CHAPTER20 Entropy and Second Law of Thermodynamics CHAPTER20




- Slides: 13

CHAPTER-20 Entropy and Second Law of Thermodynamics

CHAPTER-20 Entropy and Second Law of Thermodynamics 1. 2. 3. 4. 5. 6. Topics to be covered: Reversible processes Entropy The Carnot engine Refrigerators Real engines

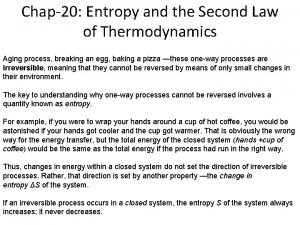
Ch 20 -2 Irreversible Process and Entropy q q q Irreversible Process: One way process If the irreversible process proceed in a closed system in a reverse way, we will wonder about it. A melted piece of ice freezes by itself. Although from energy conservation, the process can proceed in the reverse path i. e. heat released in in the environment in melting ice can be recovered back into ice piece to solidify it. Energy conservation does not prohibit the process to proceed one way or the reverse way. Which parameter the one ay direction of the process? . Entropy set the direction of irreversible process and irreversible process proceed through that path in which entropy of the system always increases Entropy Postulate: If an irreversible process occurs in a closed system, the entropy S of the system always increases

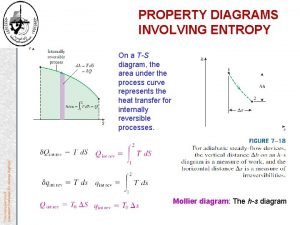
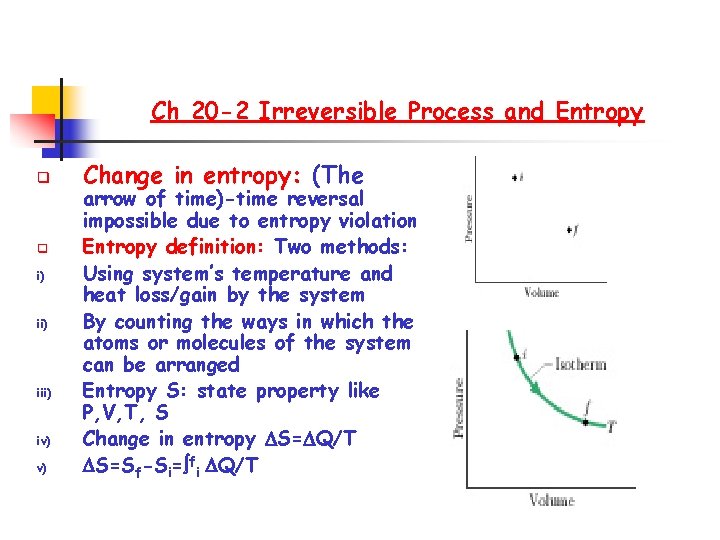
Ch 20 -2 Irreversible Process and Entropy q q i) iii) iv) v) Change in entropy: (The arrow of time)-time reversal impossible due to entropy violation Entropy definition: Two methods: Using system’s temperature and heat loss/gain by the system By counting the ways in which the atoms or molecules of the system can be arranged Entropy S: state property like P, V, T, S Change in entropy S= Q/T S=Sf-Si= fi Q/T

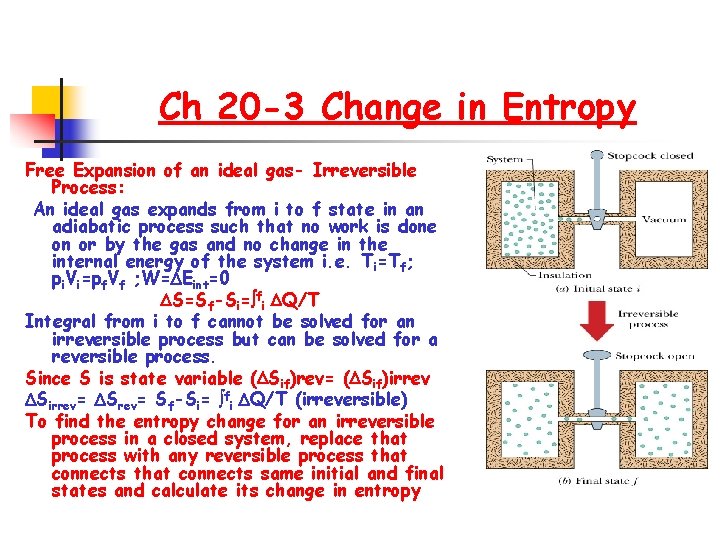
Ch 20 -3 Change in Entropy Free Expansion of an ideal gas- Irreversible Process: An ideal gas expands from i to f state in an adiabatic process such that no work is done on or by the gas and no change in the internal energy of the system i. e. Ti=Tf; pi. Vi=pf. Vf ; W= Eint=0 S=Sf-Si= fi Q/T Integral from i to f cannot be solved for an irreversible process but can be solved for a reversible process. Since S is state variable ( Sif)rev= ( Sif)irrev Sirrev= Sf-Si= fi Q/T (irreversible) To find the entropy change for an irreversible process in a closed system, replace that process with any reversible process that connects same initial and final states and calculate its change in entropy

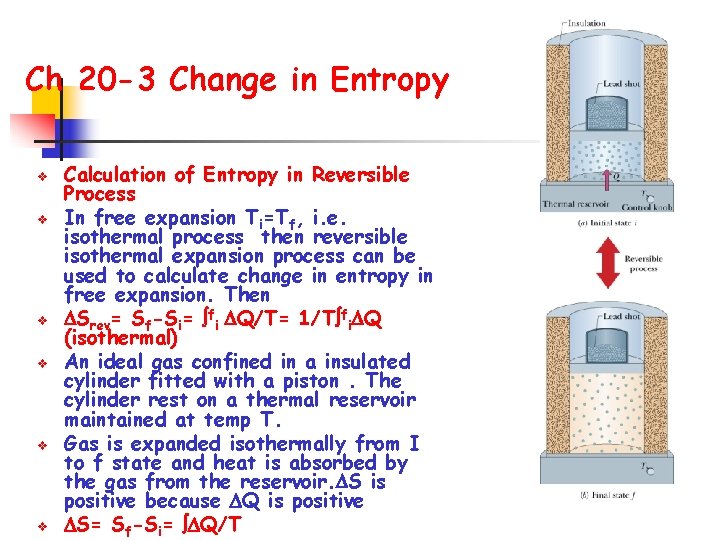
Ch 20 -3 Change in Entropy v v v Calculation of Entropy in Reversible Process In free expansion Ti=Tf, i. e. isothermal process then reversible isothermal expansion process can be used to calculate change in entropy in free expansion. Then Srev= Sf-Si= fi Q/T= 1/T fi Q (isothermal) An ideal gas confined in a insulated cylinder fitted with a piston. The cylinder rest on a thermal reservoir maintained at temp T. Gas is expanded isothermally from I to f state and heat is absorbed by the gas from the reservoir. S is positive because Q is positive S= Sf-Si= Q/T

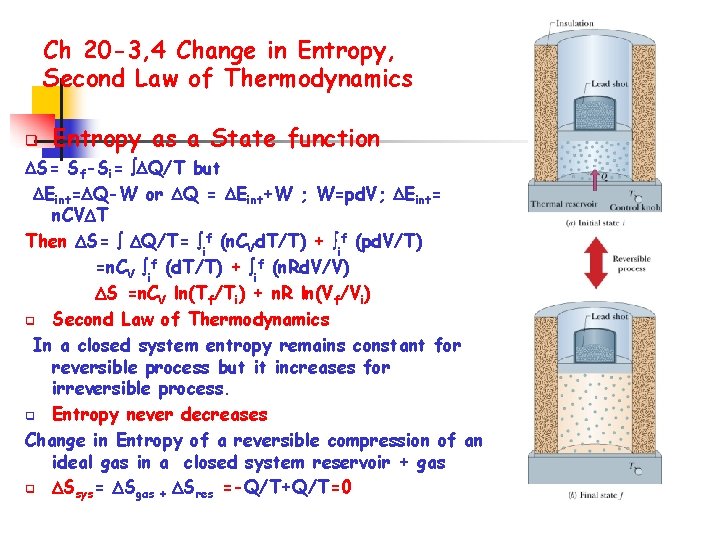
Ch 20 -3, 4 Change in Entropy, Second Law of Thermodynamics q Entropy as a State function S= Sf-Si= Q/T but Eint= Q-W or Q = Eint+W ; W=pd. V; Eint= n. CV T Then S= Q/T= if (n. CVd. T/T) + if (pd. V/T) =n. CV if (d. T/T) + if (n. Rd. V/V) S =n. CV ln(Tf/Ti) + n. R ln(Vf/Vi) q Second Law of Thermodynamics In a closed system entropy remains constant for reversible process but it increases for irreversible process. q Entropy never decreases Change in Entropy of a reversible compression of an ideal gas in a closed system reservoir + gas q Ssys= Sgas + Sres =-Q/T+Q/T=0

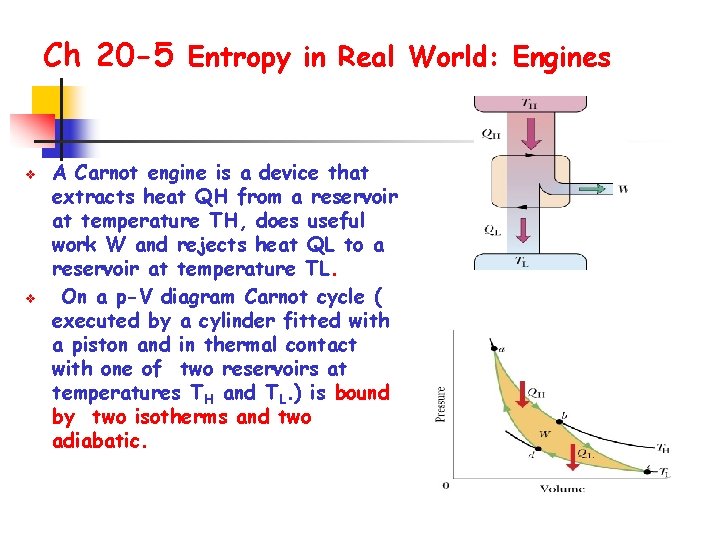
Ch 20 -5 Entropy in Real World: Engines v v A Carnot engine is a device that extracts heat QH from a reservoir at temperature TH, does useful work W and rejects heat QL to a reservoir at temperature TL. On a p-V diagram Carnot cycle ( executed by a cylinder fitted with a piston and in thermal contact with one of two reservoirs at temperatures TH and TL. ) is bound by two isotherms and two adiabatic.

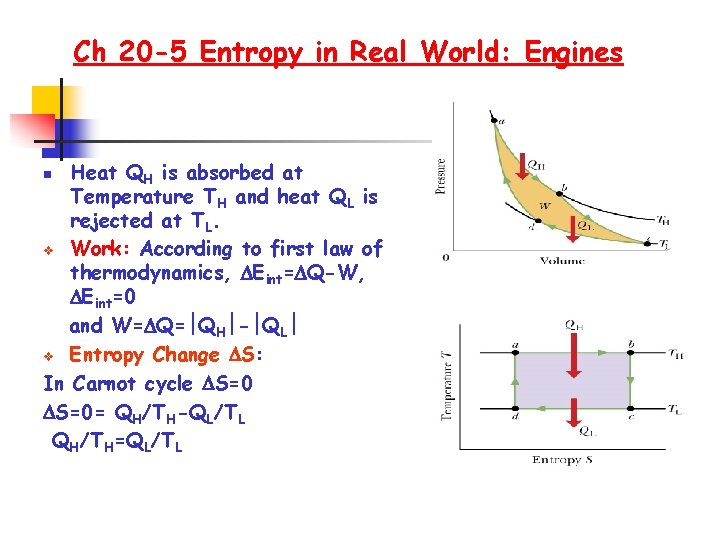
Ch 20 -5 Entropy in Real World: Engines Heat QH is absorbed at Temperature TH and heat QL is rejected at TL. v Work: According to first law of thermodynamics, Eint= Q-W, Eint=0 and W= Q= QH - QL v Entropy Change S: In Carnot cycle S=0= QH/TH-QL/TL QH/TH=QL/TL n

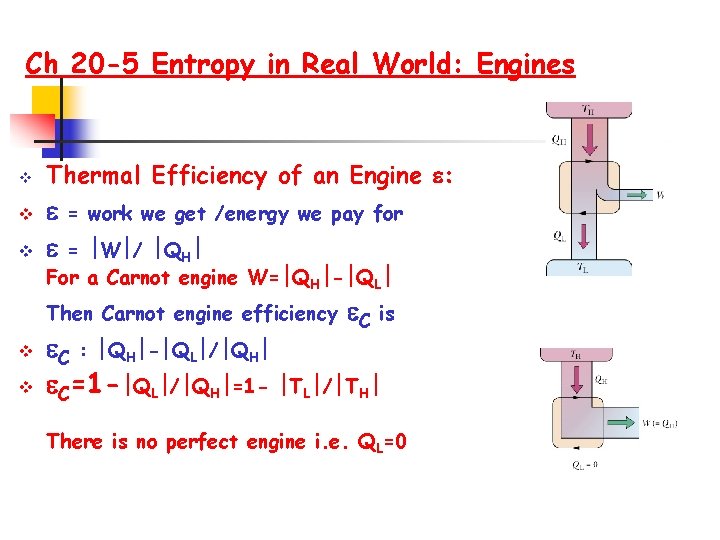
Ch 20 -5 Entropy in Real World: Engines v v v Thermal Efficiency of an Engine : = work we get /energy we pay for = W / QH For a Carnot engine W= QH - QL Then Carnot engine efficiency v v C is C : QH - QL / QH C=1 - QL / QH =1 - TL / TH There is no perfect engine i. e. QL=0

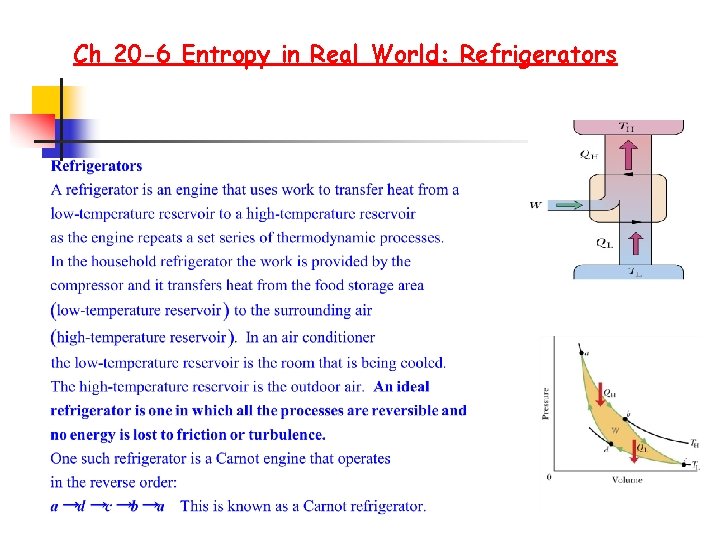
Ch 20 -6 Entropy in Real World: Refrigerators

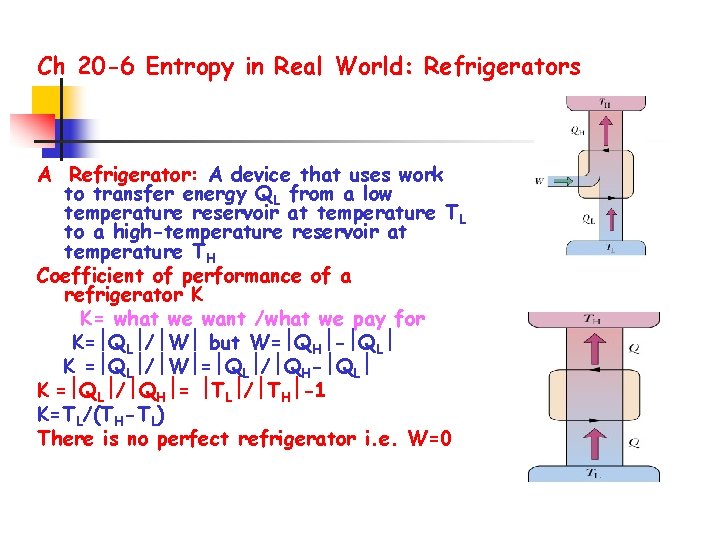
Ch 20 -6 Entropy in Real World: Refrigerators A Refrigerator: A device that uses work to transfer energy QL from a low temperature reservoir at temperature TL to a high-temperature reservoir at temperature TH Coefficient of performance of a refrigerator K K= what we want /what we pay for K= QL / W but W= QH - QL K = QL / W = QL / QH- QL K = QL / QH = TL / TH -1 K=TL/(TH-TL) There is no perfect refrigerator i. e. W=0

Suggested problems Chapter 20 n
 Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law and second law and third law
Newton's first law and second law and third law Kelvin statement
Kelvin statement Zeroth law of thermodynamics
Zeroth law of thermodynamics What is second law of thermodynamics
What is second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics 2nd law of thermodynamics
2nd law of thermodynamics 2 nd law of thermodynamics
2 nd law of thermodynamics State the second law of thermodynamics
State the second law of thermodynamics Entropy thermodynamics
Entropy thermodynamics Classical entropy
Classical entropy What is entropy in thermodynamics
What is entropy in thermodynamics Pzmore
Pzmore Law of entropy
Law of entropy