AP Chapter 17 Spontaneity Entropy and Free Energy
































- Slides: 55

AP* Chapter 17 Spontaneity, Entropy, and Free Energy

AP Learning Objectives § LO 2. 15 The student is able to explain observations regarding the solubility of ionic solids and molecules in water and other solvents on the basis of particle views that include intermolecular interactions and entropic effects. (Sec 17. 1) § LO 5. 3 The student can generate explanations or make predictions about the transfer of thermal energy between systems based on this transfer being due to a kinetic energy transfer between systems arising from molecular collisions. (Sec 17. 3) § LO 5. 12 The student is able to use representations and models to predict the sign and relative magnitude of the entropy change associated with chemical or physical processes. (Sec 17. 1 -17. 3, 17. 5) § LO 5. 13 The student is able to predict whether or not a physical or chemical process is thermodynamically favored by determination of (either quantitatively or qualitatively) the signs of both �Ho and �So, and calculation or estimation of �Go when needed. (Sec 17. 4, 17. 6) § LO 5. 14 The student is able to determine whether a chemical or physical process is thermodynamically favorable by calculating the change in standard Gibbs free energy. (Sec 17. 4, 17. 6)

AP Learning Objectives § LO 5. 15 The student is able to explain how the application of external energy sources or the coupling of favorable with unfavorable reactions can be used to cause processes that are not thermodynamically favorable to become favorable. (Sec 17. 6, 17. 9) § LO 5. 16 The student can use Le Châtelier’s principle to make qualitative predictions for systems in which coupled reactions that share a common intermediate drive formation of a product. (Sec 17. 6) § LO 5. 17 The student can make quantitative predictions for systems involving coupled reactions that share a common intermediate, based on the equilibrium constant for the combined reaction. (Sec 17. 6) § LO 5. 18 The student can explain why a thermodynamically favored chemical reaction may not produce large amounts of product (based on consideration of both initial conditions and kinetic effects), or why a thermodynamically unfavored chemical reaction can produce large amounts of product for certain sets of initial conditions. (Sec 17. 1, 17. 6 -17. 8)

AP Learning Objectives § LO 6. 25 The student is able to express the equilibrium constant in terms of �Go and RT and use this relationship to estimate the magnitude of K and, consequently, thermodynamic favorability of the process. (Sec 17. 8)

Section 17. 1 Spontaneous Processes and Entropy AP Learning Objectives, Margin Notes and References § Learning Objectives § § § LO 2. 15 The student is able to explain observations regarding the solubility of ionic solids and molecules in water and other solvents on the basis of particle views that include intermolecular interactions and entropic effects. LO 5. 12 The student is able to use representations and models to predict the sign and relative magnitude of the entropy change associated with chemical or physical processes. LO 5. 18 The student can explain why a thermodynamically favored chemical reaction may not produce large amounts of product (based on consideration of both initial conditions and kinetic effects), or why a thermodynamically unfavored chemical reaction can produce large amounts of product for certain sets of initial conditions. § Additional AP References § LO 5. 12 (see Appendix 7. 11, “Non-Spontaneous Reactions”)

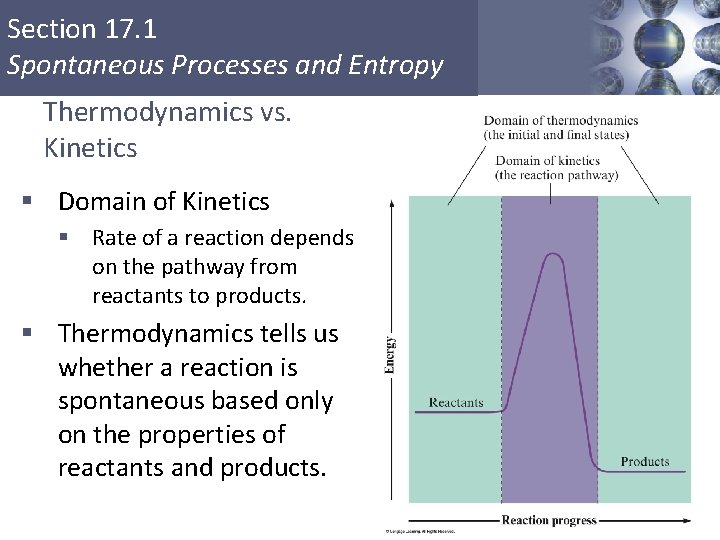
Section 17. 1 Spontaneous Processes and Entropy Thermodynamics vs. Kinetics § Domain of Kinetics § Rate of a reaction depends on the pathway from reactants to products. § Thermodynamics tells us whether a reaction is spontaneous based only on the properties of reactants and products. Copyright © Cengage Learning. All rights reserved 6

Section 17. 1 Spontaneous Processes and Entropy § Thermodynamics lets us predict the direction in which a process will occur but gives no information about the speed of the process. § A spontaneous process is one that occurs without outside intervention. Copyright © Cengage Learning. All rights reserved 7

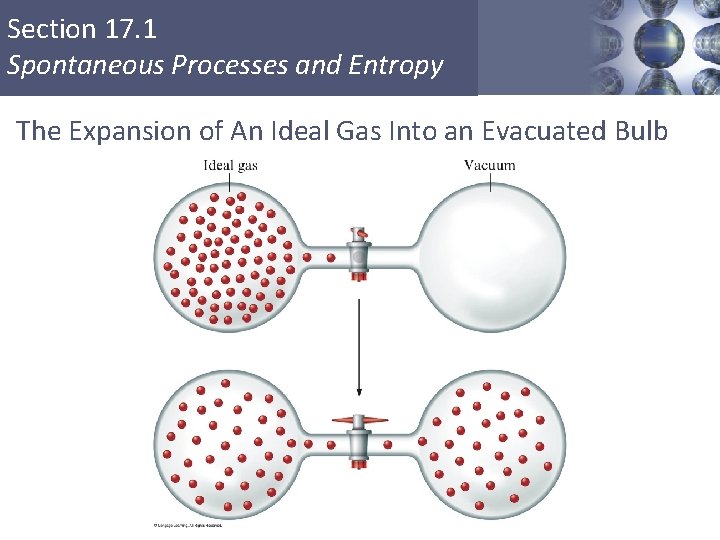
Section 17. 1 Spontaneous Processes and Entropy CONCEPT CHECK! Consider 2. 4 moles of a gas contained in a 4. 0 L bulb at a constant temperature of 32°C. This bulb is connected by a valve to an evacuated 20. 0 L bulb. Assume the temperature is constant. a) What should happen to the gas when you open the valve? Copyright © Cengage Learning. All rights reserved 8

Section 17. 1 Spontaneous Processes and Entropy CONCEPT CHECK! Consider 2. 4 moles of a gas contained in a 4. 0 L bulb at a constant temperature of 32°C. This bulb is connected by a valve to an evacuated 20. 0 L bulb. Assume the temperature is constant. b) Calculate ΔH, ΔE, q, and w for the process you described above. All are equal to zero. Copyright © Cengage Learning. All rights reserved 9

Section 17. 1 Spontaneous Processes and Entropy CONCEPT CHECK! Consider 2. 4 moles of a gas contained in a 4. 0 L bulb at a constant temperature of 32°C. This bulb is connected by a valve to an evacuated 20. 0 L bulb. Assume the temperature is constant. c) Given your answer to part b, what is the driving force for the process? Entropy Copyright © Cengage Learning. All rights reserved 10

Section 17. 1 Spontaneous Processes and Entropy The Expansion of An Ideal Gas Into an Evacuated Bulb Copyright © Cengage Learning. All rights reserved 11

Section 17. 1 Spontaneous Processes and Entropy § The driving force for a spontaneous process is an increase in the entropy of the universe. § A measure of molecular randomness or disorder. Copyright © Cengage Learning. All rights reserved 12

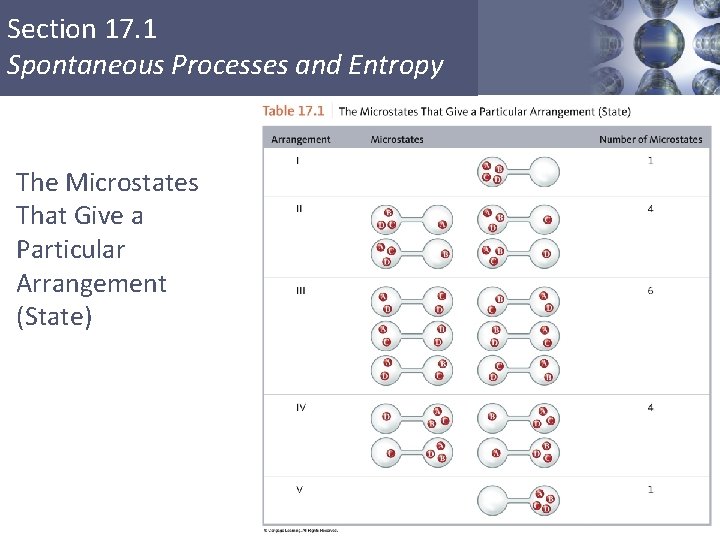
Section 17. 1 Spontaneous Processes and Entropy § Thermodynamic function that describes the number of arrangements that are available to a system existing in a given state. § Nature spontaneously proceeds toward the states that have the highest probabilities of existing. Copyright © Cengage Learning. All rights reserved 13

Section 17. 1 Spontaneous Processes and Entropy The Microstates That Give a Particular Arrangement (State)

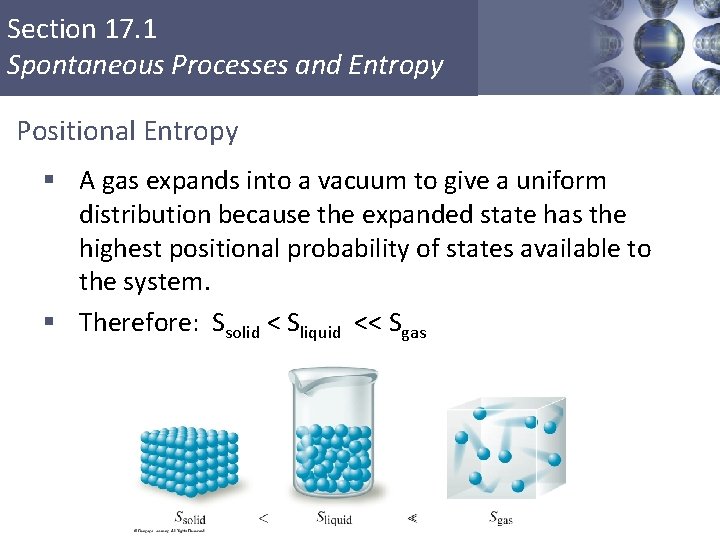
Section 17. 1 Spontaneous Processes and Entropy Positional Entropy § A gas expands into a vacuum to give a uniform distribution because the expanded state has the highest positional probability of states available to the system. § Therefore: Ssolid < Sliquid << Sgas

Section 17. 1 Spontaneous Processes and Entropy CONCEPT CHECK! Predict the sign of ΔS for each of the following, and explain: + a) The evaporation of alcohol – b) The freezing of water – c) Compressing an ideal gas at constant temperature + d) Heating an ideal gas at constant pressure + e) Dissolving Na. Cl in water Copyright © Cengage Learning. All rights reserved 16

Section 17. 2 Entropy and the Second Law of Thermodynamics AP Learning Objectives, Margin Notes and References § Learning Objectives § LO 5. 12 The student is able to use representations and models to predict the sign and relative magnitude of the entropy change associated with chemical or physical processes. § Additional AP References § LO 5. 12 (see Appendix 7. 11, “Non-Spontaneous Reactions”)

Section 17. 2 Entropy and the Second Law of Thermodynamics § In any spontaneous process there is always an increase in the entropy of the universe. § The entropy of the universe is increasing. § The total energy of the universe is constant, but the entropy is increasing. Suniverse = ΔSsystem + ΔSsurroundings Copyright © Cengage Learning. All rights reserved 18

Section 17. 2 Entropy and the Second Law of Thermodynamics ΔSsurr § ΔSsurr = +; entropy of the universe increases § ΔSsurr = -; process is spontaneous in opposite direction § ΔSsurr = 0; process has no tendency to occur Copyright © Cengage Learning. All rights reserved 19

Section 17. 3 The Effect of Temperature on Spontaneity AP Learning Objectives, Margin Notes and References § Learning Objectives § § LO 5. 3 The student can generate explanations or make predictions about the transfer of thermal energy between systems based on this transfer being due to a kinetic energy transfer between systems arising from molecular collisions. LO 5. 12 The student is able to use representations and models to predict the sign and relative magnitude of the entropy change associated with chemical or physical processes. § Additional AP References § § LO 5. 3 (see Appendix 7. 2, “Thermal Equilibrium, the Kinetic Molecular Theory, and the Process of Heat”) LO 5. 12 (see Appendix 7. 11, “Non-Spontaneous Reactions”)

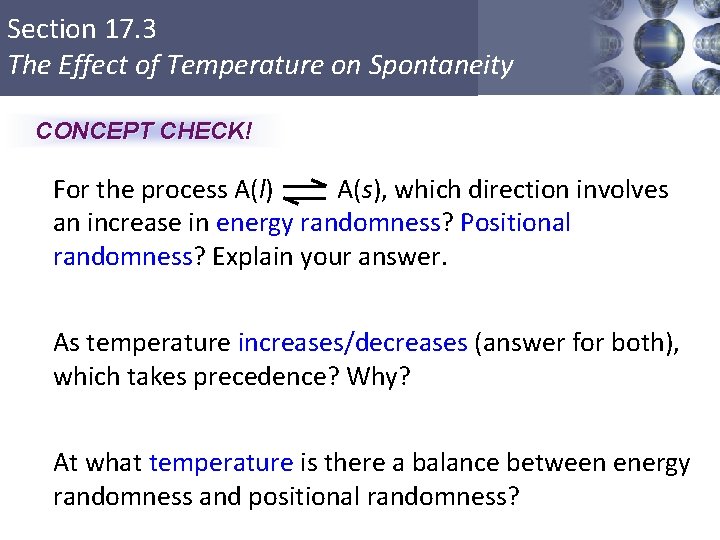
Section 17. 3 The Effect of Temperature on Spontaneity CONCEPT CHECK! For the process A(l) A(s), which direction involves an increase in energy randomness? Positional randomness? Explain your answer. As temperature increases/decreases (answer for both), which takes precedence? Why? At what temperature is there a balance between energy randomness and positional randomness? Copyright © Cengage Learning. All rights reserved 21

Section 17. 3 The Effect of Temperature on Spontaneity CONCEPT CHECK! Describe the following as spontaneous/non-spontaneous/cannot tell, and explain. A reaction that is: a) Exothermic and becomes more positionally random Spontaneous b) Exothermic and becomes less positionally random Cannot tell a) Endothermic and becomes more positionally random Cannot tell a) Endothermic and becomes less positionally random Not spontaneous Explain how temperature affects your answers.

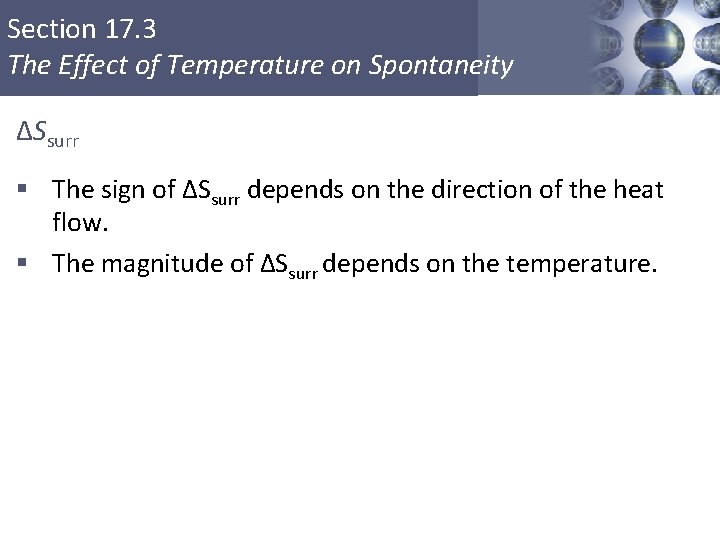
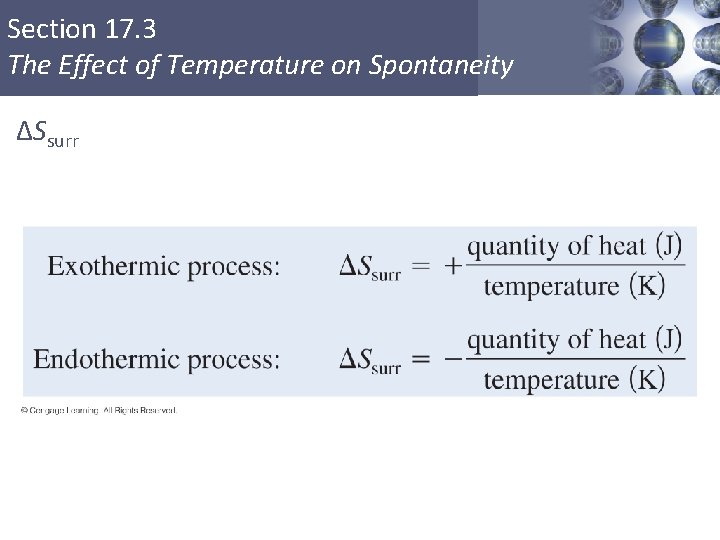
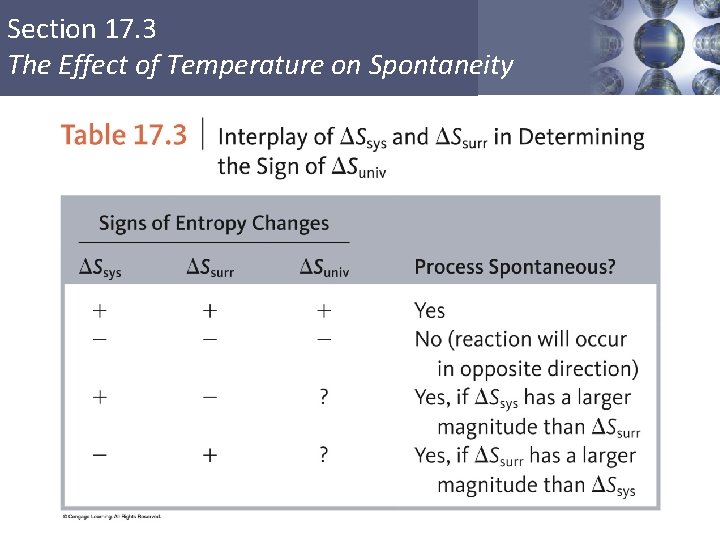
Section 17. 3 The Effect of Temperature on Spontaneity ΔSsurr § The sign of ΔSsurr depends on the direction of the heat flow. § The magnitude of ΔSsurr depends on the temperature. Copyright © Cengage Learning. All rights reserved 23

Section 17. 3 The Effect of Temperature on Spontaneity ΔSsurr Copyright © Cengage Learning. All rights reserved 24

Section 17. 3 The Effect of Temperature on Spontaneity ΔSsurr Copyright © Cengage Learning. All rights reserved 25

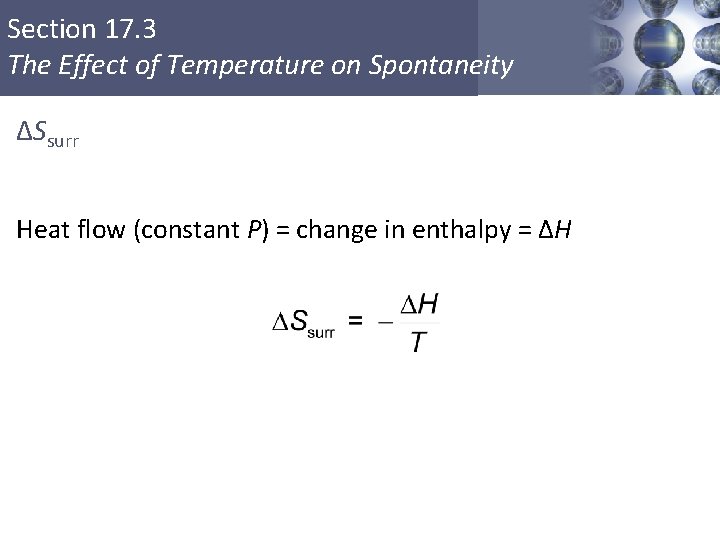
Section 17. 3 The Effect of Temperature on Spontaneity ΔSsurr Heat flow (constant P) = change in enthalpy = ΔH Copyright © Cengage Learning. All rights reserved 26

Section 17. 3 The Effect of Temperature on Spontaneity Copyright © Cengage Learning. All rights reserved 27

Section 17. 4 Free Energy AP Learning Objectives, Margin Notes and References § Learning Objectives § § LO 5. 13 The student is able to predict whether or not a physical or chemical process is thermodynamically favored by determination of (either quantitatively or qualitatively) the signs of both �Ho and �So, and calculation or estimation of �Go when needed. LO 5. 14 The student is able to determine whether a chemical or physical process is thermodynamically favorable by calculating the change in standard Gibbs free energy.

Section 17. 4 Free Energy (G) § A process (at constant T and P) is spontaneous in the direction in which the free energy decreases. § Negative ΔG means positive ΔSuniv. Copyright © Cengage Learning. All rights reserved 29

Section 17. 4 Free Energy (G) § ΔG = ΔH – TΔS (at constant T and P) Copyright © Cengage Learning. All rights reserved 30

Section 17. 4 Free Energy CONCEPT CHECK! A liquid is vaporized at its boiling point. Predict the signs of: – w + q + ΔH + ΔS – ΔSsurr 0 ΔG Explain your answers. Copyright © Cengage Learning. All rights reserved 31

Section 17. 4 Free Energy EXERCISE! The value of ΔHvaporization of substance X is 45. 7 k. J/mol, and its normal boiling point is 72. 5°C. Calculate ΔS, ΔSsurr, and ΔG for the vaporization of one mole of this substance at 72. 5°C and 1 atm. ΔS = 132 J/K·mol ΔSsurr = -132 J/K·mol ΔG = 0 k. J/mol Copyright © Cengage Learning. All rights reserved 32

Section 17. 4 Free Energy Spontaneous Reactions To play movie you must be in Slide Show Mode PC Users: Please wait for content to load, then click to play Mac Users: CLICK HERE Copyright © Cengage Learning. All rights reserved 33

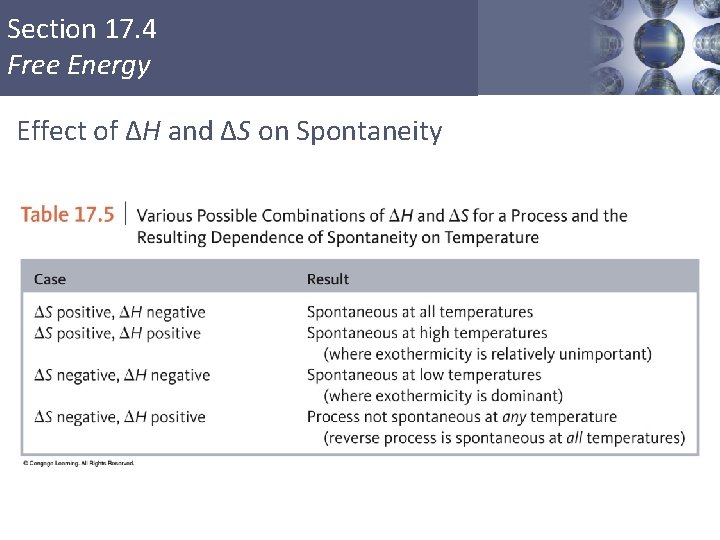
Section 17. 4 Free Energy Effect of ΔH and ΔS on Spontaneity Copyright © Cengage Learning. All rights reserved 34

Section 17. 5 Entropy Changes in Chemical Reactions AP Learning Objectives, Margin Notes and References § Learning Objectives § LO 5. 12 The student is able to use representations and models to predict the sign and relative magnitude of the entropy change associated with chemical or physical processes.

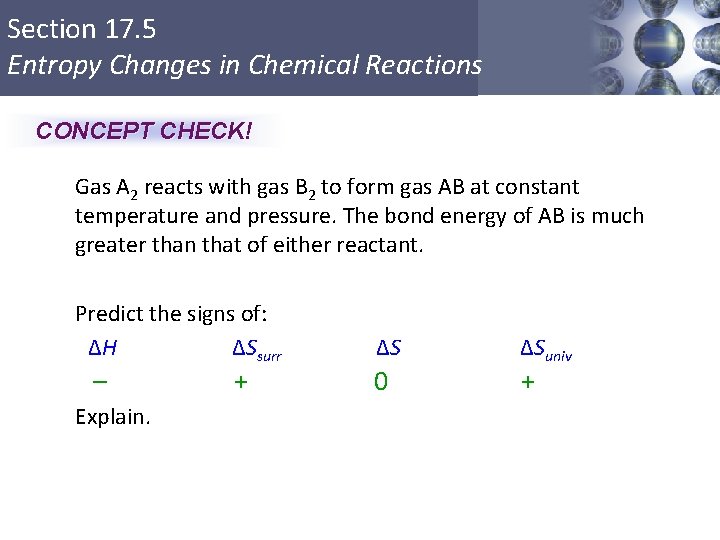
Section 17. 5 Entropy Changes in Chemical Reactions CONCEPT CHECK! Gas A 2 reacts with gas B 2 to form gas AB at constant temperature and pressure. The bond energy of AB is much greater than that of either reactant. Predict the signs of: ΔH ΔSsurr – + ΔS 0 ΔSuniv + Explain. Copyright © Cengage Learning. All rights reserved 36

Section 17. 5 Entropy Changes in Chemical Reactions Third Law of Thermodynamics § The entropy of a perfect crystal at 0 K is zero. § The entropy of a substance increases with temperature. Copyright © Cengage Learning. All rights reserved 37

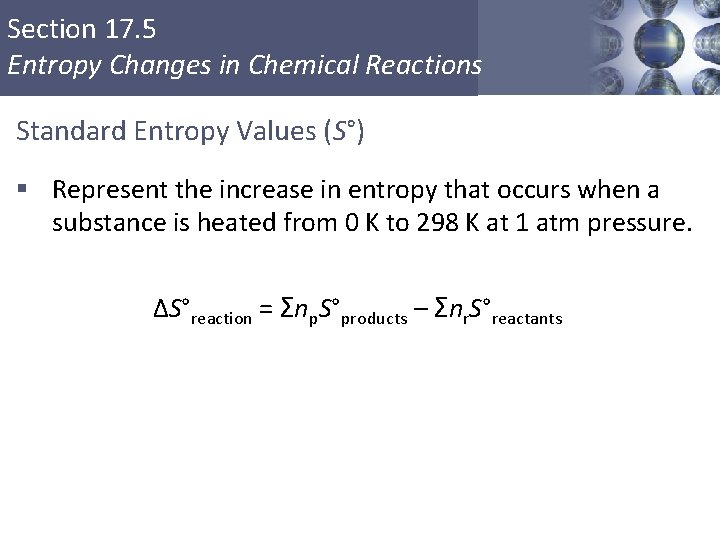
Section 17. 5 Entropy Changes in Chemical Reactions Standard Entropy Values (S°) § Represent the increase in entropy that occurs when a substance is heated from 0 K to 298 K at 1 atm pressure. ΔS°reaction = Σnp. S°products – Σnr. S°reactants Copyright © Cengage Learning. All rights reserved 38

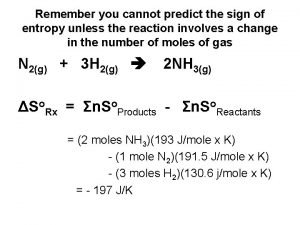
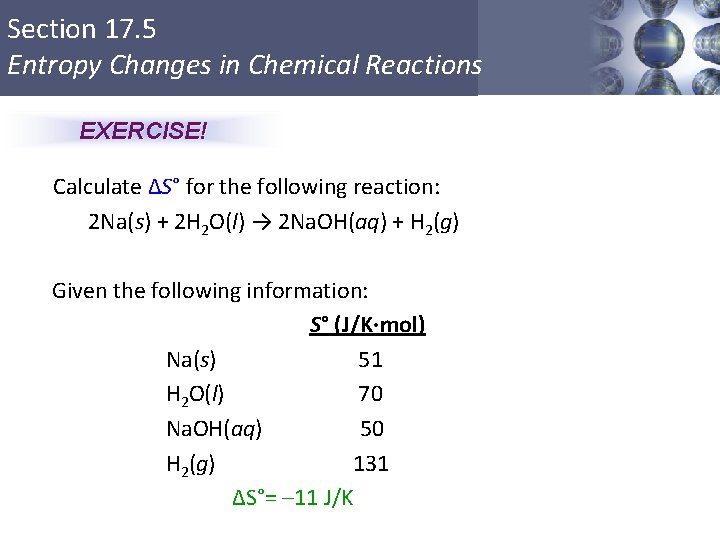
Section 17. 5 Entropy Changes in Chemical Reactions EXERCISE! Calculate ΔS° for the following reaction: 2 Na(s) + 2 H 2 O(l) → 2 Na. OH(aq) + H 2(g) Given the following information: S° (J/K·mol) Na(s) 51 H 2 O(l) 70 Na. OH(aq) 50 H 2(g) 131 ΔS°= – 11 J/K Copyright © Cengage Learning. All rights reserved 39

Section 17. 6 Free Energy and Chemical Reactions AP Learning Objectives, Margin Notes and References § Learning Objectives § § § LO 5. 13 The student is able to predict whether or not a physical or chemical process is thermodynamically favored by determination of (either quantitatively or qualitatively) the signs of both �Ho and �So, and calculation or estimation of �Go when needed. LO 5. 14 The student is able to determine whether a chemical or physical process is thermodynamically favorable by calculating the change in standard Gibbs free energy. LO 5. 15 The student is able to explain how the application of external energy sources or the coupling of favorable with unfavorable reactions can be used to cause processes that are not thermodynamically favorable to become favorable. LO 5. 16 The student can use Le Châtelier’s principle to make qualitative predictions for systems in which coupled reactions that share a common intermediate drive formation of a product. LO 5. 17 The student can make quantitative predictions for systems involving coupled reactions that share a common intermediate, based on the equilibrium constant for the combined reaction. LO 5. 18 The student can explain why a thermodynamically favored chemical reaction may not produce large amounts of product (based on consideration of both initial conditions and kinetic effects), or why a thermodynamically unfavored chemical reaction can produce large amounts of product for certain sets of initial conditions.

Section 17. 6 Free Energy and Chemical Reactions AP Learning Objectives, Margin Notes and References § Additional AP References § § LO 5. 15 (see Appendix 7. 11, “Non-Spontaneous Reactions”) LO 5. 16 (see Appendix 7. 11, “Non-Spontaneous Reactions”) LO 5. 17 (see Appendix 7. 11, “Non-Spontaneous Reactions”) LO 5. 18 (see Appendix 7. 11, “Non-Spontaneous Reactions”)

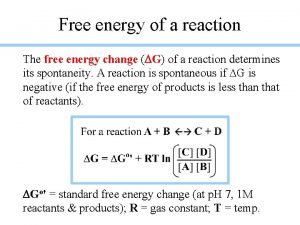
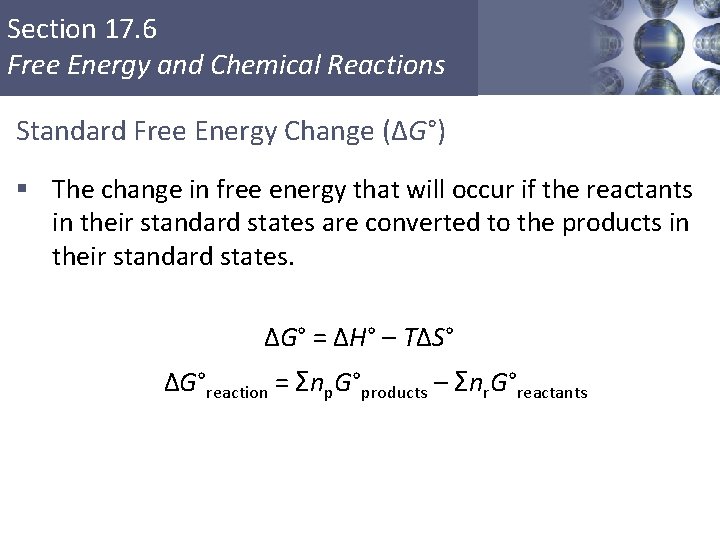
Section 17. 6 Free Energy and Chemical Reactions Standard Free Energy Change (ΔG°) § The change in free energy that will occur if the reactants in their standard states are converted to the products in their standard states. ΔG° = ΔH° – TΔS° ΔG°reaction = Σnp. G°products – Σnr. G°reactants Copyright © Cengage Learning. All rights reserved 42

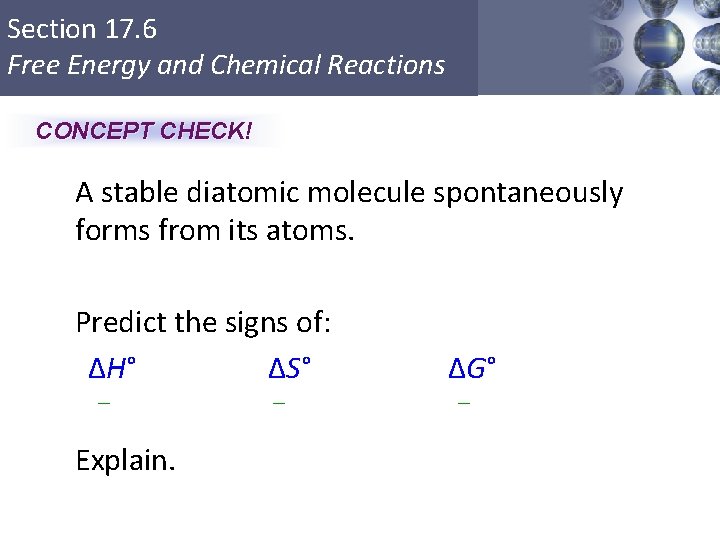
Section 17. 6 Free Energy and Chemical Reactions CONCEPT CHECK! A stable diatomic molecule spontaneously forms from its atoms. Predict the signs of: ΔH° ΔS° – – ΔG° – Explain. Copyright © Cengage Learning. All rights reserved 43

Section 17. 6 Free Energy and Chemical Reactions CONCEPT CHECK! Consider the following system at equilibrium at 25°C. PCl 3(g) + Cl 2(g) PCl 5(g) ΔG° = − 92. 50 k. J What will happen to the ratio of partial pressure of PCl 5 to partial pressure of PCl 3 if the temperature is raised? Explain. The ratio will decrease. Copyright © Cengage Learning. All rights reserved 44

Section 17. 7 The Dependence of Free Energy on Pressure AP Learning Objectives, Margin Notes and References § Learning Objectives § LO 5. 18 The student can explain why a thermodynamically favored chemical reaction may not produce large amounts of product (based on consideration of both initial conditions and kinetic effects), or why a thermodynamically unfavored chemical reaction can produce large amounts of product for certain sets of initial conditions. § Additional AP References § LO 5. 18 (see Appendix 7. 11, “Non-Spontaneous Reactions”)

Section 17. 7 The Dependence of Free Energy on Pressure Free Energy and Pressure G = G° + RT ln(P) or ΔG = ΔG° + RT ln(Q) Copyright © Cengage Learning. All rights reserved 46

Section 17. 7 The Dependence of Free Energy on Pressure CONCEPT CHECK! Sketch graphs of: 1. G vs. P 2. H vs. P 3. ln(K) vs. 1/T (for both endothermic and exothermic cases) Copyright © Cengage Learning. All rights reserved 47

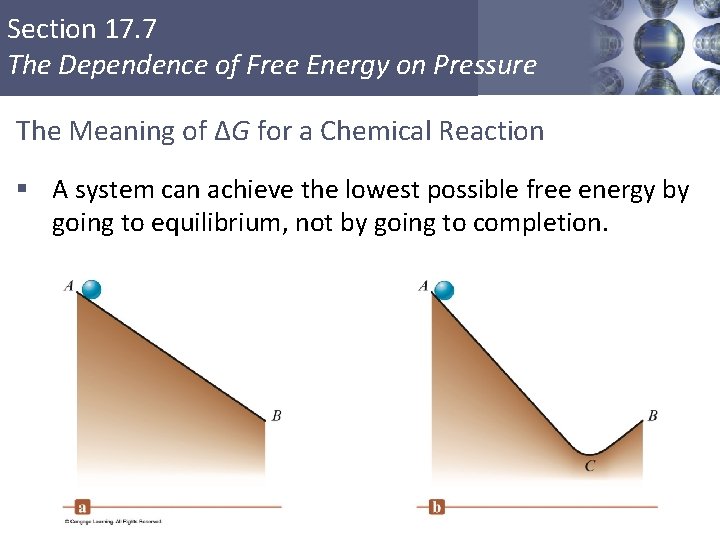
Section 17. 7 The Dependence of Free Energy on Pressure The Meaning of ΔG for a Chemical Reaction § A system can achieve the lowest possible free energy by going to equilibrium, not by going to completion. Copyright © Cengage Learning. All rights reserved 48

Section 17. 8 Free Energy and Equilibrium AP Learning Objectives, Margin Notes and References § Learning Objectives § § LO 5. 18 The student can explain why a thermodynamically favored chemical reaction may not produce large amounts of product (based on consideration of both initial conditions and kinetic effects), or why a thermodynamically unfavored chemical reaction can produce large amounts of product for certain sets of initial conditions. LO 6. 25 The student is able to express the equilibrium constant in terms of �Go and RT and use this relationship to estimate the magnitude of K and, consequently, thermodynamic favorability of the process. § Additional AP References § § LO 5. 18 (see Appendix 7. 11, “Non-Spontaneous Reactions”) LO 6. 25 (see Appendix 7. 11, “Non-Spontaneous Reactions”)

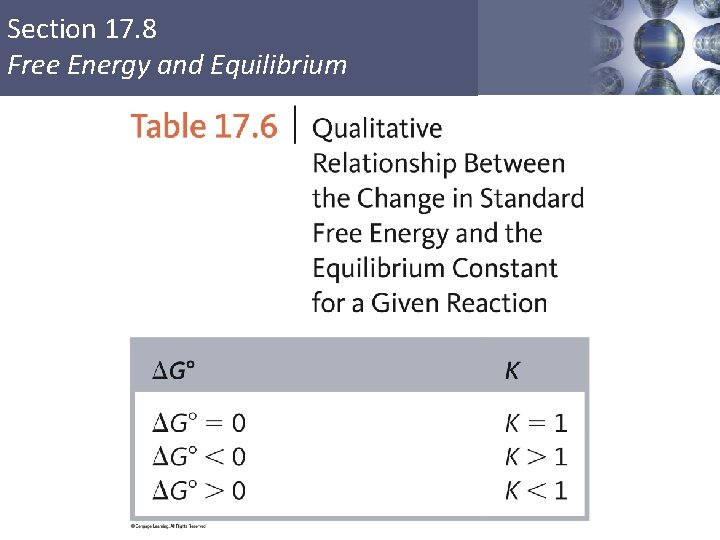
Section 17. 8 Free Energy and Equilibrium § The equilibrium point occurs at the lowest value of free energy available to the reaction system. ΔG = 0 = ΔG° + RT ln(K) ΔG° = –RT ln(K) Copyright © Cengage Learning. All rights reserved 50

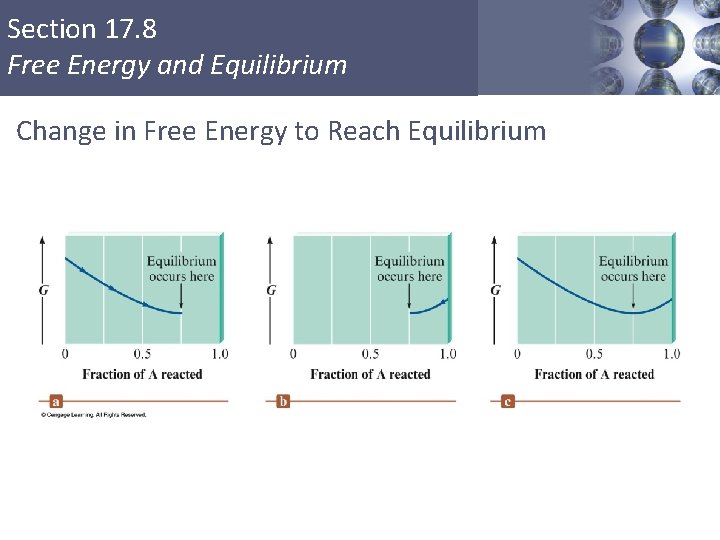
Section 17. 8 Free Energy and Equilibrium Change in Free Energy to Reach Equilibrium Copyright © Cengage Learning. All rights reserved 51

Section 17. 8 Free Energy and Equilibrium Copyright © Cengage Learning. All rights reserved 52

Section 17. 9 Free Energy and Work AP Learning Objectives, Margin Notes and References § Learning Objectives § LO 5. 15 The student is able to explain how the application of external energy sources or the coupling of favorable with unfavorable reactions can be used to cause processes that are not thermodynamically favorable to become favorable. § Additional AP References § LO 5. 15 (see Appendix 7. 11, “Non-Spontaneous Reactions”)

Section 17. 9 Free Energy and Work § Maximum possible useful work obtainable from a process at constant temperature and pressure is equal to the change in free energy. wmax = ΔG Copyright © Cengage Learning. All rights reserved 54

Section 17. 9 Free Energy and Work § Achieving the maximum work available from a spontaneous process can occur only via a hypothetical pathway. Any real pathway wastes energy. § All real processes are irreversible. § First law: You can’t win, you can only break even. § Second law: You can’t break even. § As we use energy, we degrade its usefulness. Copyright © Cengage Learning. All rights reserved 55
 Ap chemistry spontaneity entropy and free energy
Ap chemistry spontaneity entropy and free energy Spontaneous reaction
Spontaneous reaction Gibbs free energy non standard conditions
Gibbs free energy non standard conditions Gibbs free energy non standard conditions
Gibbs free energy non standard conditions Enthalpy vs entropy
Enthalpy vs entropy Dg = dg + rtlnq
Dg = dg + rtlnq Helmholtz free energy
Helmholtz free energy Negative delta g
Negative delta g Enthalpy entropy free energy
Enthalpy entropy free energy Gibbs free energy spontaneous
Gibbs free energy spontaneous G=g+rt ln(q)
G=g+rt ln(q) Thermodynamics ppt
Thermodynamics ppt Predicting spontaneity
Predicting spontaneity Concentration cell
Concentration cell Predicting spontaneity
Predicting spontaneity Predicting spontaneity
Predicting spontaneity Is the redox spontaneity rule empirical
Is the redox spontaneity rule empirical Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis The story of an hour summary
The story of an hour summary Free hearts free foreheads you and i are
Free hearts free foreheads you and i are Q system = -q surroundings
Q system = -q surroundings What is enthalpy and entropy
What is enthalpy and entropy Entropy vs enthalpy
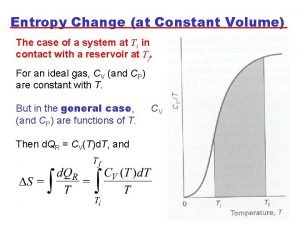
Entropy vs enthalpy Entropy change formula
Entropy change formula Entropy order parameters and complexity
Entropy order parameters and complexity Chapter 7 energy conservation of energy
Chapter 7 energy conservation of energy Allocating kernel memory in os
Allocating kernel memory in os Free-free absorption
Free-free absorption Phosphoanhydride
Phosphoanhydride Entropy equation temperature
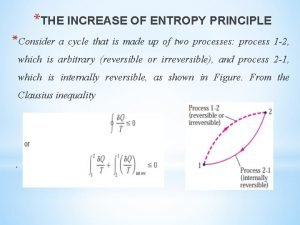
Entropy equation temperature Increase of entropy principle
Increase of entropy principle Energi bebas gibbs
Energi bebas gibbs Non spontaneous process
Non spontaneous process Kelvin statement
Kelvin statement Scalars and vectors
Scalars and vectors δhsys
δhsys Thermodynamics
Thermodynamics Thermodynamic temperature
Thermodynamic temperature Entropy in bits
Entropy in bits Huffman code entropy
Huffman code entropy Entropy rate balance equation
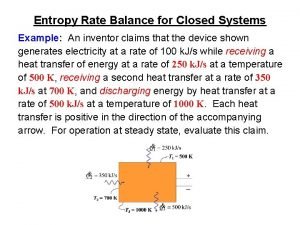
Entropy rate balance equation Total entropy
Total entropy Constant entropy
Constant entropy Physical science chapter 6 review answers
Physical science chapter 6 review answers Entropy change of surroundings formula
Entropy change of surroundings formula Refrigerator entropy
Refrigerator entropy Entropy change formula
Entropy change formula Entropy simple definition
Entropy simple definition Entropy vs enthalpy
Entropy vs enthalpy Entropy = q/t
Entropy = q/t Absolute entropy
Absolute entropy Thermodynamic second law
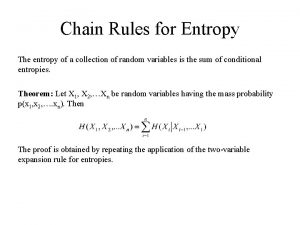
Thermodynamic second law Chain rule for entropy
Chain rule for entropy Total entropy
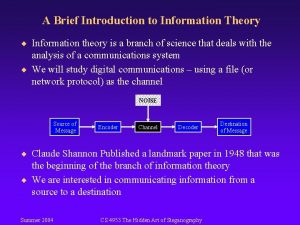
Total entropy Entropy in information theory
Entropy in information theory