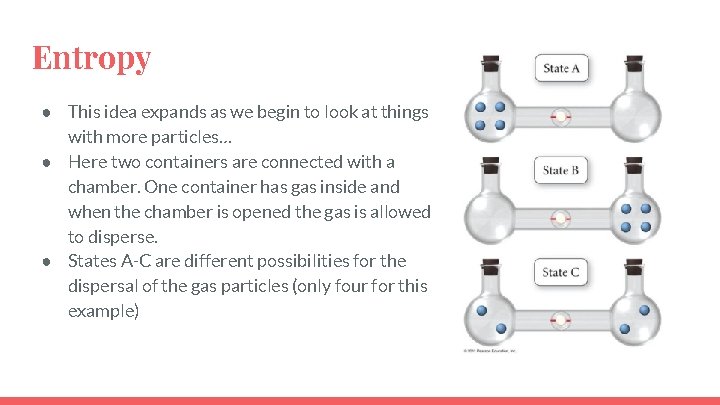
Entropy ● This idea expands as we begin to look at things with more particles… ● Here two containers are connected with a chamber. One container has gas inside and when the chamber is opened the gas is allowed to disperse. ● States A-C are different possibilities for the dispersal of the gas particles (only four for this example)

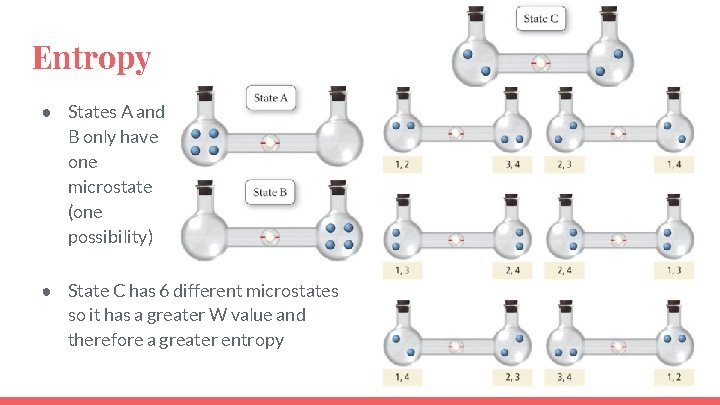
Entropy ● States A and B only have one microstate (one possibility) ● State C has 6 different microstates so it has a greater W value and therefore a greater entropy

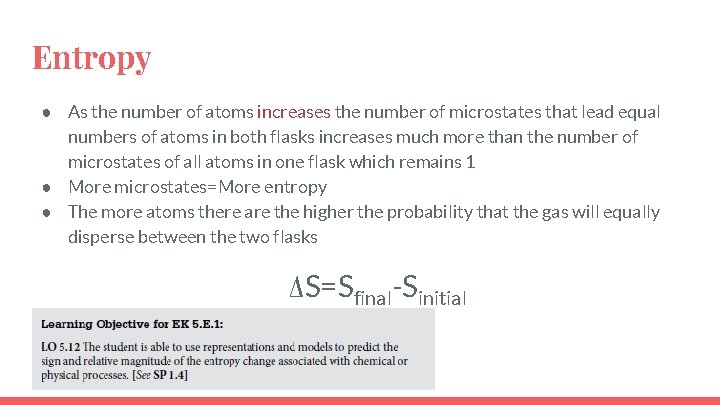
Entropy ● As the number of atoms increases the number of microstates that lead equal numbers of atoms in both flasks increases much more than the number of microstates of all atoms in one flask which remains 1 ● More microstates=More entropy ● The more atoms there are the higher the probability that the gas will equally disperse between the two flasks ΔS=Sfinal-Sinitial

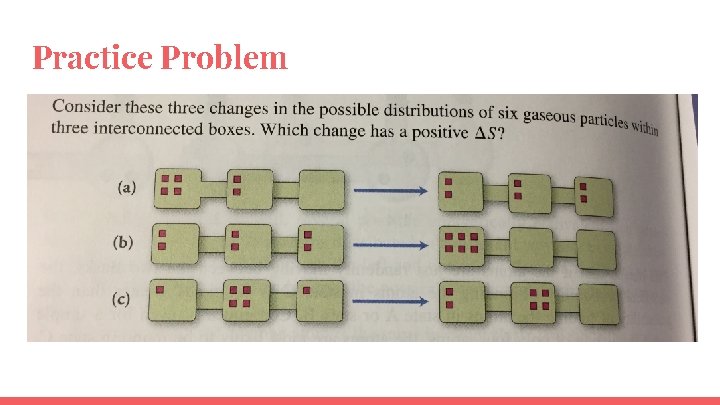
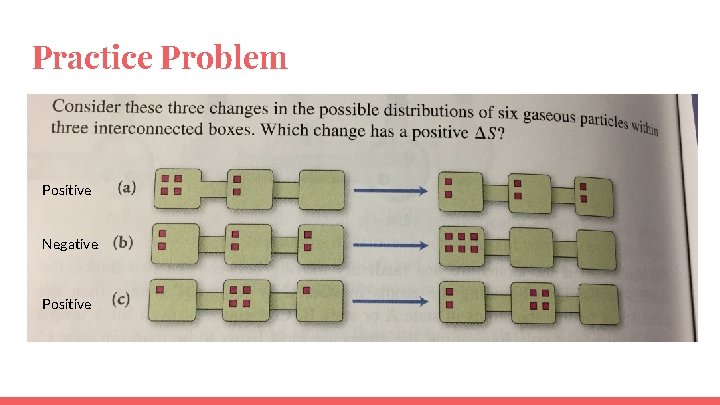
Practice Problem

Practice Problem Positive Negative Positive

Second Law of Thermodynamics For any spontaneous process, the entropy of the universe increases (ΔSuniverse > 0) ● Processes that increase the entropy of the universe (result in more disorder) occur spontaneously. ● Ted-Ed: https: //youtu. be/YM-uyk. Vfq_E

The Entropy Change Associated with a Change in State

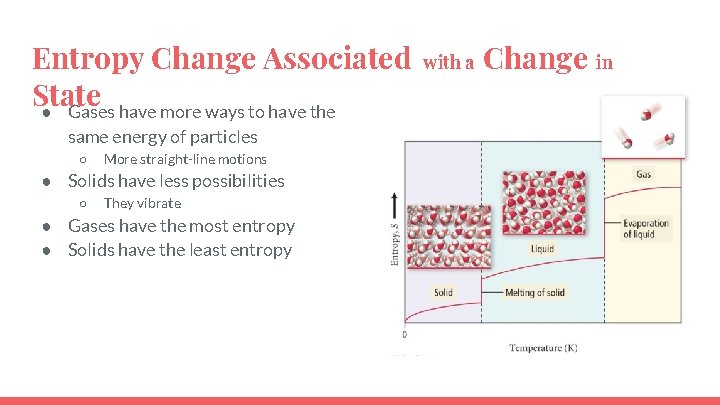
Entropy Change Associated State ● Gases have more ways to have the same energy of particles ○ More straight-line motions ● Solids have less possibilities ○ They vibrate ● Gases have the most entropy ● Solids have the least entropy with a Change in

Entropy Change Associated State with a Change in Entropy increases for the following: ● ● Phase transition from solid to liquid Phase transition from solid to gas Phase transition from liquid to gas An increase in number of moles of a gas during a chemical reaction

Sources for Images-(In order of appearance) https: //www. 1000 bulbs. com/product/67454/PLT-S 2412. html https: //shop. advanceautoparts. com/r/car-projects/how-to-remove-rust-from-your-vehicle https: //www. northerndailyleader. com. au/story/4721581/gallery-tamworth-vintage-car-clubs-north-west-rally/