Thermodynamics Spontaneity Entropy and Free Energy Spontaneity A




























- Slides: 63

Thermodynamics: Spontaneity, Entropy and Free Energy

Spontaneity A spontaneous process is one that occurs without outside intervention. Examples include: - a ball rolling downhill - ice melting at temperatures above 0 o. C - gases expanding to fill their container - iron rusts in the presence of air and water

Spontaneity Spontaneous processes can release energy (a ball rolling downhill), require energy (ice melting at temperatures above 0 o. C), or involve no energy change at all (two gases mixing).

Spontaneity There are three factors that combine to predict spontaneity. They are: 1. Energy Change 2. Temperature 3. Entropy Change

Entropy A measure of randomness or disorder

Entropy, S, is a measure of randomness or disorder. The natural tendency of things is to tend toward greater disorder. This is because there are many ways (or positions) that lead to disorder, but very few that lead to an ordered state.

Entropy The driving force for a spontaneous process is an increase in the entropy of the universe.

Entropy

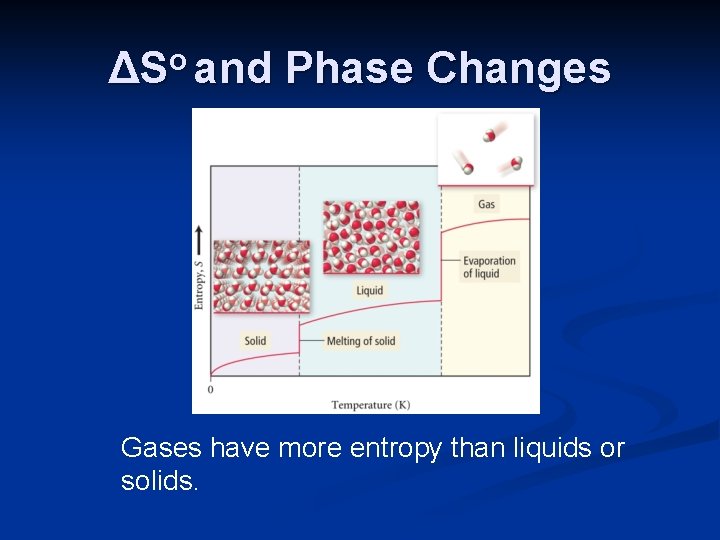
ΔSo and Phase Changes Gases have more entropy than liquids or solids.

ΔSo and Mixtures have more entropy than pure substances.

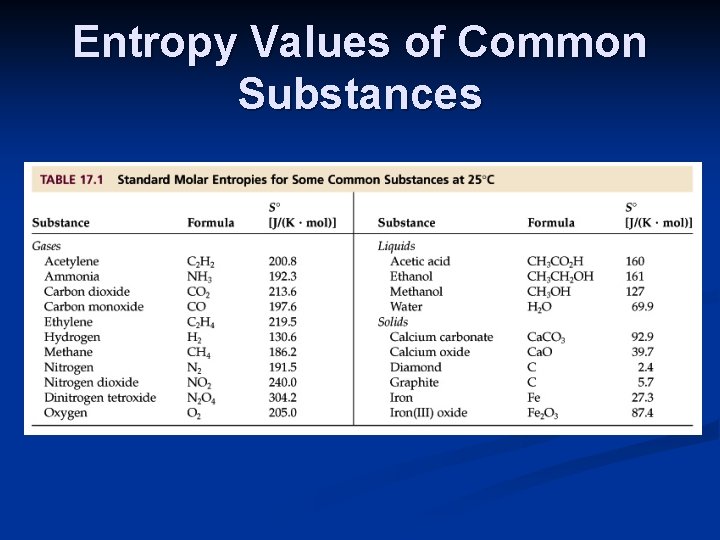
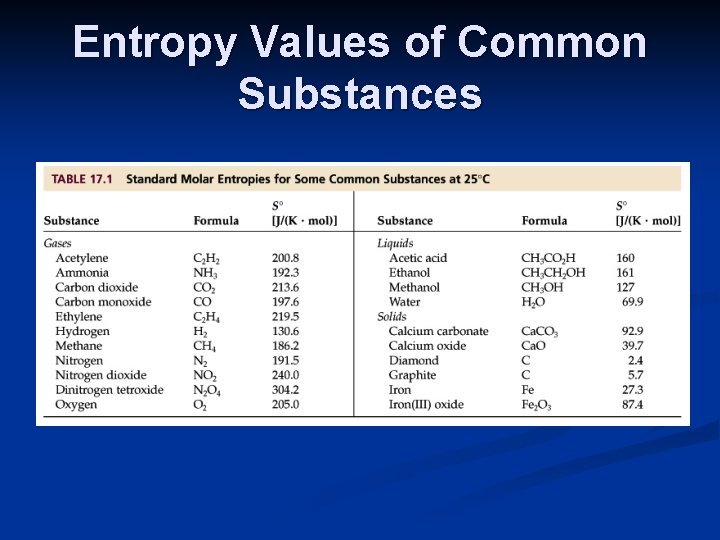
Entropy Values of Common Substances

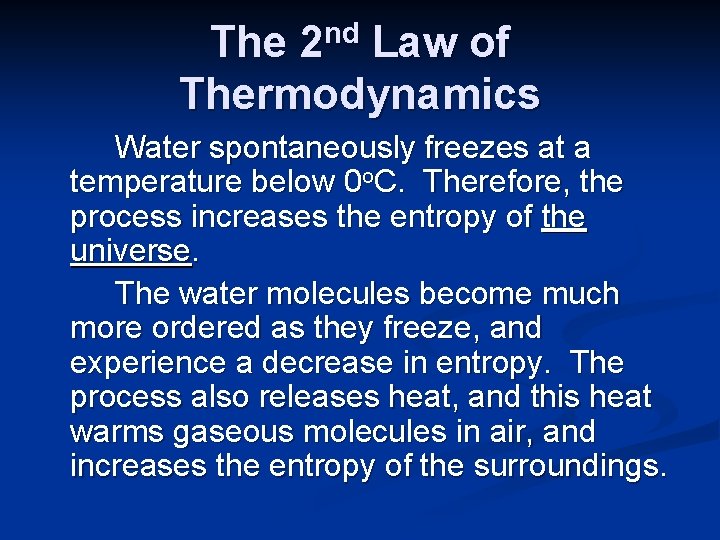
nd 2 The Law of Thermodynamics In any spontaneous process there is always an increase in the entropy of the universe.

nd 2 The Law of Thermodynamics Water spontaneously freezes at a temperature below 0 o. C. Therefore, the process increases the entropy of the universe. The water molecules become much more ordered as they freeze, and experience a decrease in entropy. The process also releases heat, and this heat warms gaseous molecules in air, and increases the entropy of the surroundings.

nd 2 The Law of Thermodynamics Since the process is spontaneous below 0 o. C, ΔSsurr, which is positive, must be greater in magnitude than ΔS of the water molecules.

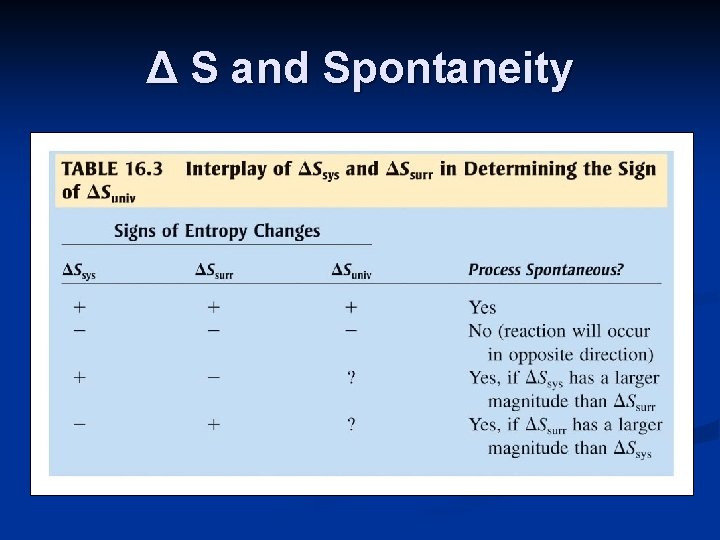
Δ S and Spontaneity

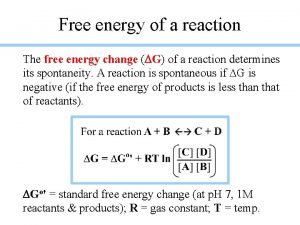
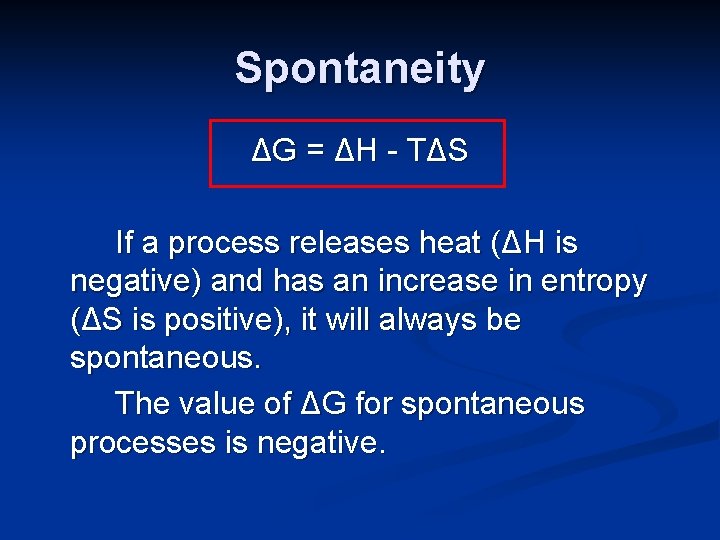
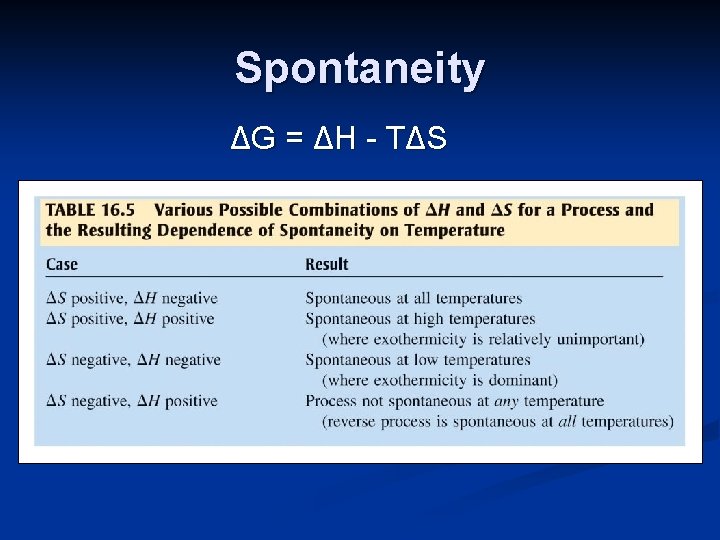
Spontaneity Entropy, temperature and heat flow all play a role in spontaneity. A thermodynamic quantity, the Gibbs Free Energy (G), combines these factors to predict the spontaneity of a process. ΔG = ΔH - T ΔS

Spontaneity ΔG = ΔH - T ΔS If a process releases heat (ΔH is negative) and has an increase in entropy (ΔS is positive), it will always be spontaneous. The value of ΔG for spontaneous processes is negative.

Spontaneity ΔG = ΔH - T ΔS

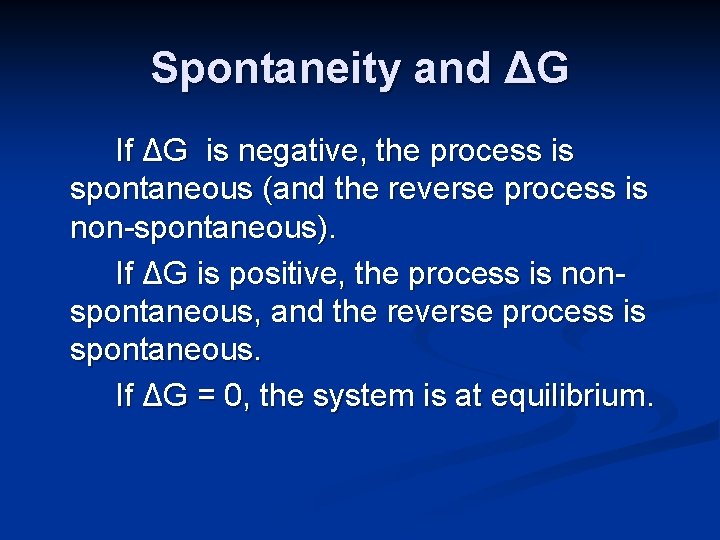
Spontaneity and ΔG If ΔG is negative, the process is spontaneous (and the reverse process is non-spontaneous). If ΔG is positive, the process is nonspontaneous, and the reverse process is spontaneous. If ΔG = 0, the system is at equilibrium.

ΔG Although ΔG can be used to predict in which direction a reaction will proceed, it does not predict the rate of the reaction. For example, the conversion of diamond to graphite has a ΔGo = -3 k. J, so diamonds should spontaneously change to graphite at standard conditions. However, kinetics shows that the reaction is extremely slow.

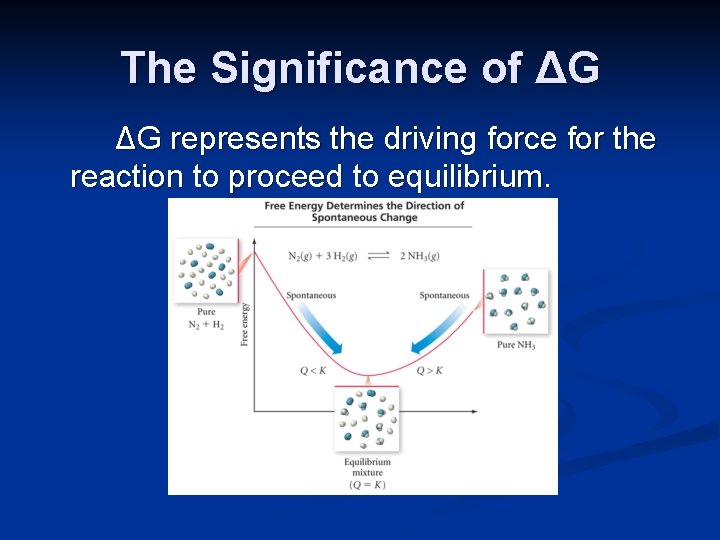
The Significance of ΔG ΔG represents the driving force for the reaction to proceed to equilibrium.

The Significance of ΔG If negative, the value of ΔG in KJ is the maximum possible useful work that can be obtained from a process or reaction at constant temperature and pressure. If positive, the value of ΔG in KJ is the minimum work that must be done to make the non-spontaneous process or reaction proceed.

Predicting the sign of ΔSo For many chemical reactions or physical changes, it is relatively easy to predict if the entropy of the system is increasing or decreasing. If a substance goes from a more ordered phase (solid) to a less ordered phase (liquid or gas), its entropy increases.

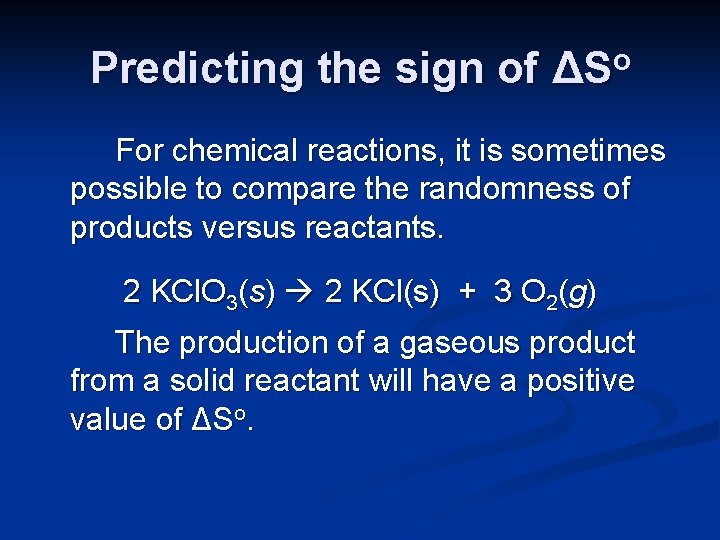
Predicting the sign of ΔSo For chemical reactions, it is sometimes possible to compare the randomness of products versus reactants. 2 KCl. O 3(s) 2 KCl(s) + 3 O 2(g) The production of a gaseous product from a solid reactant will have a positive value of ΔSo.

Calculating Entropy Changes Since entropy is a measure of randomness, it is possible to calculate absolute entropy values. This is in contrast to enthalpy values, where we can only calculate changes in enthalpy. A perfect crystal at absolute zero has an entropy value (S) =0. All other substances have positive values of entropy due to some degree of disorder.

Calculating Entropy Changes Fortunately, the entropy values of most common elements and compounds have been tabulated. Most thermodynamic tables, including the appendix in the textbook, include standard entropy values, S o.

Entropy Values of Common Substances

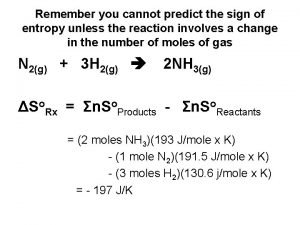
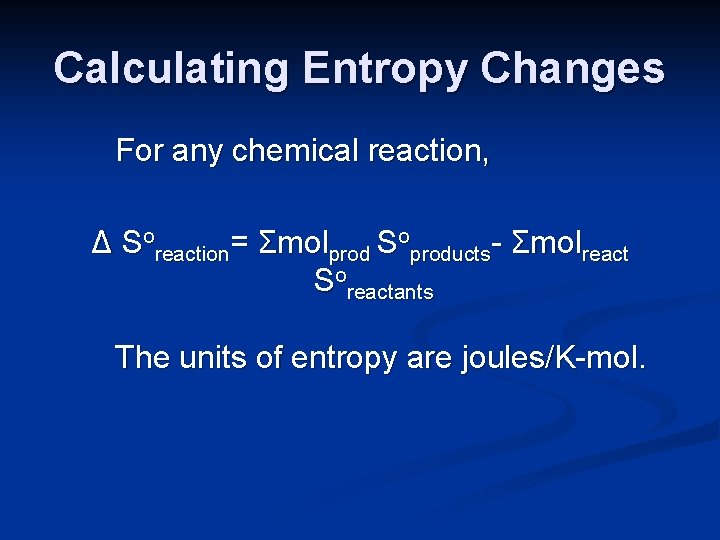
Calculating Entropy Changes For any chemical reaction, Δ Soreaction= Σmolprod Soproducts- Σmolreact Soreactants The units of entropy are joules/K-mol.

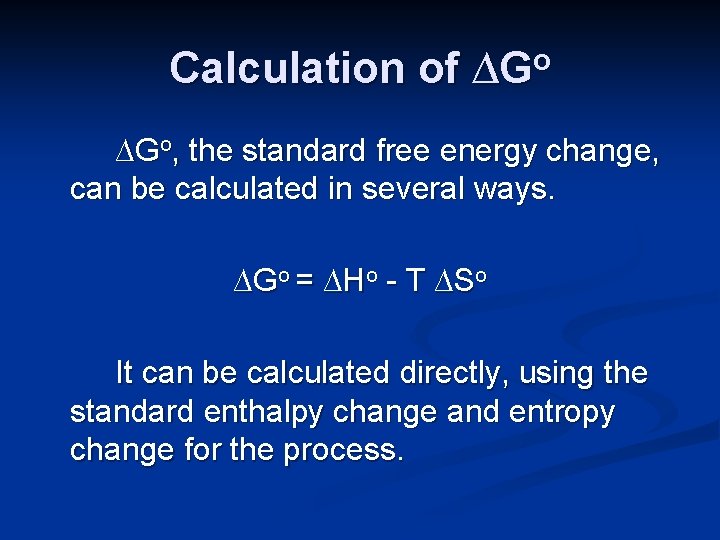
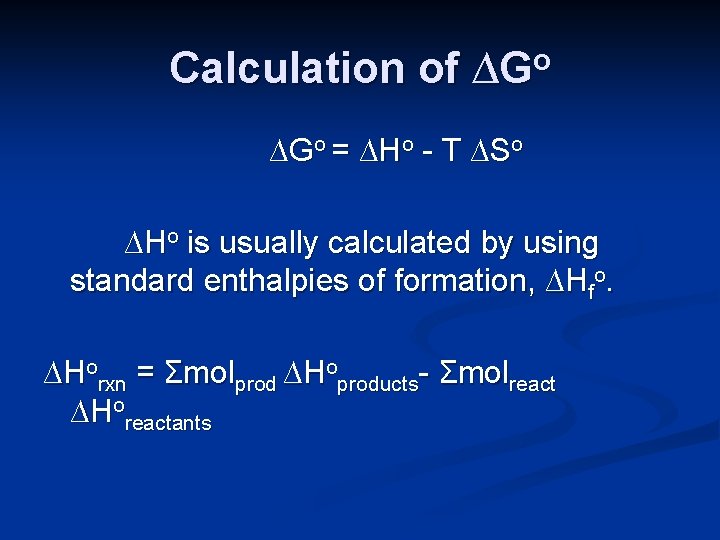
Calculation of ∆Go, the standard free energy change, can be calculated in several ways. ∆Go = ∆Ho - T ∆So It can be calculated directly, using the standard enthalpy change and entropy change for the process.

Calculation of ∆Go = ∆Ho - T ∆So ∆Ho is usually calculated by using standard enthalpies of formation, ∆Hfo. ∆Horxn = Σmolprod ∆Hoproducts- Σmolreact ∆Horeactants

Calculation of ∆Go = ∆Ho - T ∆So Once ∆Ho and ∆So have been calculated, the value of ∆Go can be calculated, using the temperature in Kelvins.

Calculation of ∆Go can also be calculated by combining the free energy changes of related reactions. This is the same method used in Hess’ Law to calculate enthalpy changes. If the sum of the reactions gives the reaction of interest, the sum of the ∆Go values gives ∆Go for the reaction.

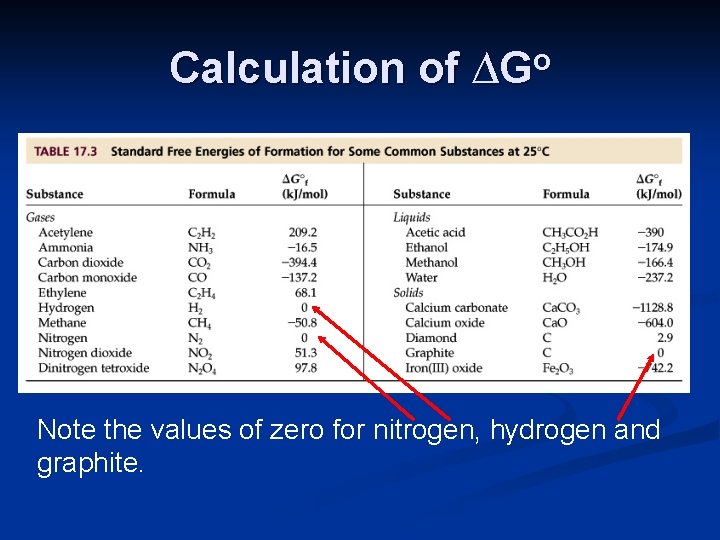
Calculation of ∆Go Lastly, ∆Go can be calculated using standard free energies of formation, ∆Gfo. Some tables of thermodynamic data, including the appendix of your textbook, include values of ∆Gfo. ∆Grxno = Σmolprod ∆Gfo prod - Σmolreact ∆Gfo react

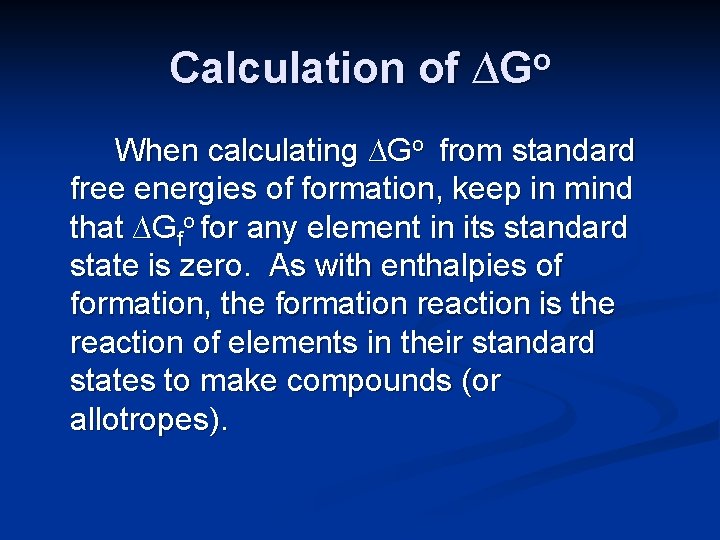
Calculation of ∆Go When calculating ∆Go from standard free energies of formation, keep in mind that ∆Gfo for any element in its standard state is zero. As with enthalpies of formation, the formation reaction is the reaction of elements in their standard states to make compounds (or allotropes).

Calculation of ∆Go

Calculation of ∆Go Note the values of zero for nitrogen, hydrogen and graphite.

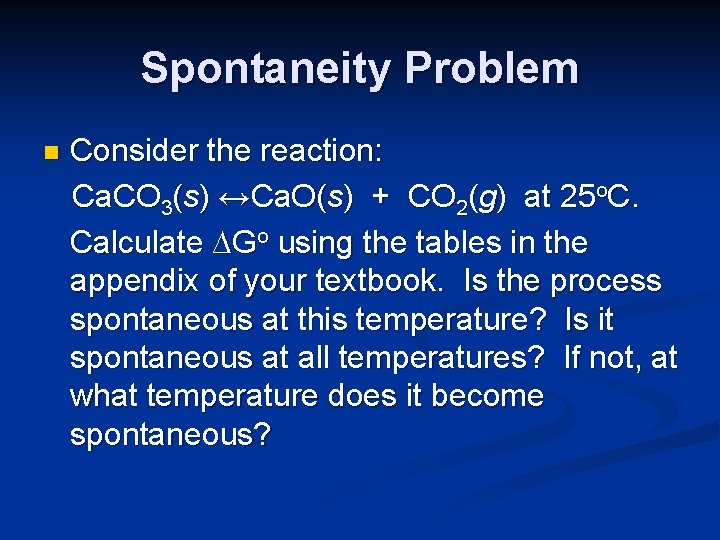
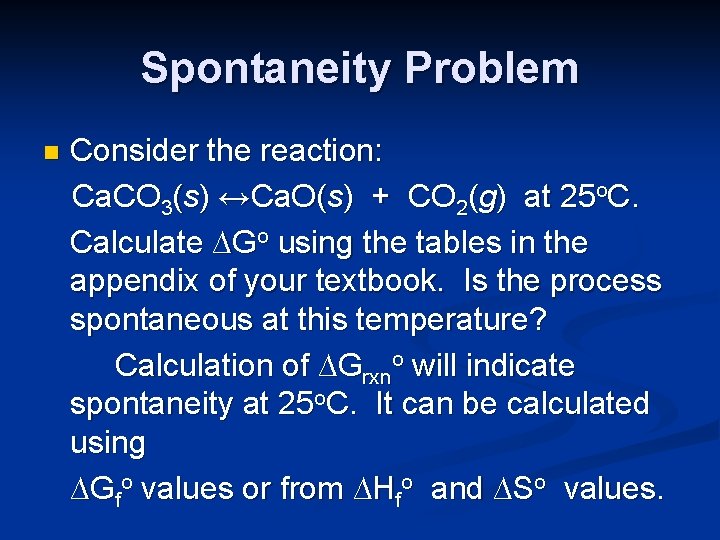
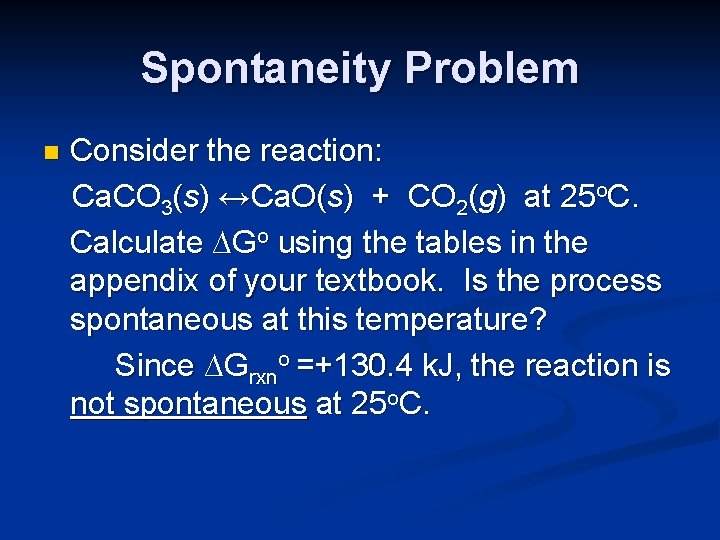
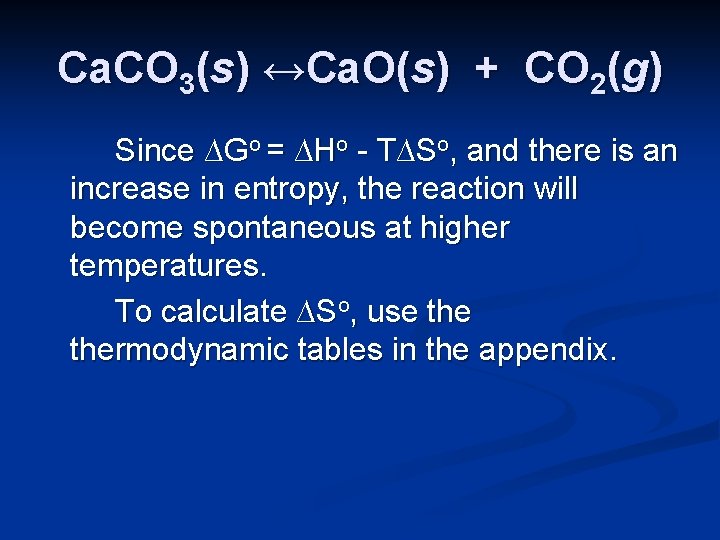
Spontaneity Problem n Consider the reaction: Ca. CO 3(s) ↔Ca. O(s) + CO 2(g) at 25 o. C. Calculate ∆Go using the tables in the appendix of your textbook. Is the process spontaneous at this temperature? Is it spontaneous at all temperatures? If not, at what temperature does it become spontaneous?

Spontaneity Problem n Consider the reaction: Ca. CO 3(s) ↔Ca. O(s) + CO 2(g) at 25 o. C. Calculate ∆Go using the tables in the appendix of your textbook. Is the process spontaneous at this temperature? Calculation of ∆Grxno will indicate spontaneity at 25 o. C. It can be calculated using ∆Gfo values or from ∆Hfo and ∆So values.

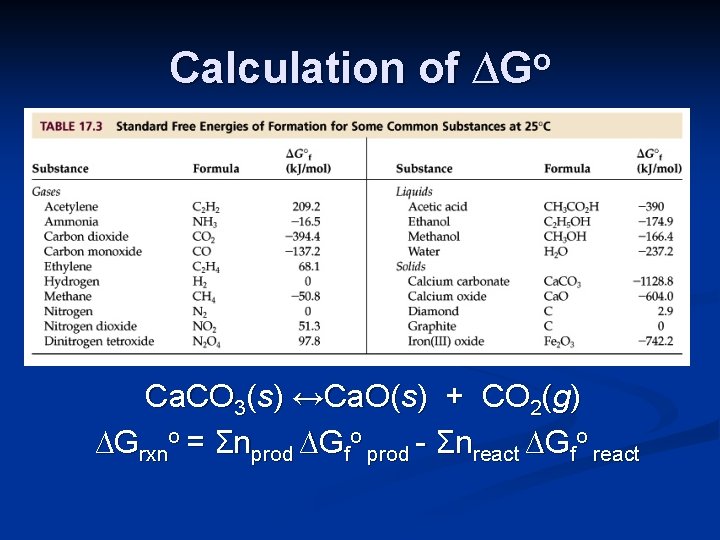
Calculation of ∆Go Ca. CO 3(s) ↔Ca. O(s) + CO 2(g) ∆Grxno = Σnprod ∆Gfo prod - Σnreact ∆Gfo react

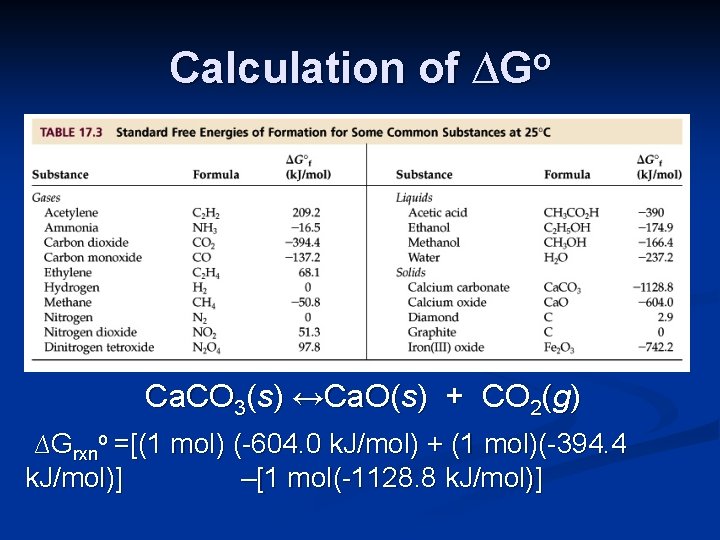
Calculation of ∆Go Ca. CO 3(s) ↔Ca. O(s) + CO 2(g) ∆Grxno =[(1 mol) (-604. 0 k. J/mol) + (1 mol)(-394. 4 k. J/mol)] –[1 mol(-1128. 8 k. J/mol)]

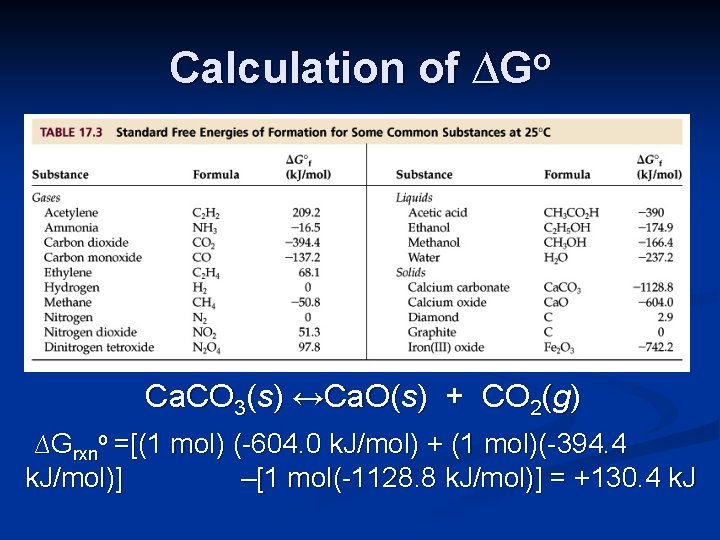
Calculation of ∆Go Ca. CO 3(s) ↔Ca. O(s) + CO 2(g) ∆Grxno =[(1 mol) (-604. 0 k. J/mol) + (1 mol)(-394. 4 k. J/mol)] –[1 mol(-1128. 8 k. J/mol)] = +130. 4 k. J

Spontaneity Problem n Consider the reaction: Ca. CO 3(s) ↔Ca. O(s) + CO 2(g) at 25 o. C. Calculate ∆Go using the tables in the appendix of your textbook. Is the process spontaneous at this temperature? Since ∆Grxno =+130. 4 k. J, the reaction is not spontaneous at 25 o. C.

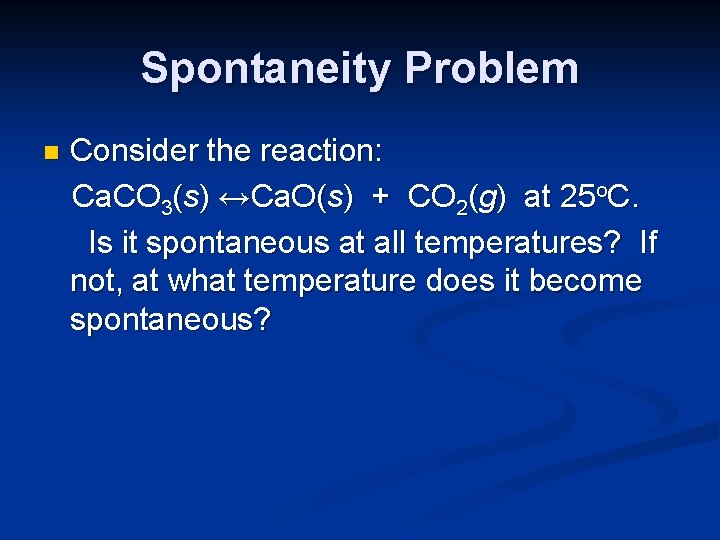
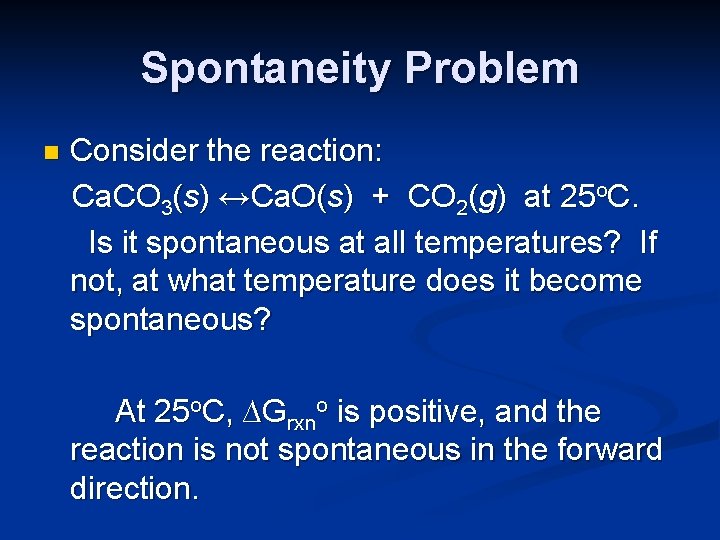
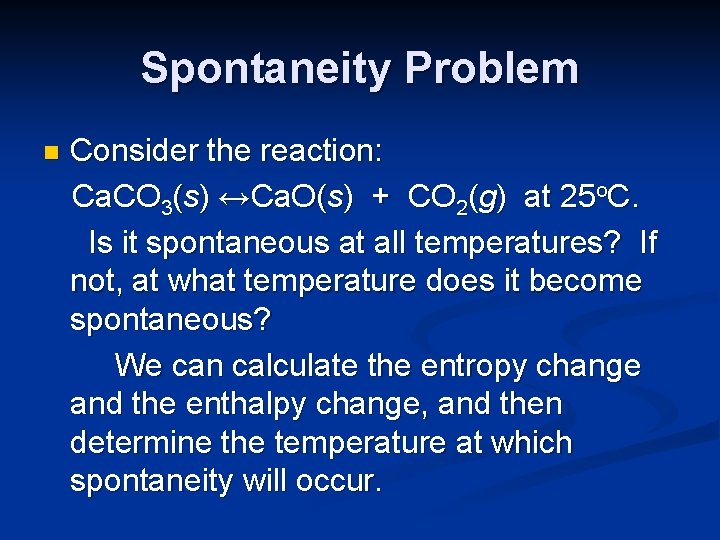
Spontaneity Problem n Consider the reaction: Ca. CO 3(s) ↔Ca. O(s) + CO 2(g) at 25 o. C. Is it spontaneous at all temperatures? If not, at what temperature does it become spontaneous?

Spontaneity Problem n Consider the reaction: Ca. CO 3(s) ↔Ca. O(s) + CO 2(g) at 25 o. C. Is it spontaneous at all temperatures? If not, at what temperature does it become spontaneous? At 25 o. C, ∆Grxno is positive, and the reaction is not spontaneous in the forward direction.

Spontaneity Problem n Consider the reaction: Ca. CO 3(s) ↔Ca. O(s) + CO 2(g) at 25 o. C. Is it spontaneous at all temperatures? If not, at what temperature does it become spontaneous? Inspection of the reaction shows that it involves an increase in entropy due to production of a gas from a solid.

Spontaneity Problem n Consider the reaction: Ca. CO 3(s) ↔Ca. O(s) + CO 2(g) at 25 o. C. Is it spontaneous at all temperatures? If not, at what temperature does it become spontaneous? We can calculate the entropy change and the enthalpy change, and then determine the temperature at which spontaneity will occur.

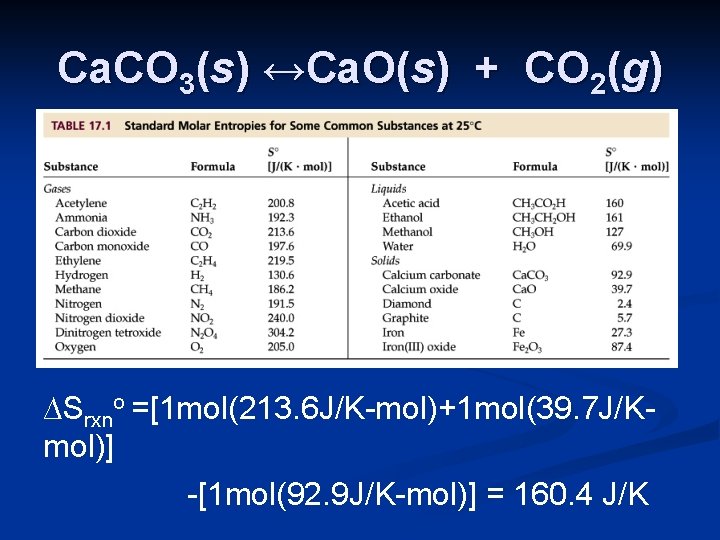
Ca. CO 3(s) ↔Ca. O(s) + CO 2(g) Since ∆Go = ∆Ho - T∆So, and there is an increase in entropy, the reaction will become spontaneous at higher temperatures. To calculate ∆So, use thermodynamic tables in the appendix.

Ca. CO 3(s) ↔Ca. O(s) + CO 2(g) ∆Srxno =[1 mol(213. 6 J/K-mol)+1 mol(39. 7 J/Kmol)] -[1 mol(92. 9 J/K-mol)] = 160. 4 J/K

Ca. CO 3(s) ↔Ca. O(s) + CO 2(g) ∆Go = ∆Ho - T∆So Since we know the value of ∆Go (+130. 4 k. J) and ∆So (160. 4 J/K), we can calculate the value of ∆Ho at 25 o. C. 130. 4 k. J = ∆Ho –(298 K) (160. 4 J/K) ( ∆Ho = 130. 4 k. J + (298 K) (. 1604 k. J/K) (. ∆Ho = + 178. 2 k. J

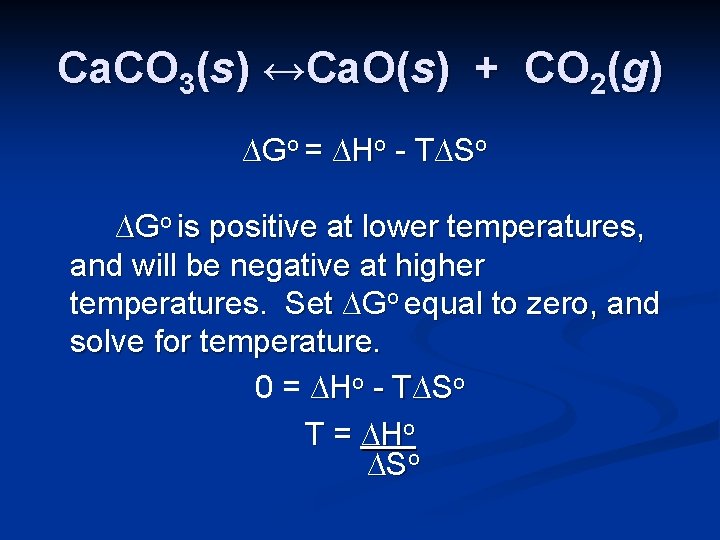
Ca. CO 3(s) ↔Ca. O(s) + CO 2(g) ∆Go = ∆Ho - T∆So If we assume that the values of ∆Ho and ∆So don’t change much with temperature, we can estimate the temperature at which the reaction will become spontaneous.

Ca. CO 3(s) ↔Ca. O(s) + CO 2(g) ∆Go = ∆Ho - T∆So ∆Go is positive at lower temperatures, and will be negative at higher temperatures. Set ∆Go equal to zero, and solve for temperature. 0 = ∆Ho - T∆So T = ∆Ho ∆So

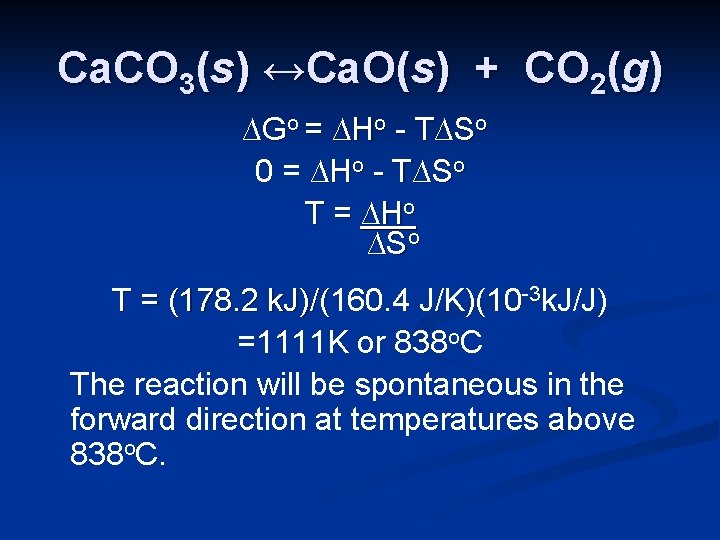
Ca. CO 3(s) ↔Ca. O(s) + CO 2(g) ∆Go = ∆Ho - T∆So 0 = ∆Ho - T∆So T = ∆Ho ∆So T = (178. 2 k. J)/(160. 4 J/K)(10 -3 k. J/J) k. J)/( =1111 K or 838 o. C The reaction will be spontaneous in the forward direction at temperatures above 838 o. C.

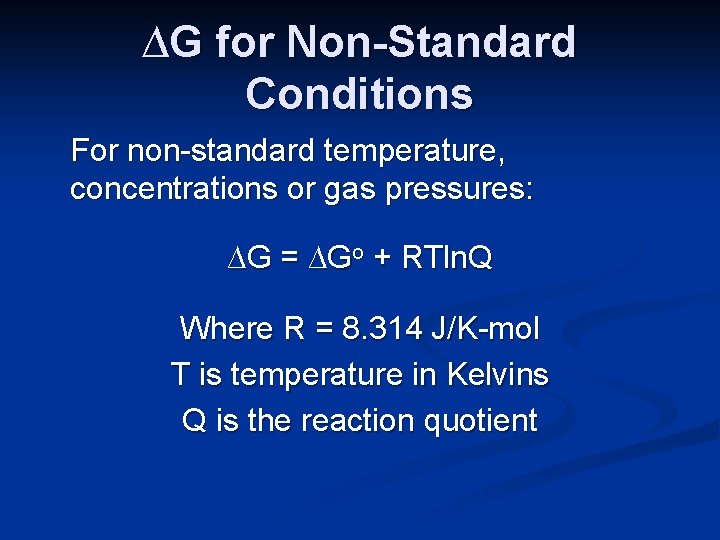
∆G for Non-Standard Conditions The thermodynamic tables are for standard conditions. This includes having all reactants and products present initially at a temperature of 25 o. C. All gases are at a pressure of 1 atm, and all solutions are 1 M.

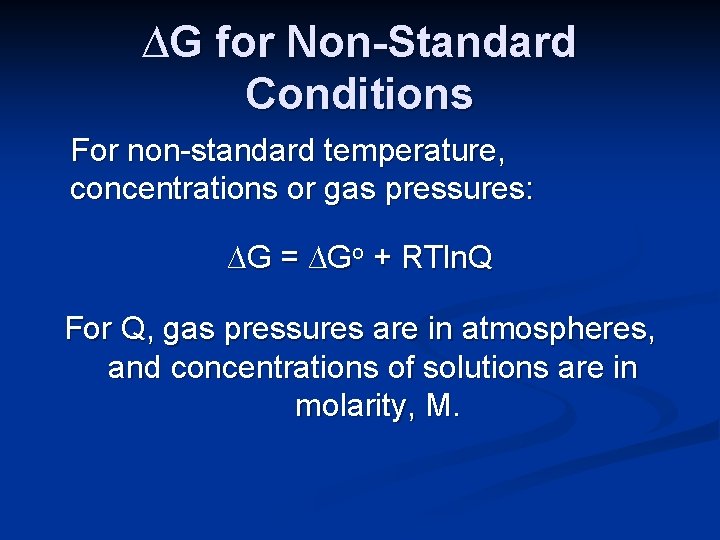
∆G for Non-Standard Conditions For non-standard temperature, concentrations or gas pressures: ∆G = ∆Go + RTln. Q Where R = 8. 314 J/K-mol T is temperature in Kelvins Q is the reaction quotient

∆G for Non-Standard Conditions For non-standard temperature, concentrations or gas pressures: ∆G = ∆Go + RTln. Q For Q, gas pressures are in atmospheres, and concentrations of solutions are in molarity, M.

∆Go and Equilibrium A large negative value of ∆Go indicates that the forward reaction or process is spontaneous. That is, there is a large driving force for the forward reaction. This also means that the equilibrium constant for the reaction will be large.

∆Go and Equilibrium A large positive value of ∆Go indicates that the reverse reaction or process is spontaneous. That is, there is a large driving force for the reverse reaction. This also means that the equilibrium constant for the reaction will be small. When a reaction or process is at equilibrium, ∆Go = zero.

∆Go and Equilibrium

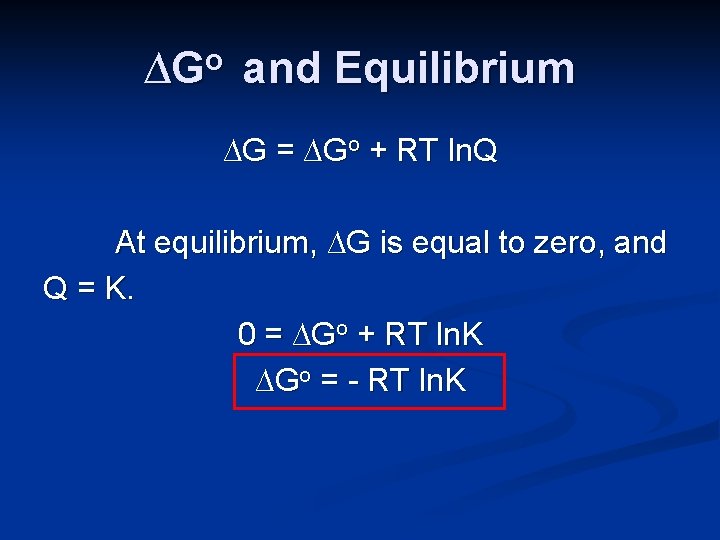
∆Go and Equilibrium ∆G = ∆Go + RT ln. Q At equilibrium, ∆G is equal to zero, and Q = K. 0 = ∆Go + RT ln. K ∆Go = - RT ln. K

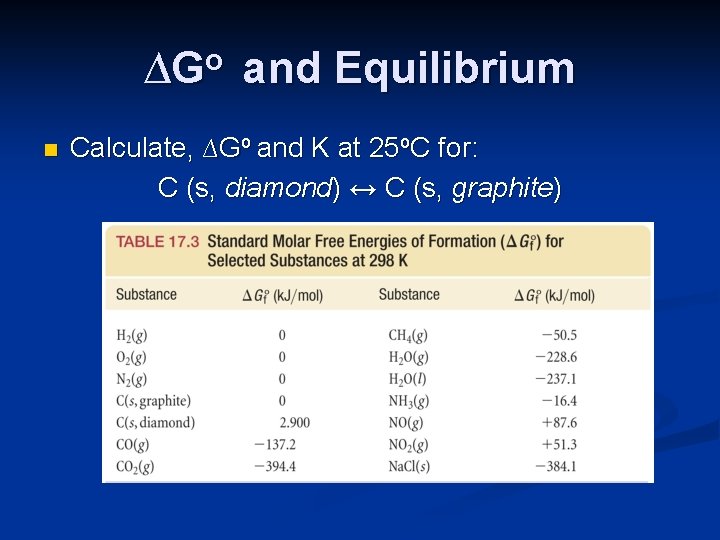
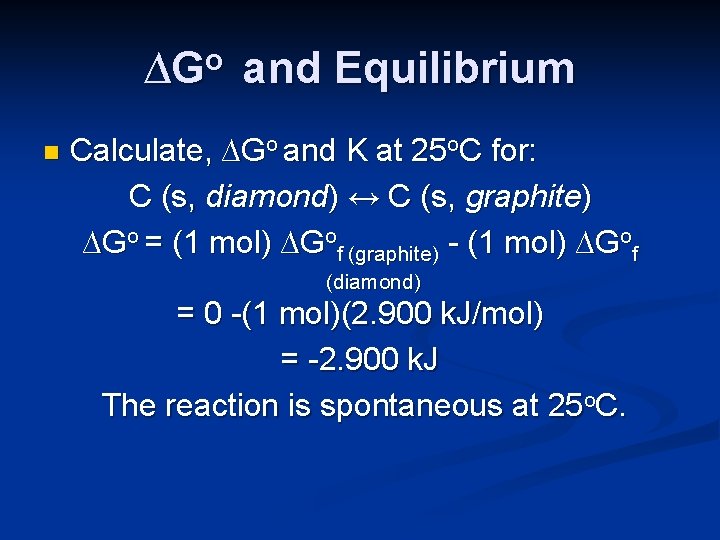
∆Go and Equilibrium n Calculate, ∆Go and K at 25 o. C for: C (s, diamond) ↔ C (s, graphite)

∆Go and Equilibrium n Calculate, ∆Go and K at 25 o. C for: C (s, diamond) ↔ C (s, graphite) ∆Go = (1 mol) ∆Gof (graphite) - (1 mol) ∆Gof (diamond) = 0 -(1 mol)(2. 900 k. J/mol) = -2. 900 k. J The reaction is spontaneous at 25 o. C.

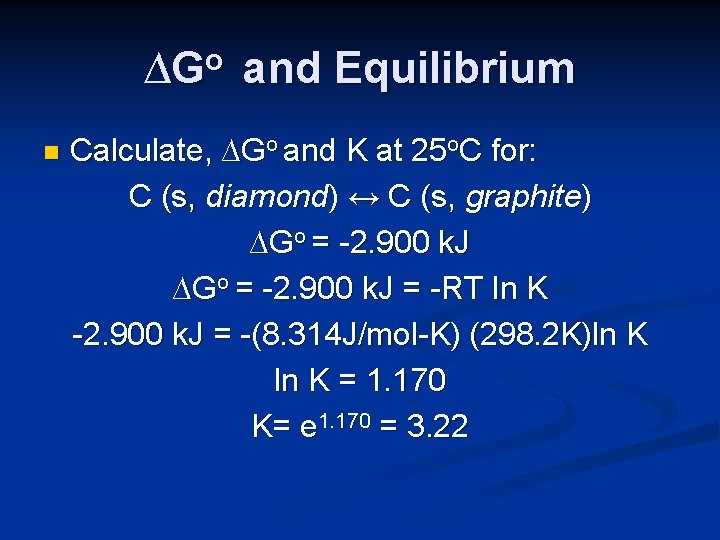
∆Go and Equilibrium n Calculate, ∆Go and K at 25 o. C for: C (s, diamond) ↔ C (s, graphite) ∆Go = -2. 900 k. J = -RT ln K -2. 900 k. J = -(8. 314 J/mol-K) (298. 2 K)ln K = 1. 170 K= e 1. 170 = 3. 22

C (s, diamond) ↔ C (s, graphite) The negative value of ∆Go and the equilibrium constant >1 suggest that diamonds can spontaneously react to form graphite. Although the reaction is thermodynamically favored, the rate constant is extremely small due to a huge activation energy. The disruption of the bonding in the diamond to form planar sp 2 hybridized carbon atoms is kinetically unfavorable.
 Ap chem spontaneity entropy and free energy
Ap chem spontaneity entropy and free energy Gibbs free energy and spontaneity
Gibbs free energy and spontaneity Free energy practice problems
Free energy practice problems Gibbs free energy non standard conditions
Gibbs free energy non standard conditions Relationship between entropy and free energy
Relationship between entropy and free energy Dg = dg + rtlnq
Dg = dg + rtlnq Helmholtz free energy and gibbs free energy
Helmholtz free energy and gibbs free energy Negative delta g
Negative delta g Enthalpy entropy free energy
Enthalpy entropy free energy Thermodynamics
Thermodynamics Entropy in thermodynamics
Entropy in thermodynamics Law of entropy
Law of entropy Unit of entropy
Unit of entropy Gibbs free energy biology
Gibbs free energy biology Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Predicting spontaneity
Predicting spontaneity Predicting spontaneity
Predicting spontaneity Half cell reaction
Half cell reaction Predicting spontaneity
Predicting spontaneity Predicting spontaneity
Predicting spontaneity Is the redox spontaneity rule empirical
Is the redox spontaneity rule empirical Entropy equation
Entropy equation Steady flow energy equation thermodynamics
Steady flow energy equation thermodynamics What is internal energy
What is internal energy Quasi static process
Quasi static process First law of energy conservation
First law of energy conservation Internal energy formula
Internal energy formula Heating energy formula
Heating energy formula Free body and soul free
Free body and soul free Optimistic poem
Optimistic poem Entropy and heat transfer
Entropy and heat transfer What is enthalpy and entropy
What is enthalpy and entropy Minimum enthalpy and maximum entropy
Minimum enthalpy and maximum entropy Entropy of system and surroundings
Entropy of system and surroundings Entropy order parameters and complexity
Entropy order parameters and complexity The allocation map
The allocation map Free free absorption
Free free absorption Phosphoanhydride bond
Phosphoanhydride bond Increase of entropy principle
Increase of entropy principle Nilai entropi propana
Nilai entropi propana Reversible process
Reversible process Kelvin planck statement
Kelvin planck statement Scalars and vectors
Scalars and vectors δhsys
δhsys Change of entropy formula
Change of entropy formula Yeytex
Yeytex Huffman coding visualization
Huffman coding visualization Entropy balance for closed system
Entropy balance for closed system Entropy change at constant volume formula
Entropy change at constant volume formula Physical science chapter 6 review answers
Physical science chapter 6 review answers Standard entropy change formula
Standard entropy change formula Refrigerator entropy
Refrigerator entropy Units of entropu
Units of entropu L
L G=h-ts
G=h-ts Entropy = q/t
Entropy = q/t Absolute entropy
Absolute entropy Entropy chain rule
Entropy chain rule Total entropy
Total entropy Entropy in information theory
Entropy in information theory Enthalpy vs entropy
Enthalpy vs entropy Entropy of ideal gas
Entropy of ideal gas Entropy balance equation
Entropy balance equation