Chemical Thermodynamics 1 Lecture 23 1 In This


























































- Slides: 162

Chemical Thermodynamics -1 Lecture 23 1

In This Chapter, We Will Discuss: 1. Spontaneous porcesses 2. Entropy and the 2 nd law of thermodynamics 3. 4. 5. 6. 7. 2 Molecular interpretation of entropy Entropy changes in chemical reactions Gibb’s free energy Free energy and temperature Free energy and equilibrium constant

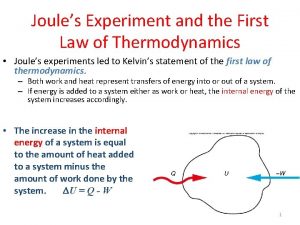
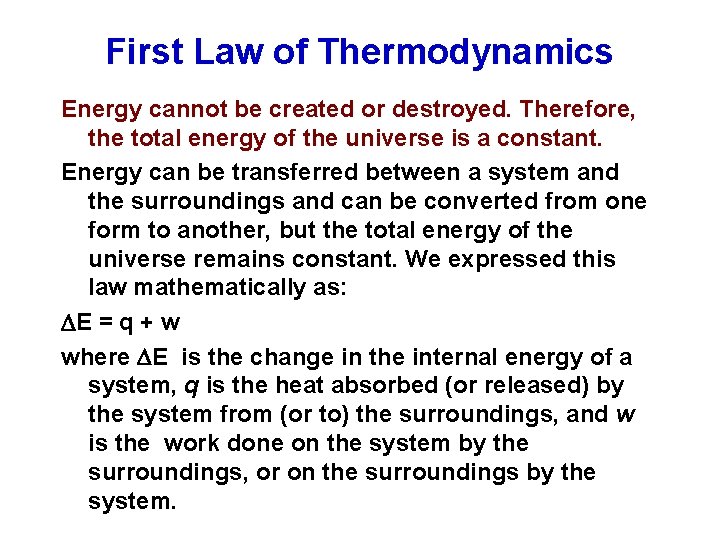
First Law of Thermodynamics Energy cannot be created or destroyed. Therefore, the total energy of the universe is a constant. Energy can be transferred between a system and the surroundings and can be converted from one form to another, but the total energy of the universe remains constant. We expressed this law mathematically as: E = q + w where E is the change in the internal energy of a system, q is the heat absorbed (or released) by the system from (or to) the surroundings, and w is the work done on the system by the surroundings, or on the surroundings by the system.

Remember that q>0 means that the system is absorbing heat from the surroundings, and w>0 means that the surroundings are doing work on the system. Experience tells us that certain processes always occur, even though the energy of the universe is conserved. Ice melts when left outside a freezer, for instance, and if you touch a hot object, heat is transferred to your hand. The first law guarantees that energy is conserved in these processes, and yet they occur without any outside intervention. We say that these processes are spontaneous. A spontaneous process is one that proceeds on its own without any outside assistance. 4

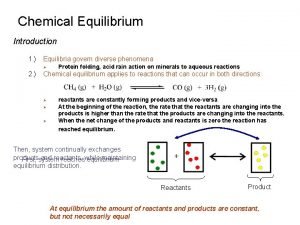
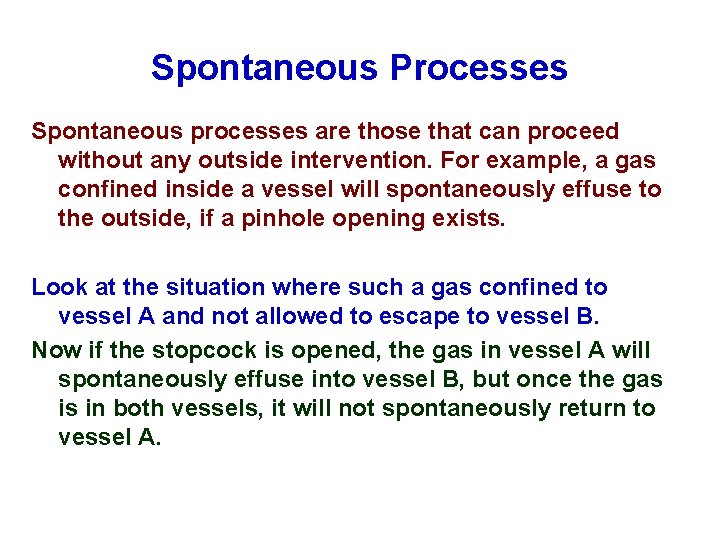
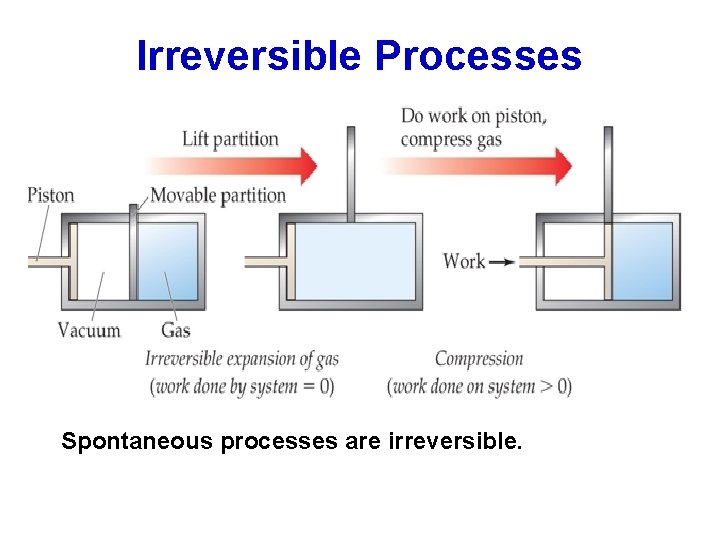
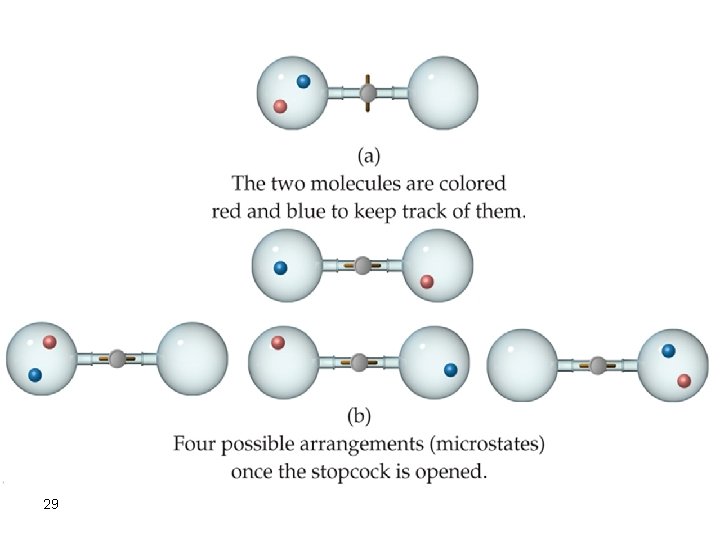
Spontaneous Processes Spontaneous processes are those that can proceed without any outside intervention. For example, a gas confined inside a vessel will spontaneously effuse to the outside, if a pinhole opening exists. Look at the situation where such a gas confined to vessel A and not allowed to escape to vessel B. Now if the stopcock is opened, the gas in vessel A will spontaneously effuse into vessel B, but once the gas is in both vessels, it will not spontaneously return to vessel A.

6

Spontaneous Processes that are spontaneous in one direction are nonspontaneous in the reverse direction.

Spontaneous Processes that are spontaneous at one temperature may be nonspontaneous at other temperatures. Above 0 C, it is spontaneous for ice to melt. Below 0 C, the reverse process is spontaneous.

Identifying Spontaneous Processes Predict whether each process is spontaneous as described, spontaneous in the reverse direction, or in equilibrium: (a) Water at 40 °C gets hotter when a piece of metal heated to 150 °C is added. (b) Water at room temperature decomposes into H 2(g) and O 2(g). (a) This process is spontaneous. Whenever two objects at different temperatures are brought into contact, heat is transferred from the hotter object to the colder one. The final temperature, after the metal and water achieve the same temperature (thermal equilibrium), will be somewhere between the initial temperatures of the metal and the water. (b) Experience tells us that this process is not spontaneous —we certainly have never seen hydrogen and oxygen gases spontaneously bubbling up out of water! Rather, the reverse process—the reaction of H 2 and O 2 to form H 2 O—is spontaneous

Predict whether each process is spontaneous as described, spontaneous in the reverse direction, or in equilibrium: (a) Benzene vapor, C 6 H 6(g), at a pressure of 1 atm condenses to liquid benzene at the normal boiling point of benzene, 80. 1 °C. (b) At 1 atm pressure, CO 2(s) sublimes at – 78 °C. Is this process spontaneous at – 100 °C and 1 atm pressure? (a) The normal boiling point is the temperature at which a vapor at 1 atm is in equilibrium with its liquid. Thus, this is an equilibrium situation. If the temperature were below 80. 1 °C, condensation would be spontaneous. (b) No, the reverse process is spontaneous at this temperature

Criteria of Spontaneity A brick falling from your hand loses potential energy. The loss of some form of energy is a common feature of spontaneous change in mechanical systems. It was suggested that the direction of spontaneous changes in chemical systems is determined by the loss of energy. , and thus, all spontaneous chemical and physical changes are exothermic!!! (wrong statement). It takes only a few moments, however, to find exceptions to this generalization. For example, the melting of ice at room temperature is spontaneous and endothermic. Similarly, many spontaneous dissolution processes, such as the dissolving of NH 4 NO 3, are endothermic. 11

Spontaneity: Enthalpy is NOT the Whole Story!!! We will see that reactions involve not only changes in enthalpy but also changes in entropy. Our discussion of entropy will lead us to the second law of thermodynamics, which provides insight into why physical and chemical changes tend to favor one direction over another. We do not expect a brick to spontaneously rise from the ground to our hand, or to gather with other bricks to form an organized building!!!. Thermodynamics helps us understand the significance of this directional character of processes, regardless of whether they are exothermic or endothermic. 12

Reversible Processes Carnot considered what an ideal engine, one with the highest possible efficiency, would be like. He observed that it is impossible to convert the energy content of a fuel completely to work because a significant amount of heat is always lost to the surroundings. Carnot’s analysis gave insight into how to build better, more efficient engines, and it was one of the earliest studies in what has developed into the discipline of thermodynamics. An ideal engine operates under an ideal set of conditions in which all the processes are reversible. A reversible process is a specific way in which a system changes its state. Carnot concluded that a reversible change produces the maximum amount of work that can be done by a system on its surroundings. 13

Reversible Processes In a reversible process the system changes in such a way that the system and surroundings can be put back in their original states by exactly reversing the process. When two objects at different temperatures are in contact, heat flows spontaneously from the hotter object to the colder one. Because it is impossible to make heat flow in the opposite direction, from colder object to hotter one, the flow of heat is an irreversible process. Given these facts, can we imagine any conditions under which heat transfer can be made reversible? To answer this question, we must consider temperature differences that are infinitesimally small, as opposed to the discrete temperature differences with which we are most familiar.

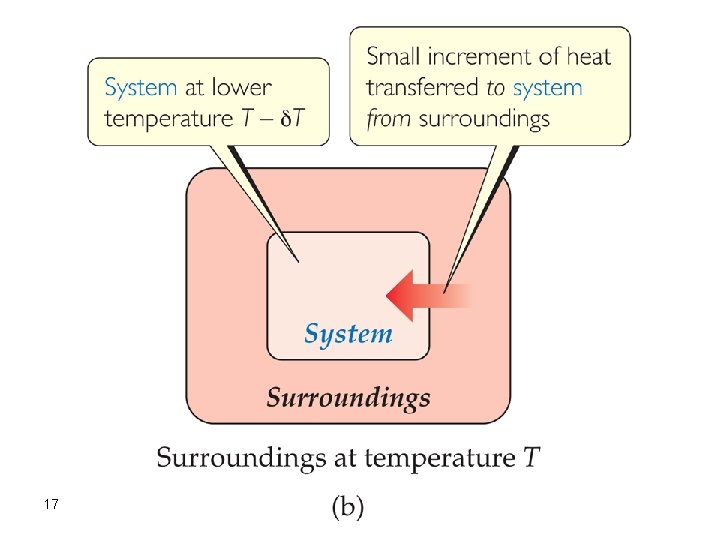
Consider a system and its surroundings at essentially the same temperature, with just an infinitesimal temperature difference d. T between them. If the surroundings are at temperature T and the system is at the infinitesimally higher temperature T+ d. T, then an infinitesimal amount of heat flows from system to surroundings. We can reverse the direction of heat flow by making an infinitesimal change of temperature in the opposite direction, lowering the system temperature to T- d. T. Now the direction of heat flow is from surroundings to system. Reversible processes are those that reverse direction whenever an infinitesimal change is made in some property of the system. 15

16

17

Irreversible Processes Spontaneous processes are irreversible.

For a process to be truly reversible, the amounts of heat must be infinitesimally small and the transfer of heat must occur infinitely slowly; thus, no process that we can observe is truly reversible. Because real processes can, at best, only approximate the infinitely slow change associated with reversible processes, all real processes are irreversible. Further, a nonspontaneous process can occur only if the surroundings do work on the system (compression of a gas). Thus, any spontaneous process is irreversible. 19

Entropy and the 2 nd Law of Thermodynamics How can we use the fact that any spontaneous process is irreversible to make predictions about the spontaneity of an unfamiliar process? Understanding spontaneity requires us to examine the entropy in more details. In general, entropy is associated either with the extent of randomness in a system or with the extent to which energy is distributed among the various motions of the molecules of the system. 20

Entropy (S) is a term introduced by Rudolph Clausius in the nineteenth century. Clausius was convinced of the significance of the ratio of heat delivered and the temperature at which it is delivered, (q/T), which he called entropy. Like total energy, E, and enthalpy, H, entropy is a state function. Therefore, S = Sfinal Sinitial

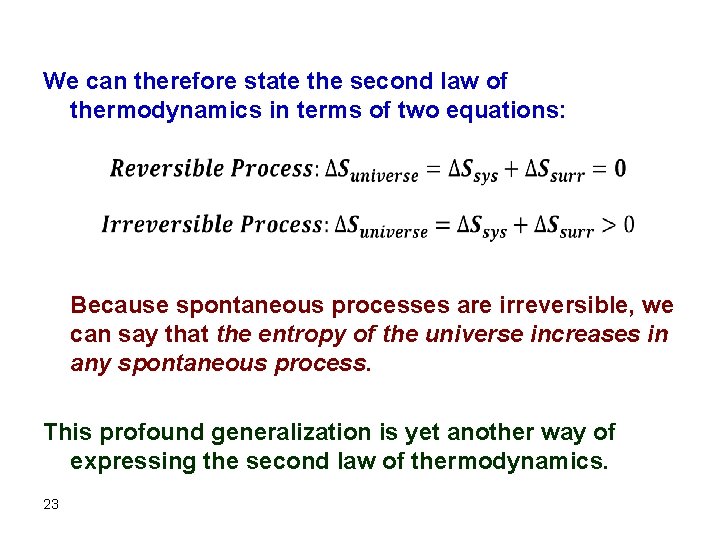
The Second Law of Thermodynamics In general, any irreversible process results in an increase in total entropy, whereas any reversible process results in no overall change in entropy of the universe. This statement is known as the second law of thermodynamics. The sum of the entropy of a system plus the entropy of the surroundings is what we refer to as the total entropy change, or the entropy change of the universe, SUniverse. 22

We can therefore state the second law of thermodynamics in terms of two equations: Because spontaneous processes are irreversible, we can say that the entropy of the universe increases in any spontaneous process. This profound generalization is yet another way of expressing the second law of thermodynamics. 23

The second law of thermodynamics tells us the essential character of any spontaneous change—it is always accompanied by an increase in the entropy of the universe. We can use this criterion to predict whether a given process is spontaneous or not. Before seeing how this is done, however, we will find it useful to explore entropy from a molecular perspective. Throughout most of the remainder of this chapter, we will focus on systems rather than surroundings. To simplify the notation, we will usually refer to the entropy change of the system as S rather than explicitly indicating Ssys. 24

The Concept of a Microstate Imagine taking a snapshot of the positions and speeds of all the molecules at a given instant. The speed of each molecule tells us its kinetic energy. That particular set of positions and kinetic energies of the individual gas molecules is what we call a microstate of the system. A microstate is a single possible arrangement of the positions and kinetic energies of the gas molecules when the gas is in a specific thermodynamic state. We could envision continuing to take snapshots of our system to see other possible microstates. 25

It is essential to define the concepts of microstate and macrostate. Lets us consider N unbiased identifiable coins. Each coin has two states: head (h) or tail (t). Lets us make N tosses. The strings of h and t are microstates. The number of microstates is 2 N. Each microstate has probability (1/2)N. The sum of all microstates gives us a macrostate. 26

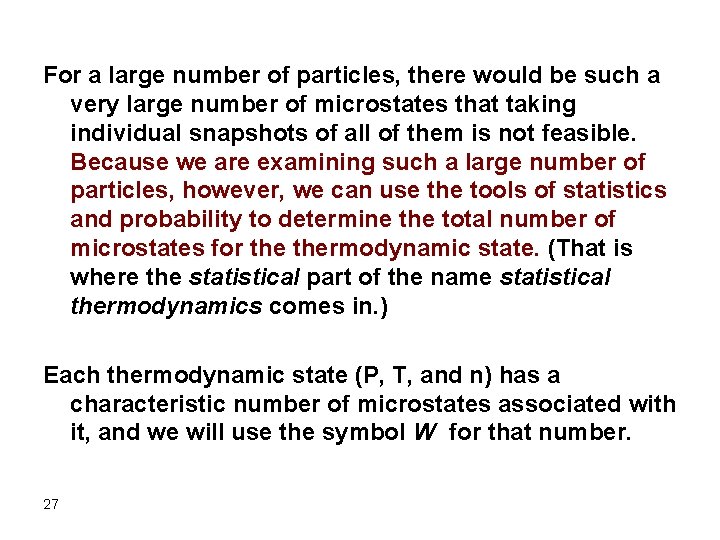
For a large number of particles, there would be such a very large number of microstates that taking individual snapshots of all of them is not feasible. Because we are examining such a large number of particles, however, we can use the tools of statistics and probability to determine the total number of microstates for thermodynamic state. (That is where the statistical part of the name statistical thermodynamics comes in. ) Each thermodynamic state (P, T, and n) has a characteristic number of microstates associated with it, and we will use the symbol W for that number. 27

Molecular Interpretation of Entropy To understand the concept of spontaneity, let us look at the expansion of a gas into a vacuum, which is a spontaneous process. Now, what really makes this expansion spontaneous? The answer is simple as it relates to the continuous motion of gas molecules described by the kinetic molecular theory. The expansion can be viewed as the ultimate result of the random movement of the gas molecules, throughout the larger volume. 28

29

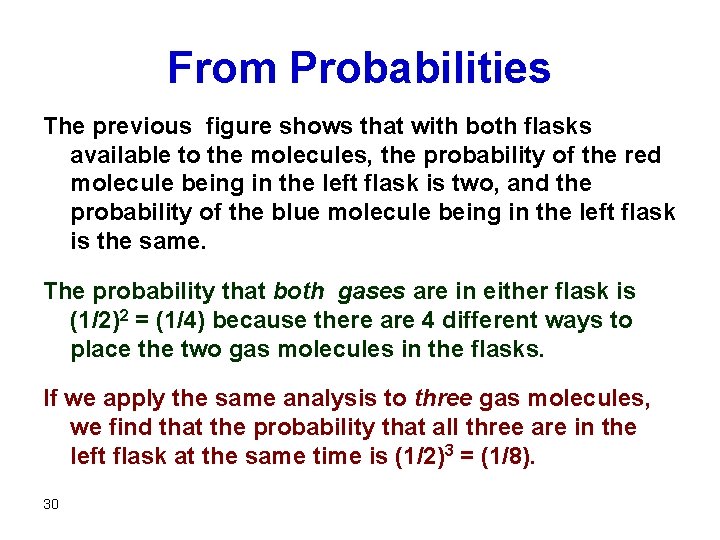
From Probabilities The previous figure shows that with both flasks available to the molecules, the probability of the red molecule being in the left flask is two, and the probability of the blue molecule being in the left flask is the same. The probability that both gases are in either flask is (1/2)2 = (1/4) because there are 4 different ways to place the two gas molecules in the flasks. If we apply the same analysis to three gas molecules, we find that the probability that all three are in the left flask at the same time is (1/2)3 = (1/8). 30

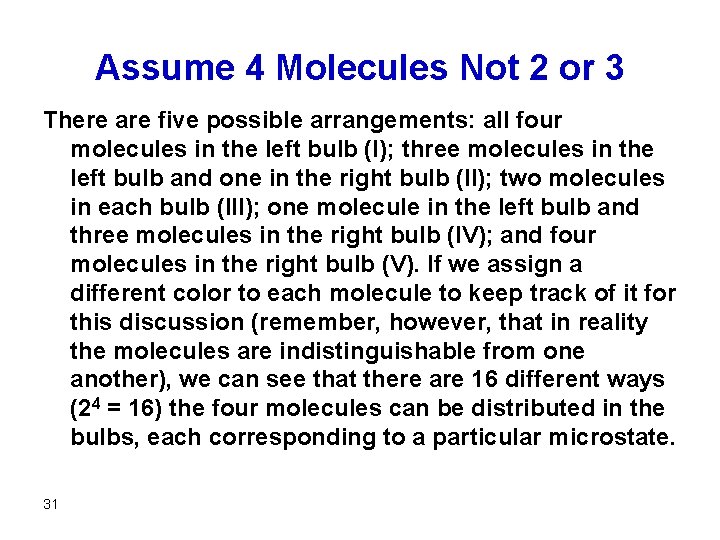
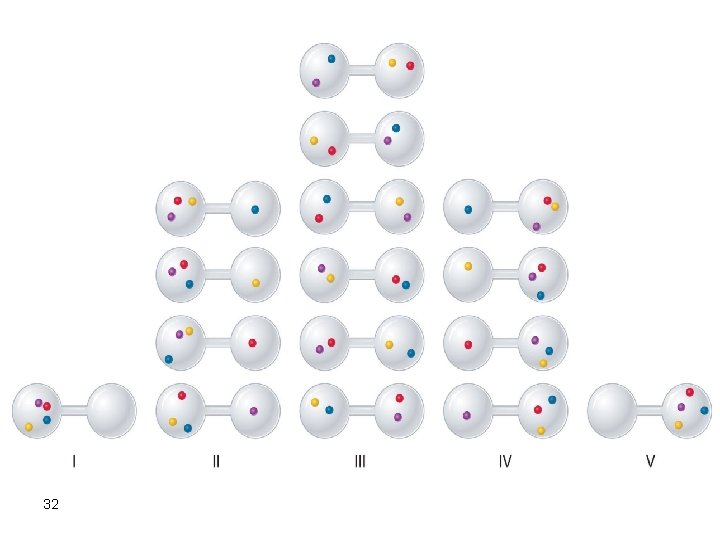
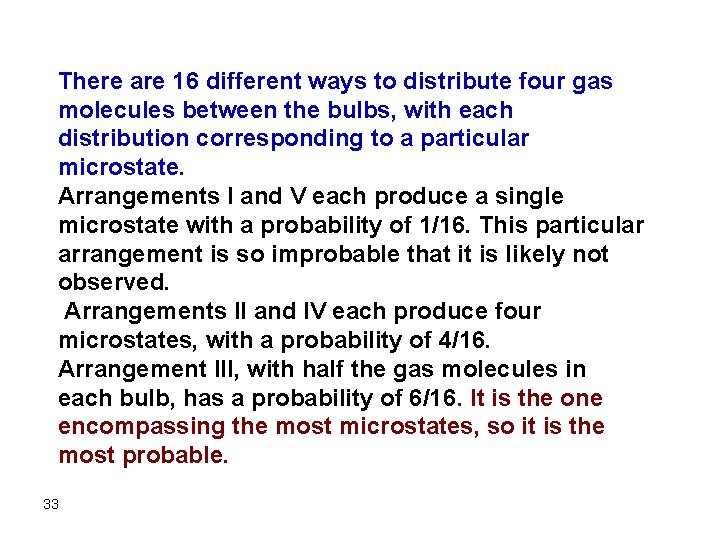
Assume 4 Molecules Not 2 or 3 There are five possible arrangements: all four molecules in the left bulb (I); three molecules in the left bulb and one in the right bulb (II); two molecules in each bulb (III); one molecule in the left bulb and three molecules in the right bulb (IV); and four molecules in the right bulb (V). If we assign a different color to each molecule to keep track of it for this discussion (remember, however, that in reality the molecules are indistinguishable from one another), we can see that there are 16 different ways (24 = 16) the four molecules can be distributed in the bulbs, each corresponding to a particular microstate. 31

32

There are 16 different ways to distribute four gas molecules between the bulbs, with each distribution corresponding to a particular microstate. Arrangements I and V each produce a single microstate with a probability of 1/16. This particular arrangement is so improbable that it is likely not observed. Arrangements II and IV each produce four microstates, with a probability of 4/16. Arrangement III, with half the gas molecules in each bulb, has a probability of 6/16. It is the one encompassing the most microstates, so it is the most probable. 33

What about a mole of gas? Now let’s consider a mole of gas. The probability that all the molecules are in the left flask at the same time is (1/2)N, where N = 6. 02*1023 molecules. This is a vanishingly small number! Thus, there is essentially zero likelihood that all the gas molecules will be in the left flask at the same time. This analysis of the microscopic behavior of the gas molecules leads to the expected macroscopic behavior: The gas spontaneously expands to fill both the left and right flasks, and it does not spontaneously all reside in one flask. 34

Boltzmann Distribution and Microstates The science of thermodynamics developed as a means of describing the properties of matter in our macroscopic world without regard to microscopic structure. In fact, thermodynamics was a well-developed field before the modern view of atomic and molecular structure was even known. The thermodynamic properties of water, for example, addressed the behavior of bulk water (or ice or water vapor) as a substance without considering any specific properties of individual H 2 O molecules. 35

To connect the microscopic and macroscopic descriptions of matter, scientists have developed the field of statistical thermodynamics, which uses the tools of statistics and probability to link the microscopic and macroscopic worlds. Here we show entropy, which is a property of bulk matter, can be connected to the behavior of atoms and molecules. Suppose we now consider one mole of an ideal gas in a particular thermodynamic state, which we can define by specifying the temperature, T, and volume, V, of the gas. What is happening to this gas at the microscopic level, and how does what is going on at the microscopic level relate to the entropy of the gas? 36

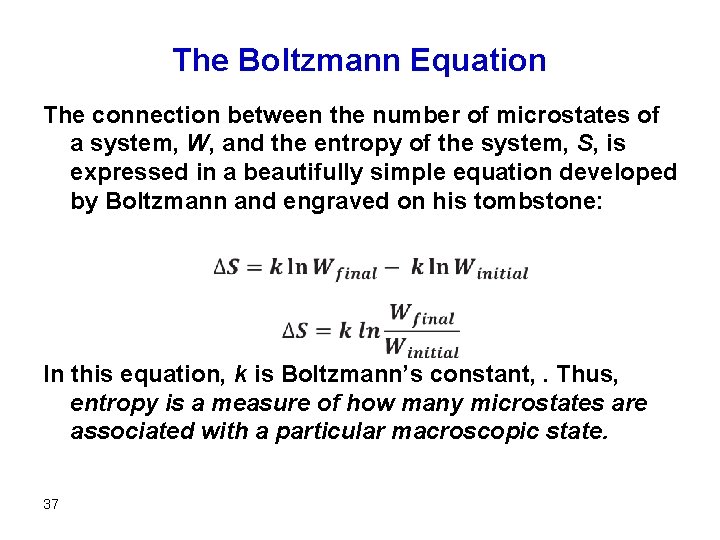
The Boltzmann Equation The connection between the number of microstates of a system, W, and the entropy of the system, S, is expressed in a beautifully simple equation developed by Boltzmann and engraved on his tombstone: In this equation, k is Boltzmann’s constant, . Thus, entropy is a measure of how many microstates are associated with a particular macroscopic state. 37


Entropy Change as Related to V and T suppose we increase the volume of the system, which is analogous to allowing the gas to expand isothermally. A greater volume means a greater number of positions available to the gas atoms and therefore a greater number of microstates. The entropy therefore increases as the volume increases. Second, suppose we keep the volume fixed but increase the temperature. How does this change affect the entropy of the system? An increase in temperature increases the most probable speed of the molecules and also broadens the distribution of speeds. Hence, the molecules have a greater number of possible kinetic energies, and the number of microstates increases. Thus, the entropy of the system increases with increasing temperature. 39

Molecular Motions and Energy Any real molecule can undergo three kinds of complex motion. The entire molecule can move in one direction, which is the simple motion we visualize for an ideal particle and see in a macroscopic object, such as a thrown baseball. We call such movement translational motion. The molecules in a gas have more freedom of translational motion than those in a liquid, which have more freedom of translational motion than the molecules of a solid. A real molecule can also undergo vibrational motion, in which the atoms in the molecule move periodically toward and away from one another, and rotational motion, in which the molecule spins about an axis. 40

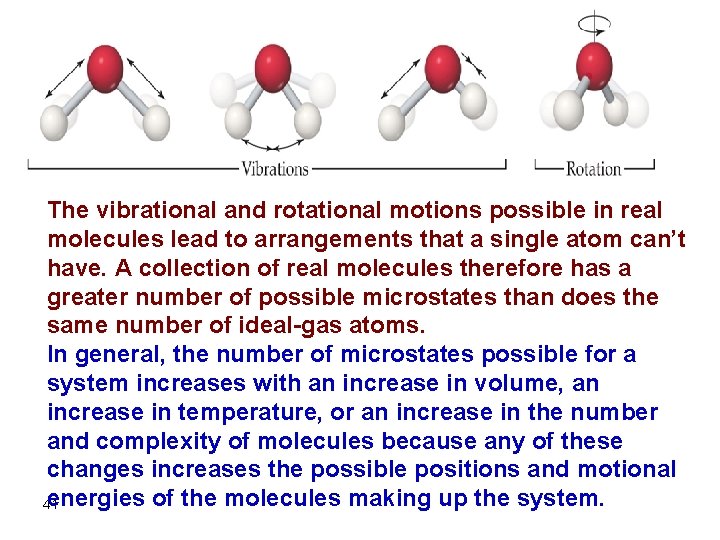
The vibrational and rotational motions possible in real molecules lead to arrangements that a single atom can’t have. A collection of real molecules therefore has a greater number of possible microstates than does the same number of ideal-gas atoms. In general, the number of microstates possible for a system increases with an increase in volume, an increase in temperature, or an increase in the number and complexity of molecules because any of these changes increases the possible positions and motional energies of the molecules making up the system. 41

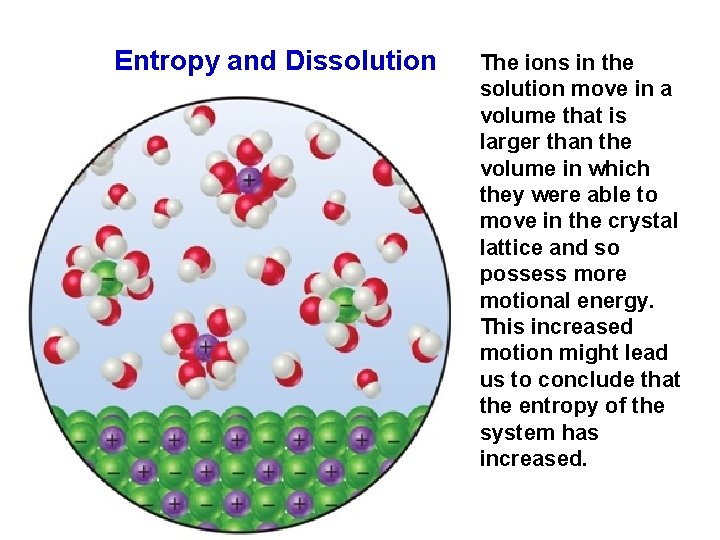
Entropy and Dissolution 42 The ions in the solution move in a volume that is larger than the volume in which they were able to move in the crystal lattice and so possess more motional energy. This increased motion might lead us to conclude that the entropy of the system has increased.

We have to be careful, however, because some of the water molecules have lost some freedom of motion because they are now held around the ions as water of hydration. These water molecules are in a more ordered state than before because they are now confined to the immediate environment of the ions. Therefore, the dissolving of a salt involves both a disordering process (the ions become less confined) and an ordering process (some water molecules become more confined). The disordering processes are usually dominant, and so the overall effect is an increase in the randomness of the system when salts dissolve in water. 43

Chemical Thermodynamics -2 Lecture 24 44

Making Qualitative Predictions About S In summary, we generally expect the entropy of a system to increase for processes in which: 1. The volume of a system increases. 2. The temperature increases. 3. The number of independently moving particles increases. 4. The number of atoms in a molecule increases Remember that: entropy increases when: 1. Solids or liquids are converted to gases. 2. Solids are converted to liquids or solutions. 3. The number of gas molecules increases during a chemical reaction. 45

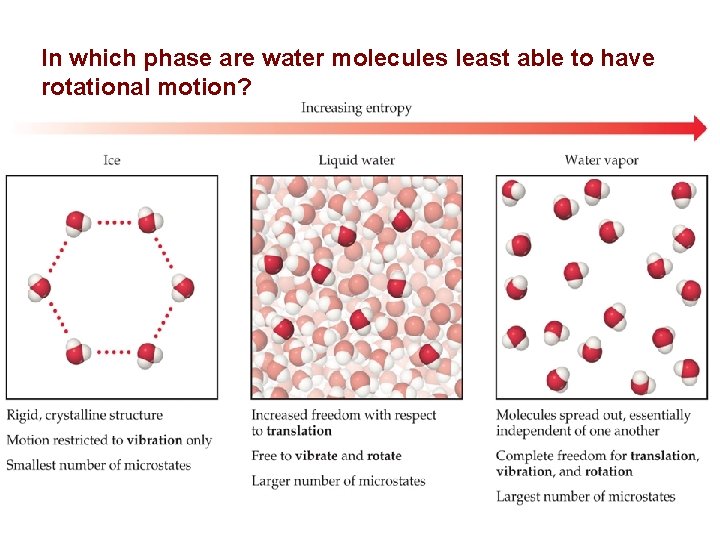
In which phase are water molecules least able to have rotational motion? 46

If a process is nonspontaneous, does that mean the process cannot occur under any circumstances? A. Yes. Nonspontaneous processes can never occur under any circumstances. B. Nonspontaneous processes can occur with some continuous external assistance.

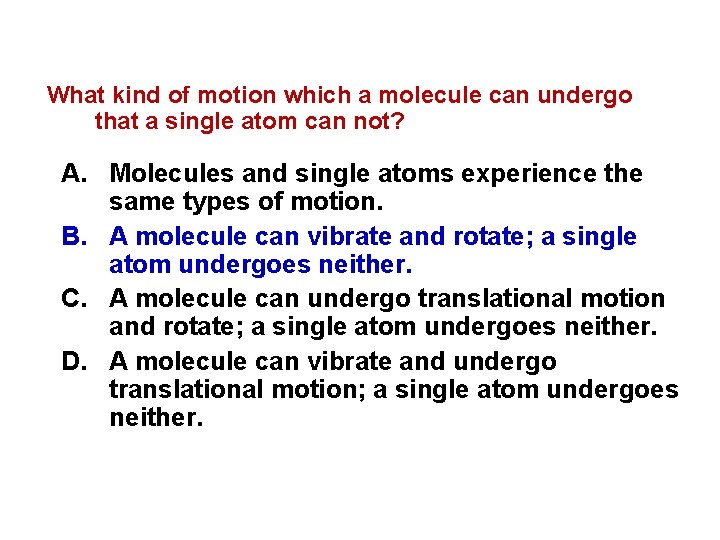
What kind of motion which a molecule can undergo that a single atom can not? A. Molecules and single atoms experience the same types of motion. B. A molecule can vibrate and rotate; a single atom undergoes neither. C. A molecule can undergo translational motion and rotate; a single atom undergoes neither. D. A molecule can vibrate and undergo translational motion; a single atom undergoes neither.

If a process is exothermic, does the entropy of the surroundings: A) Always increase B) Always decrease C) Sometimes increase and sometimes decrease, depending on the process? A. Always increase B. Always decrease C. Sometimes increases and sometimes decreases, depending on the process

Consider a pure crystalline solid that is heated from absolute zero to a temperature above the boiling point of the liquid. Which of the following processes produces the greatest increase in the entropy of the substance? A. melting the solid B. heating the liquid C. heating the gas D. heating the solid E. vaporizing the liquid 50

The rusting of iron is spontaneous and is accompanied by a decrease in the entropy of the system (the iron and oxygen). What can we conclude about the entropy change of the surroundings? A. We need to know if the change involves a closed or open system to make a conclusion. B. The entropy of the surroundings must increase by the same amount as the entropy decrease of the system. C. The entropy of the surroundings must increase by a greater amount than the entropy decrease of the system. For spontaneous processes, Suniv > 0 D. The entropy of the surroundings must decrease by a smaller amount than the entropy decrease of the system.

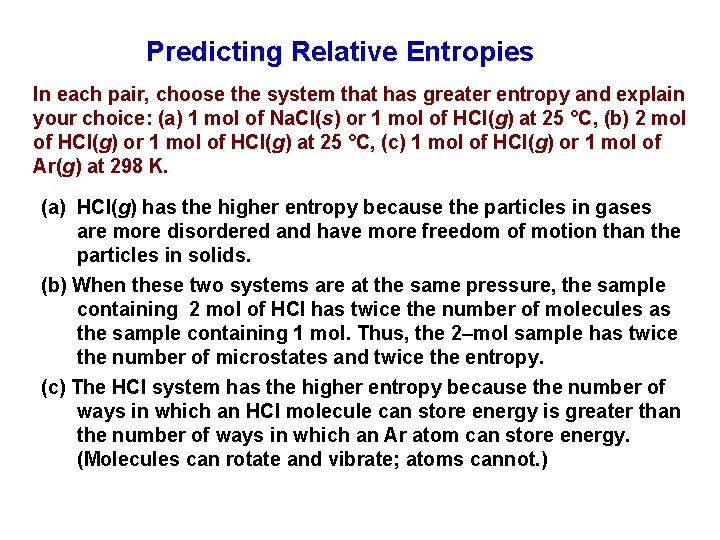
Predicting Relative Entropies In each pair, choose the system that has greater entropy and explain your choice: (a) 1 mol of Na. Cl(s) or 1 mol of HCl(g) at 25 °C, (b) 2 mol of HCl(g) or 1 mol of HCl(g) at 25 °C, (c) 1 mol of HCl(g) or 1 mol of Ar(g) at 298 K. (a) HCl(g) has the higher entropy because the particles in gases are more disordered and have more freedom of motion than the particles in solids. (b) When these two systems are at the same pressure, the sample containing 2 mol of HCl has twice the number of molecules as the sample containing 1 mol. Thus, the 2–mol sample has twice the number of microstates and twice the entropy. (c) The HCl system has the higher entropy because the number of ways in which an HCl molecule can store energy is greater than the number of ways in which an Ar atom can store energy. (Molecules can rotate and vibrate; atoms cannot. )

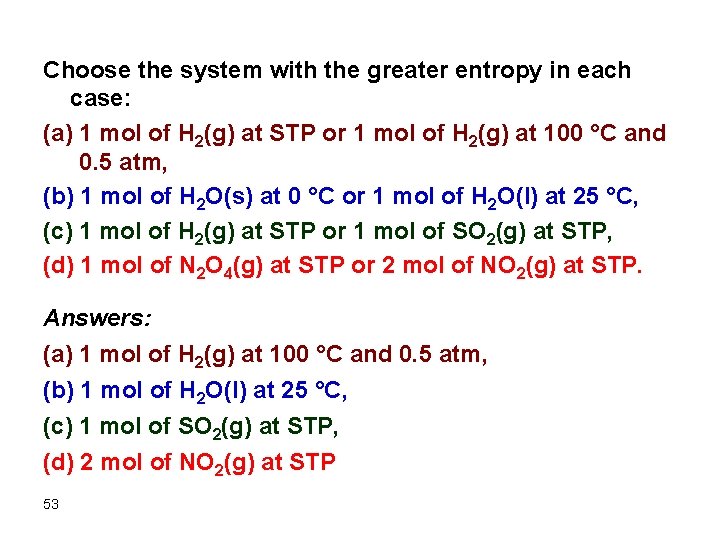
Choose the system with the greater entropy in each case: (a) 1 mol of H 2(g) at STP or 1 mol of H 2(g) at 100 °C and 0. 5 atm, (b) 1 mol of H 2 O(s) at 0 °C or 1 mol of H 2 O(l) at 25 °C, (c) 1 mol of H 2(g) at STP or 1 mol of SO 2(g) at STP, (d) 1 mol of N 2 O 4(g) at STP or 2 mol of NO 2(g) at STP. Answers: (a) 1 mol of H 2(g) at 100 °C and 0. 5 atm, (b) 1 mol of H 2 O(l) at 25 °C, (c) 1 mol of SO 2(g) at STP, (d) 2 mol of NO 2(g) at STP 53

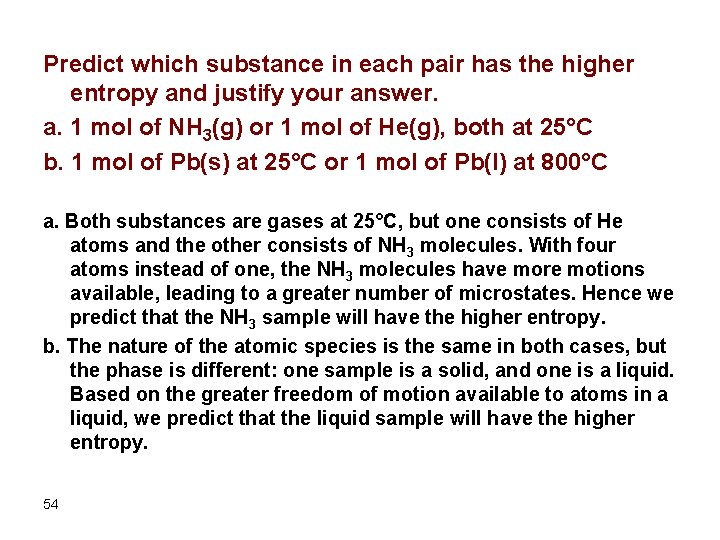
Predict which substance in each pair has the higher entropy and justify your answer. a. 1 mol of NH 3(g) or 1 mol of He(g), both at 25°C b. 1 mol of Pb(s) at 25°C or 1 mol of Pb(l) at 800°C a. Both substances are gases at 25°C, but one consists of He atoms and the other consists of NH 3 molecules. With four atoms instead of one, the NH 3 molecules have more motions available, leading to a greater number of microstates. Hence we predict that the NH 3 sample will have the higher entropy. b. The nature of the atomic species is the same in both cases, but the phase is different: one sample is a solid, and one is a liquid. Based on the greater freedom of motion available to atoms in a liquid, we predict that the liquid sample will have the higher entropy. 54

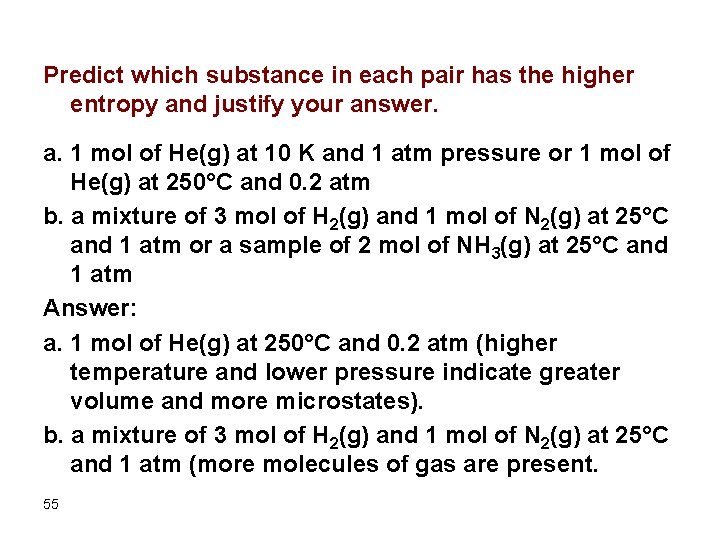
Predict which substance in each pair has the higher entropy and justify your answer. a. 1 mol of He(g) at 10 K and 1 atm pressure or 1 mol of He(g) at 250°C and 0. 2 atm b. a mixture of 3 mol of H 2(g) and 1 mol of N 2(g) at 25°C and 1 atm or a sample of 2 mol of NH 3(g) at 25°C and 1 atm Answer: a. 1 mol of He(g) at 250°C and 0. 2 atm (higher temperature and lower pressure indicate greater volume and more microstates). b. a mixture of 3 mol of H 2(g) and 1 mol of N 2(g) at 25°C and 1 atm (more molecules of gas are present. 55

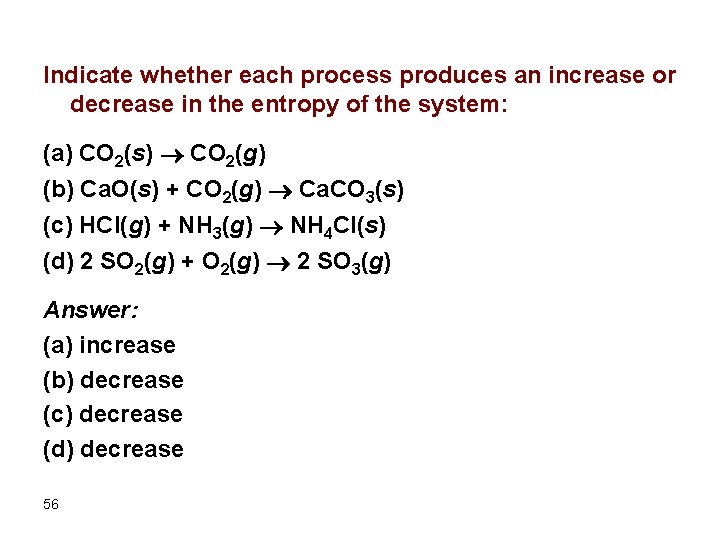
Indicate whether each process produces an increase or decrease in the entropy of the system: (a) CO 2(s) CO 2(g) (b) Ca. O(s) + CO 2(g) Ca. CO 3(s) (c) HCl(g) + NH 3(g) NH 4 Cl(s) (d) 2 SO 2(g) + O 2(g) 2 SO 3(g) Answer: (a) increase (b) decrease (c) decrease (d) decrease 56

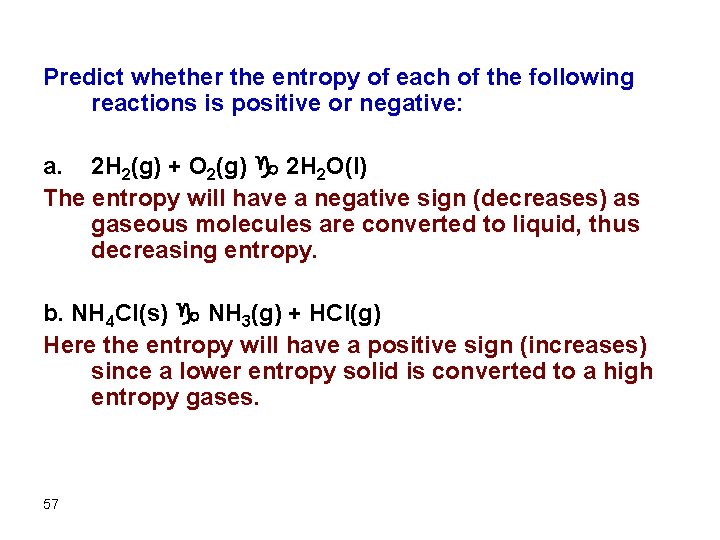
Predict whether the entropy of each of the following reactions is positive or negative: a. 2 H 2(g) + O 2(g) g 2 H 2 O(l) The entropy will have a negative sign (decreases) as gaseous molecules are converted to liquid, thus decreasing entropy. b. NH 4 Cl(s) g NH 3(g) + HCl(g) Here the entropy will have a positive sign (increases) since a lower entropy solid is converted to a high entropy gases. 57

Predicting the Sign of ΔS Predict whether ΔS is positive or negative for each process, assuming each occurs at constant temperature: (a) H 2 O(l) H 2 O(g) (b) Ag+(aq) + Cl–(aq) Ag. Cl(s) (a) Evaporation involves a large increase in volume as liquid changes to gas. One mole of water (18 g) occupies about 18 m. L as a liquid and if it could exist as a gas at STP it would occupy 22. 4 L. Because the molecules are distributed throughout a much larger volume in the gaseous state, vaporization results in an entropy and thus ΔS is positive. (b) In this process, ions, which are free to move throughout the volume of the solution, form a solid, in which they are confined to a smaller volume and restricted to more highly ordered positions. Thus, ΔS is negative.

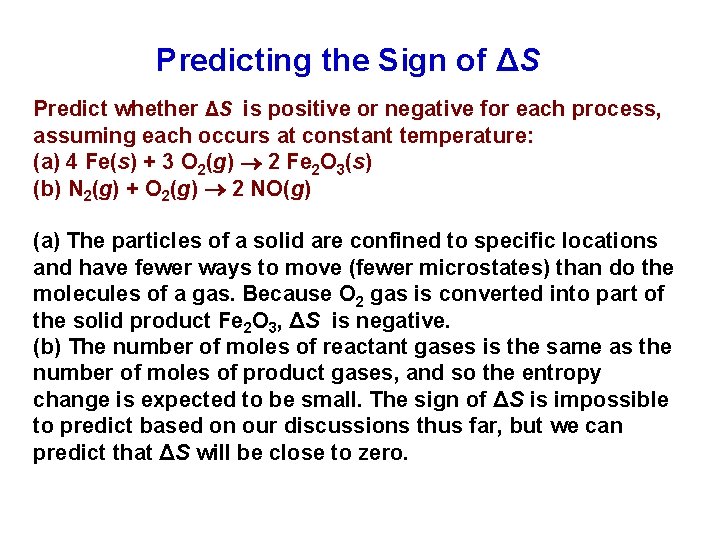
Predicting the Sign of ΔS Predict whether ΔS is positive or negative for each process, assuming each occurs at constant temperature: (a) 4 Fe(s) + 3 O 2(g) 2 Fe 2 O 3(s) (b) N 2(g) + O 2(g) 2 NO(g) (a) The particles of a solid are confined to specific locations and have fewer ways to move (fewer microstates) than do the molecules of a gas. Because O 2 gas is converted into part of the solid product Fe 2 O 3, ΔS is negative. (b) The number of moles of reactant gases is the same as the number of moles of product gases, and so the entropy change is expected to be small. The sign of ΔS is impossible to predict based on our discussions thus far, but we can predict that ΔS will be close to zero.

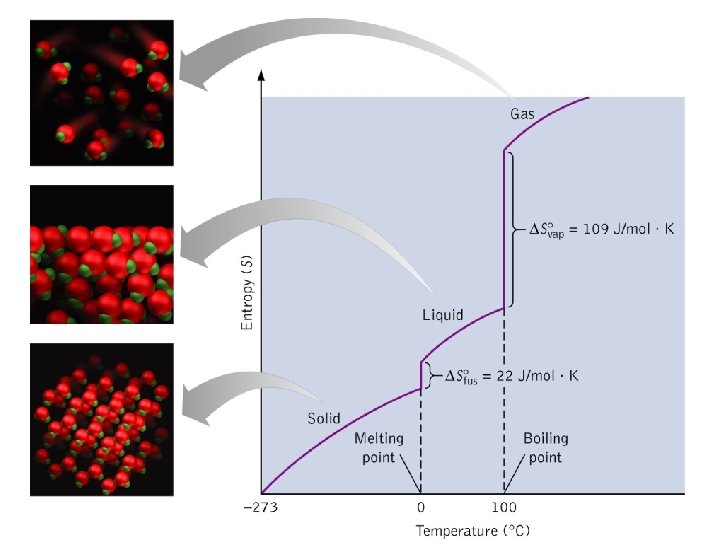
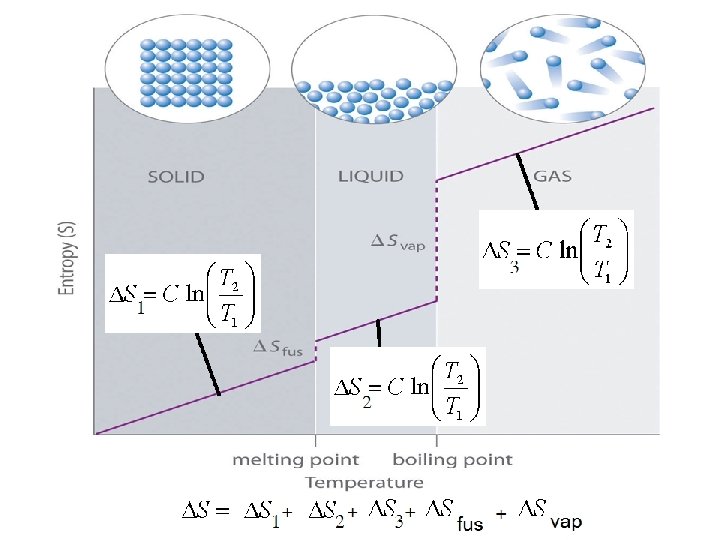
Entropy for a Phase Change As you know, a crystalline solid is composed of an ordered array of molecules, ions, or atoms that occupy fixed positions in a lattice, whereas the molecules in a liquid are free to move within the volume of the liquid; molecules in a gas have even more freedom to move than those in a liquid. The freedom of motion increases the number of available microstates, resulting in a higher entropy. Thus the entropy of a system must increase during melting (ΔSfus > 0). Similarly, when a liquid is converted to a vapor, the greater freedom of motion of the molecules in the gas phase means that ΔSvap > 0. Conversely, the reverse processes (condensing a vapor to form a liquid or freezing a liquid to form a solid) must be accompanied by a decrease in the entropy of the system: ΔS < 0. 60

61

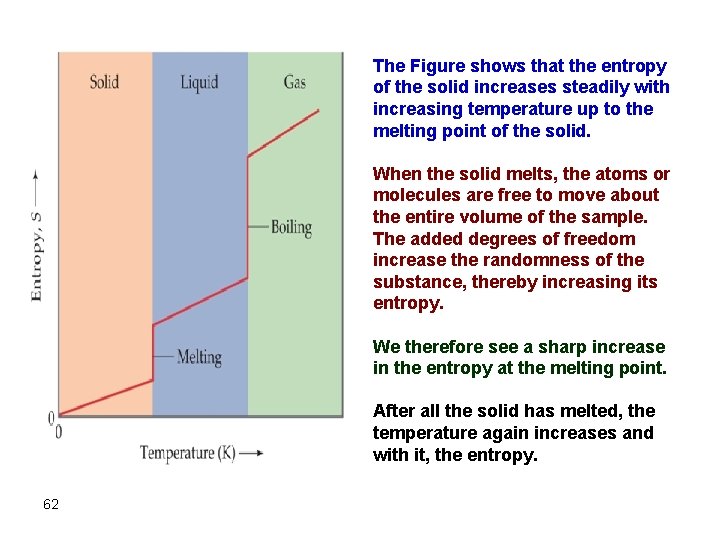
The Figure shows that the entropy of the solid increases steadily with increasing temperature up to the melting point of the solid. When the solid melts, the atoms or molecules are free to move about the entire volume of the sample. The added degrees of freedom increase the randomness of the substance, thereby increasing its entropy. We therefore see a sharp increase in the entropy at the melting point. After all the solid has melted, the temperature again increases and with it, the entropy. 62

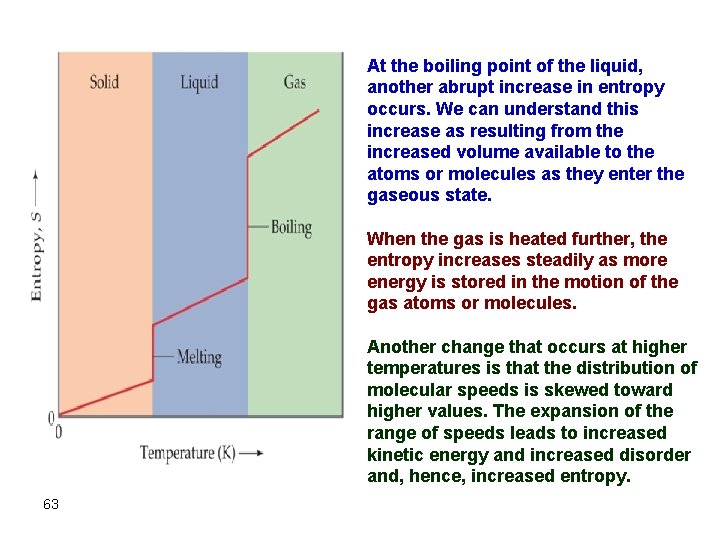
At the boiling point of the liquid, another abrupt increase in entropy occurs. We can understand this increase as resulting from the increased volume available to the atoms or molecules as they enter the gaseous state. When the gas is heated further, the entropy increases steadily as more energy is stored in the motion of the gas atoms or molecules. Another change that occurs at higher temperatures is that the distribution of molecular speeds is skewed toward higher values. The expansion of the range of speeds leads to increased kinetic energy and increased disorder and, hence, increased entropy. 63

64

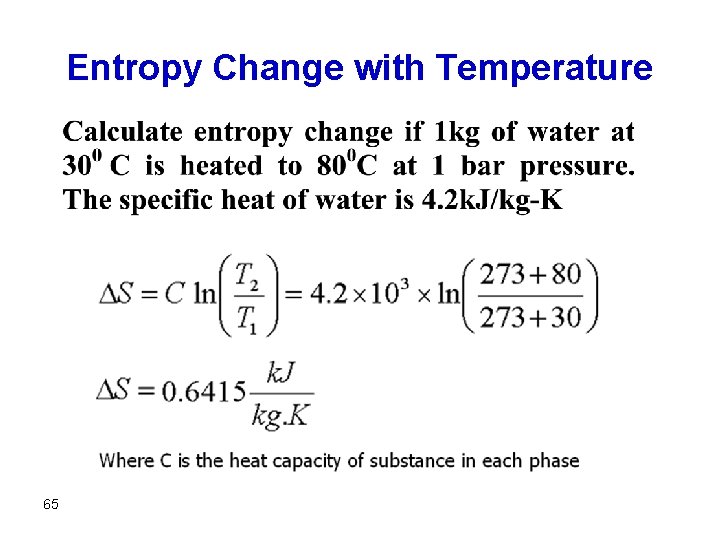
Entropy Change with Temperature 65

66

The Relationship between Internal Energy and Entropy Because the quantity of heat transferred (qrev) is directly proportional to the absolute temperature of an object (qrev ∝ T), the hotter the object, the greater the amount of heat transferred. Moreover, adding heat to a system increases the kinetic energy of the component atoms and molecules and hence their disorder (ΔS ∝ qrev). Combining these relationships for any reversible process, qrev =TΔS and Because the numerator (qrev) is expressed in units of energy (joules), the units of ΔS are joules/kelvin (J/K). We can write: ΔE = qrev + wrev = TΔS − PΔV 67

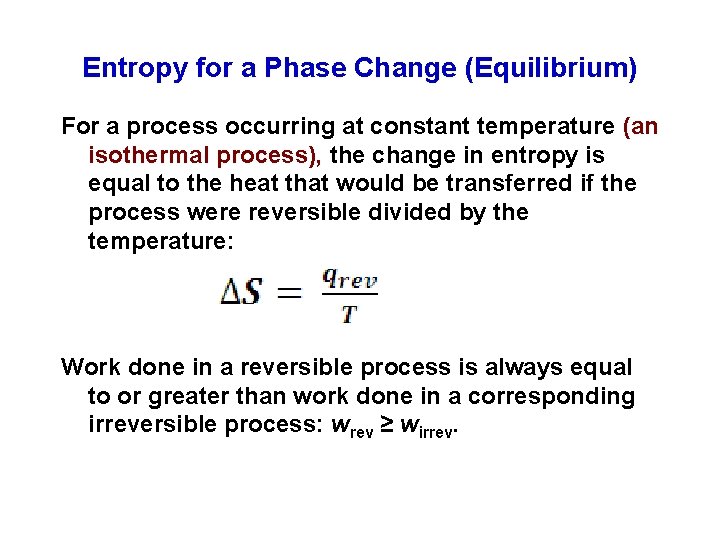
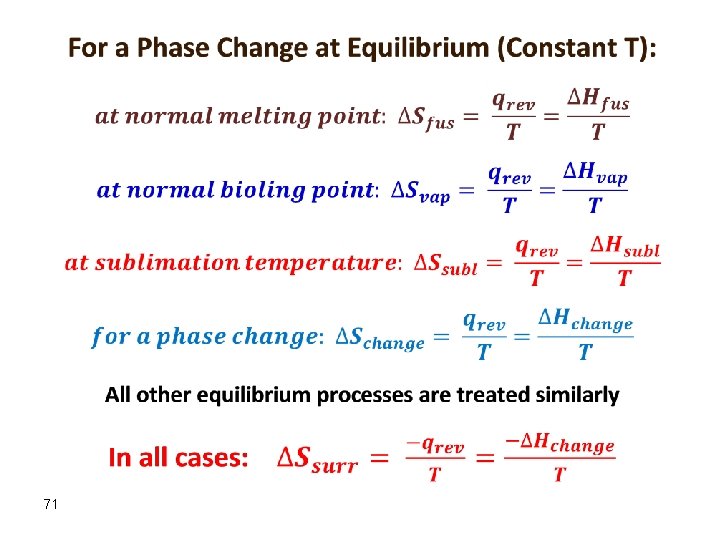
Entropy for a Phase Change (Equilibrium) For a process occurring at constant temperature (an isothermal process), the change in entropy is equal to the heat that would be transferred if the process were reversible divided by the temperature: Work done in a reversible process is always equal to or greater than work done in a corresponding irreversible process: wrev ≥ wirrev.

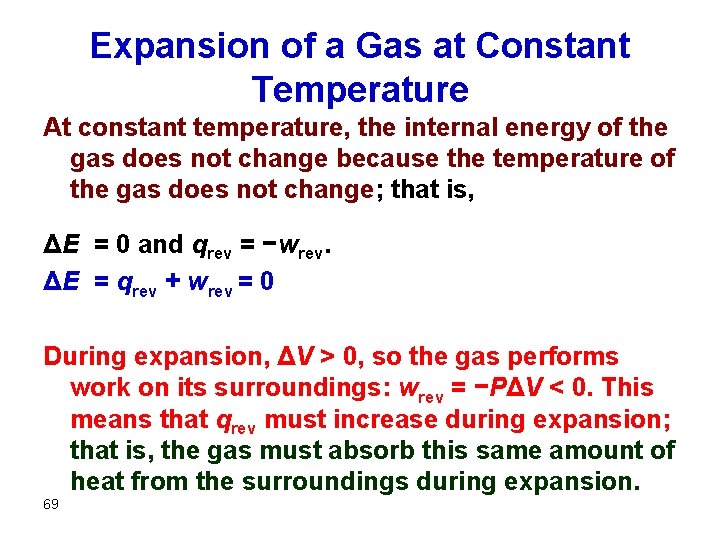
Expansion of a Gas at Constant Temperature At constant temperature, the internal energy of the gas does not change because the temperature of the gas does not change; that is, ΔE = 0 and qrev = −wrev. ΔE = qrev + wrev = 0 During expansion, ΔV > 0, so the gas performs work on its surroundings: wrev = −PΔV < 0. This means that qrev must increase during expansion; that is, the gas must absorb this same amount of heat from the surroundings during expansion. 69

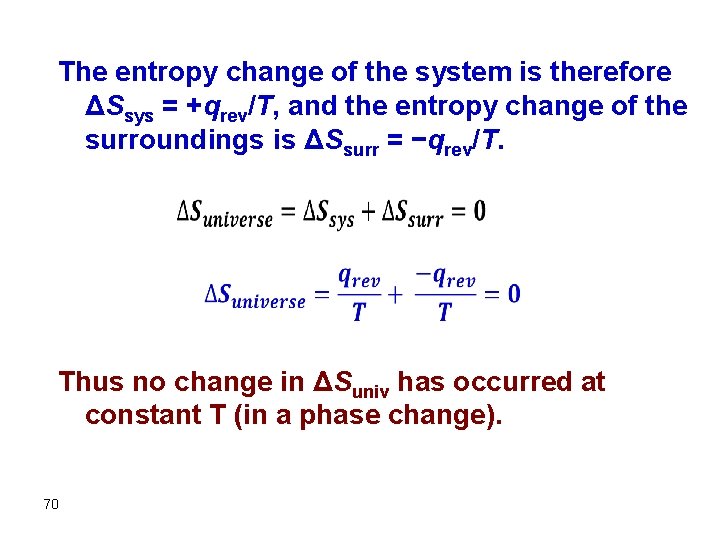
The entropy change of the system is therefore ΔSsys = +qrev/T, and the entropy change of the surroundings is ΔSsurr = −qrev/T. Thus no change in ΔSuniv has occurred at constant T (in a phase change). 70

71

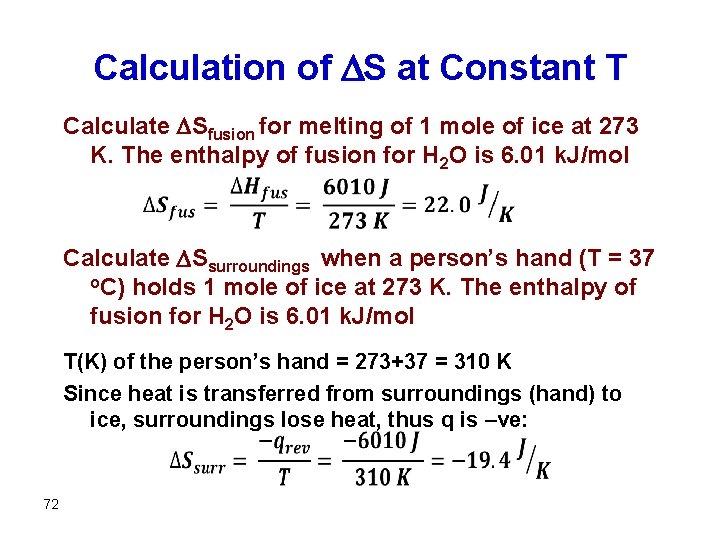
Calculation of S at Constant T Calculate Sfusion for melting of 1 mole of ice at 273 K. The enthalpy of fusion for H 2 O is 6. 01 k. J/mol Calculate Ssurroundings when a person’s hand (T = 37 o. C) holds 1 mole of ice at 273 K. The enthalpy of fusion for H 2 O is 6. 01 k. J/mol T(K) of the person’s hand = 273+37 = 310 K Since heat is transferred from surroundings (hand) to ice, surroundings lose heat, thus q is –ve: 72

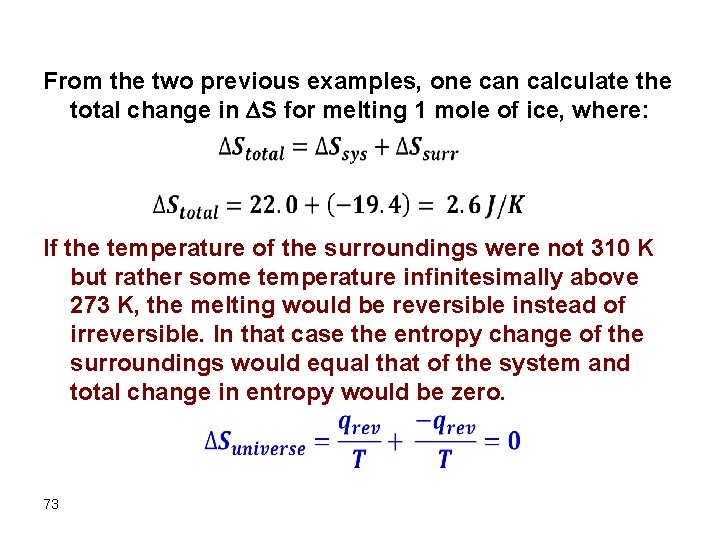
From the two previous examples, one can calculate the total change in S for melting 1 mole of ice, where: If the temperature of the surroundings were not 310 K but rather some temperature infinitesimally above 273 K, the melting would be reversible instead of irreversible. In that case the entropy change of the surroundings would equal that of the system and total change in entropy would be zero. 73

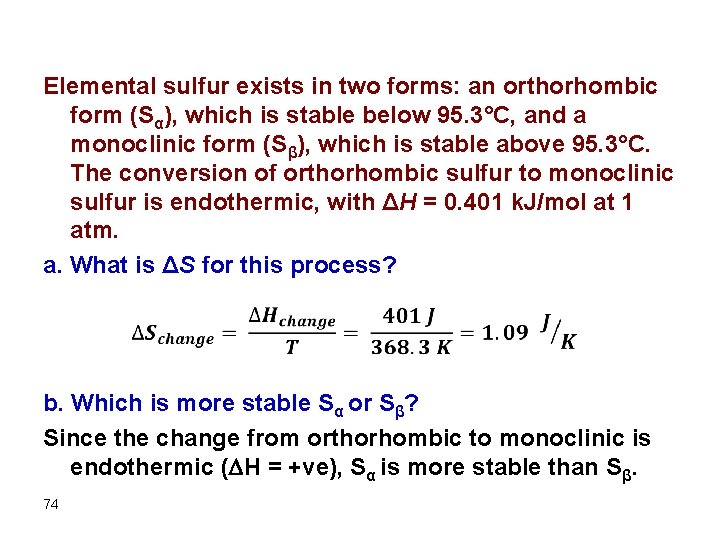
Elemental sulfur exists in two forms: an orthorhombic form (Sα), which is stable below 95. 3°C, and a monoclinic form (Sβ), which is stable above 95. 3°C. The conversion of orthorhombic sulfur to monoclinic sulfur is endothermic, with ΔH = 0. 401 k. J/mol at 1 atm. a. What is ΔS for this process? b. Which is more stable Sα or Sβ? Since the change from orthorhombic to monoclinic is endothermic ( H = +ve), Sα is more stable than Sβ. 74

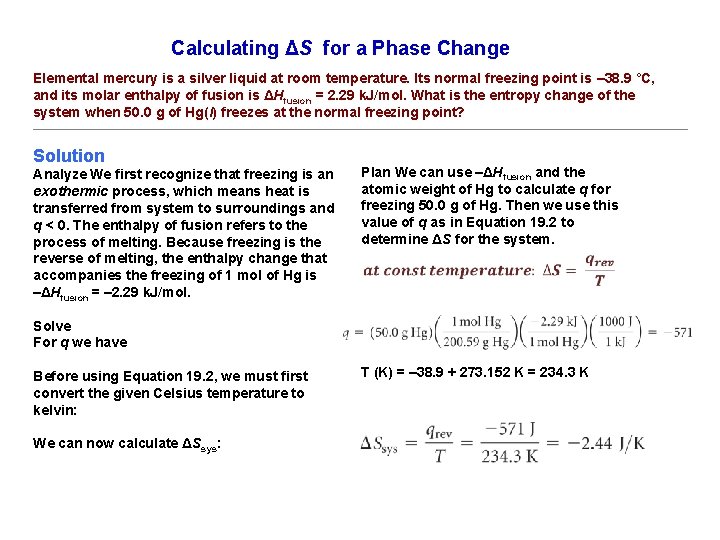
Calculating ΔS for a Phase Change Elemental mercury is a silver liquid at room temperature. Its normal freezing point is – 38. 9 °C, and its molar enthalpy of fusion is ΔHfusion = 2. 29 k. J/mol. What is the entropy change of the system when 50. 0 g of Hg(l) freezes at the normal freezing point? Solution Analyze We first recognize that freezing is an exothermic process, which means heat is transferred from system to surroundings and q < 0. The enthalpy of fusion refers to the process of melting. Because freezing is the reverse of melting, the enthalpy change that accompanies the freezing of 1 mol of Hg is –ΔHfusion = – 2. 29 k. J/mol. Plan We can use –ΔHfusion and the atomic weight of Hg to calculate q for freezing 50. 0 g of Hg. Then we use this value of q as in Equation 19. 2 to determine ΔS for the system. Solve For q we have Before using Equation 19. 2, we must first convert the given Celsius temperature to kelvin: We can now calculate ΔSsys: T (K) = – 38. 9 + 273. 152 K = 234. 3 K

The normal boiling point of ethanol, C 2 H 5 OH, is 78. 3 °C, and its molar enthalpy of vaporization is 38. 56 k. J/mol. What is the change in entropy in the system when 68. 3 g of C 2 H 5 OH(g) at 1 atm condenses to liquid at the normal boiling point? Solution Analyze We first recognize that condensation is an exothermic process, which means heat is transferred from system to surroundings and q < 0. The enthalpy of vaporization refers to the process of conversion of liquid to gas. –ΔHvap = – 38. 56 k. J/mol = ΔHcond. Plan We can use –ΔHvap and the formula weight of ethanol to calculate q for condensing 68. 3 g ethanol. Then we use this value of q as in Equation 19. 2 to determine ΔS for the system. For q we have : Before using Equation 19. 2, we must first convert the given Celsius temperature to kelvin: We can now calculate ΔSsys: T (K) = 78. 3+ 273. 15 K = 351. 45 K

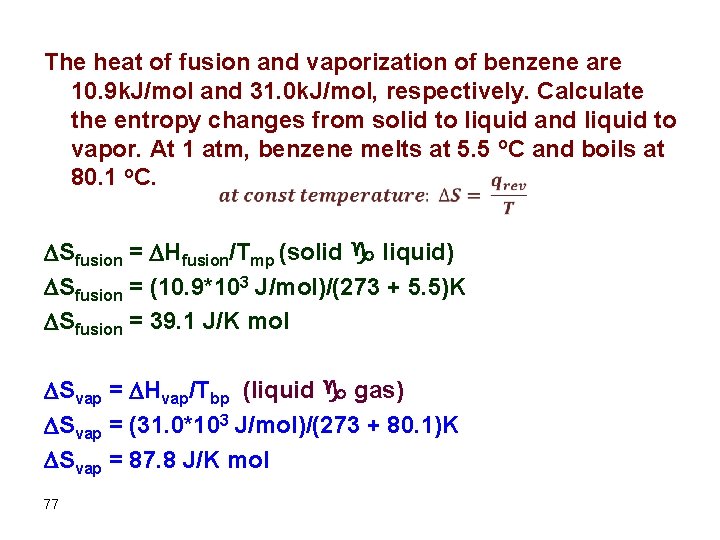
The heat of fusion and vaporization of benzene are 10. 9 k. J/mol and 31. 0 k. J/mol, respectively. Calculate the entropy changes from solid to liquid and liquid to vapor. At 1 atm, benzene melts at 5. 5 o. C and boils at 80. 1 o. C. Sfusion = Hfusion/Tmp (solid g liquid) Sfusion = (10. 9*103 J/mol)/(273 + 5. 5)K Sfusion = 39. 1 J/K mol Svap = Hvap/Tbp (liquid g gas) Svap = (31. 0*103 J/mol)/(273 + 80. 1)K Svap = 87. 8 J/K mol 77

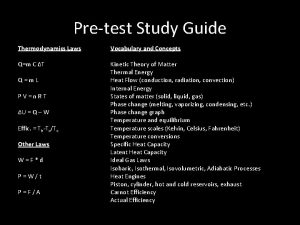
Calculate the missing data in the following table: Compound ΔHfus (k. J/mol) acetic acid 11. 7 CH 3 CN 8. 2 CH 4 0. 94 CH 3 OH formic acid 78 ΔSfus Melting [J/(mol·K)] Point (°C) 16. 6 35. 9 − 182. 5 18. 2 12. 7 45. 1 − 97. 7

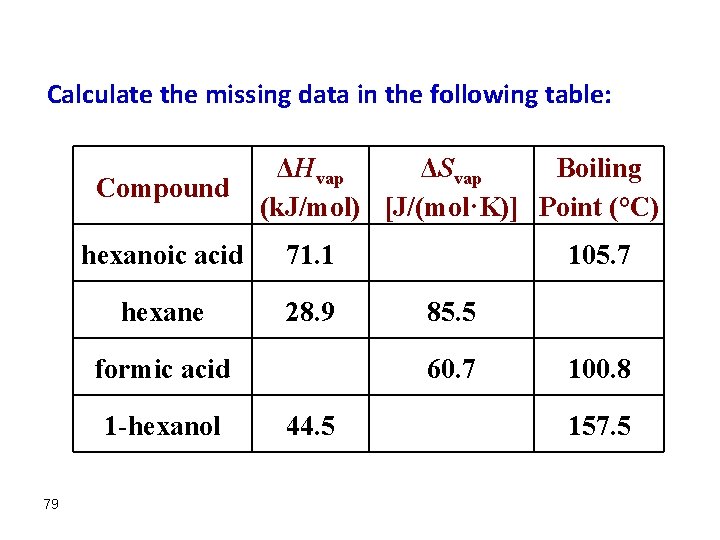
Calculate the missing data in the following table: Compound ΔHvap ΔSvap Boiling (k. J/mol) [J/(mol·K)] Point (°C) hexanoic acid 71. 1 hexane 28. 9 formic acid 1 -hexanol 79 105. 7 85. 5 60. 7 44. 5 100. 8 157. 5

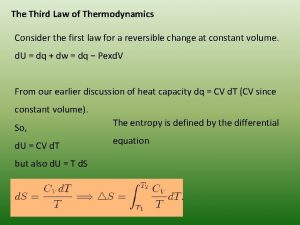
The Third Law of Thermodynamics If we decrease thermal energy of a system by lowering the temperature, the energy stored in translational, vibrational, and rotational motion decreases. As less energy is stored, the entropy of the system decreases. If we keep lowering the temperature, do we reach a state in which these motions are essentially shut down, a point described by a single microstate? This question is addressed by the third law of thermodynamics, which states that the entropy of a pure crystalline substance at absolute zero is zero {S(0 K) = 0}. 80

Consider a pure crystalline solid. At absolute zero, the individual atoms or molecules in the lattice would be perfectly ordered and in fixed positions. Because none of them would have thermal motion, there is only one possible microstate. As a result, Boltzmann equation becomes S = k ln W S = 0, since W = 1. As the temperature is increased from absolute zero, the atoms or molecules in the crystal gain energy in the form of vibrational motion about their lattice positions. This means that the entropy increases. What happens to the entropy, however, as we continue to heat the crystal? More energy results in more motions and thus entropy continues to increase. 81

What is the entropy of a system that has only one microstate? A. B. C. D. S = 0 S = 1 S > 0 S < 0

If you are told that the entropy of a system is zero, what do you know about the system? A. The system is a substance at the triple point. B. The system is pure liquid at 0 K (absolute zero). C. The contents of the system are at their standard states. D. The system must be a perfect crystal at 0 K (absolute zero).

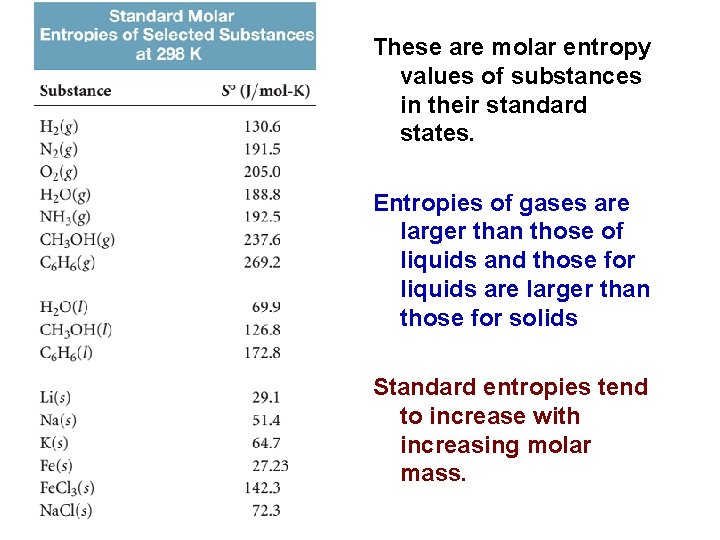
These are molar entropy values of substances in their standard states. Entropies of gases are larger than those of liquids and those for liquids are larger than those for solids Standard entropies tend to increase with increasing molar mass. 84

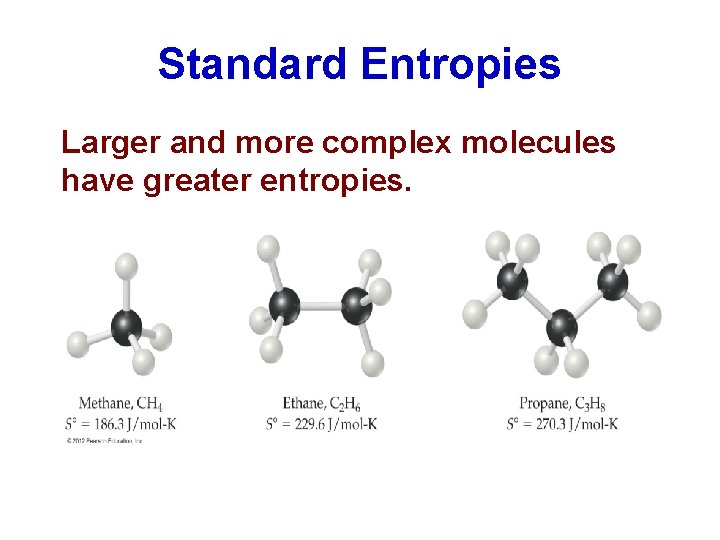
Standard Entropies Larger and more complex molecules have greater entropies.

We can make several observations about the S values: 1. Unlike enthalpies of formation, standard molar entropies of elements at the reference temperature of 298 K are not zero. 2. The standard molar entropies of gases are significantly greater than those of liquids and solids. 3. Standard molar entropies generally increase with increasing molar mass. 4. Standard molar entropies generally increase with increasing number of atoms in the formula of a substance. 86

Chemical Thermodynamics - 3 Lecture 25 87

ENTROPY CHANGES IN CHEMICAL REACTIONS We discussed earlier how calorimetry can be used to measure H for chemical reactions. No comparable methods exist for measuring S for a reaction. However, because third law establishes a zero point for entropy, we can use experimental measurements to determine the absolute value of the entropy, S for the initial and final states. Entropy changes for a reaction can be estimated in a manner analogous to that by which H is estimated: where n and m are the coefficients in the balanced chemical equation. 88

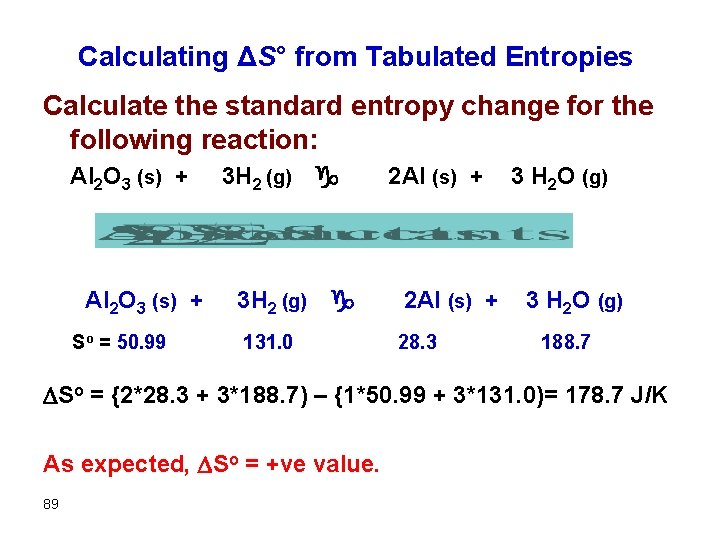
Calculating ΔS° from Tabulated Entropies Calculate the standard entropy change for the following reaction: Al 2 O 3 (s) + 3 H 2 (g) g So = 50. 99 131. 0 2 Al (s) + 3 H 2 O (g) 28. 3 188. 7 So = {2*28. 3 + 3*188. 7) – {1*50. 99 + 3*131. 0)= 178. 7 J/K As expected, So = +ve value. 89

Calculate the standard entropy change for the reaction: 2 Na. HCO 3 (s) g Na 2 CO 3 (s) + CO 2 (g) + H 2 O (g) Provided that S 0 (Na. HCO 3 (s)) = 155 J/mol K, S 0 (Na 2 CO 3 (s)) = 136 J/mol K, S 0 (CO 2 (g)) = 213. 6 J/mol K, and S 0 (H 2 O (g)) = 188. 7 J/mol K. Solution S 0 = Sn. S 0(products) - Sm. S 0(reactants) S 0 = {S 0 (Na 2 CO 3 (s)) + S 0 (CO 2 (g)) + S 0 (H 2 O (g))} – {2 S 0 (Na. HCO 3 (s))} S 0 = {136 + 213. 6 + 188. 7} – {2*155} = 228 J/K As expected, So = +ve value. 90

Calculate the standard entropy change (ΔS°) at 25°C for the reaction H 2(g)+O 2(g)⇌H 2 O 2(l). At 25°C, the absolute entropies of the products and reactants are S°(H 2 O 2) = 109. 6 J/(mol·K), S°(O 2) = 205. 2 J/(mol·K), and S°(H 2) = 130. 7 J/(mol·K). ΔS° = S°(H 2 O 2) − [S°(O 2 )+ S°(H 2)] ΔS° = [1× 109. 6] − {[1× 130. 7] + [1× 205. 2]} ΔS° = − 226. 3 J/K (per mole of H 2 O 2) As we might expect for a reaction in which 2 mol of gas are converted to 1 mol of a much more ordered liquid, ΔS° is very negative for this reaction. 91

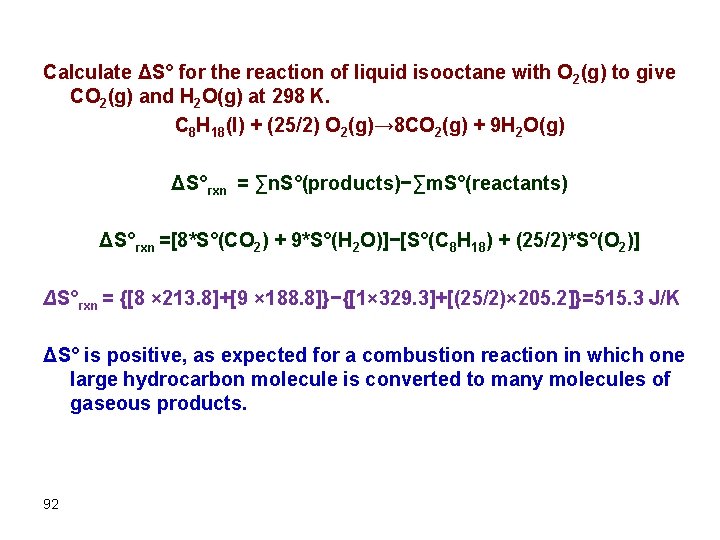
Calculate ΔS° for the reaction of liquid isooctane with O 2(g) to give CO 2(g) and H 2 O(g) at 298 K. C 8 H 18(l) + (25/2) O 2(g)→ 8 CO 2(g) + 9 H 2 O(g) ΔS°rxn = ∑n. S°(products)−∑m. S°(reactants) ΔS°rxn =[8*S°(CO 2) + 9*S°(H 2 O)]−[S°(C 8 H 18) + (25/2)*S°(O 2)] ΔS°rxn = {[8 × 213. 8]+[9 × 188. 8]}−{[1× 329. 3]+[(25/2)× 205. 2]}=515. 3 J/K ΔS° is positive, as expected for a combustion reaction in which one large hydrocarbon molecule is converted to many molecules of gaseous products. 92

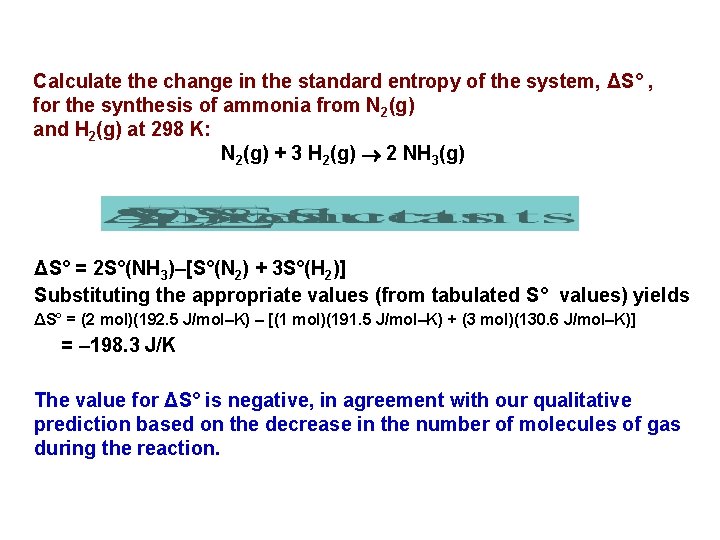
Calculate the change in the standard entropy of the system, ΔS° , for the synthesis of ammonia from N 2(g) and H 2(g) at 298 K: N 2(g) + 3 H 2(g) 2 NH 3(g) ΔS° = 2 S°(NH 3)–[S°(N 2) + 3 S°(H 2)] Substituting the appropriate values (from tabulated S° values) yields ΔS° = (2 mol)(192. 5 J/mol–K) – [(1 mol)(191. 5 J/mol–K) + (3 mol)(130. 6 J/mol–K)] = – 198. 3 J/K The value for ΔS° is negative, in agreement with our qualitative prediction based on the decrease in the number of molecules of gas during the reaction.

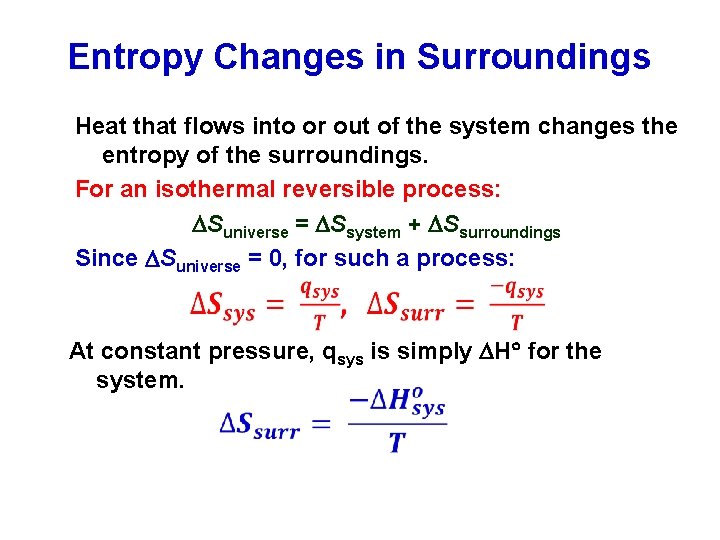
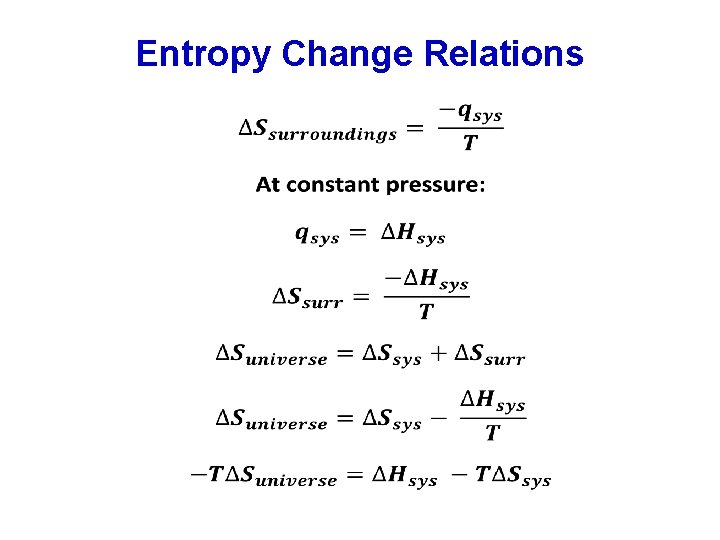
Entropy Changes in Surroundings Heat that flows into or out of the system changes the entropy of the surroundings. For an isothermal reversible process: Suniverse = Ssystem + Ssurroundings Since Suniverse = 0, for such a process: At constant pressure, qsys is simply H for the system.

Entropy Change Relations

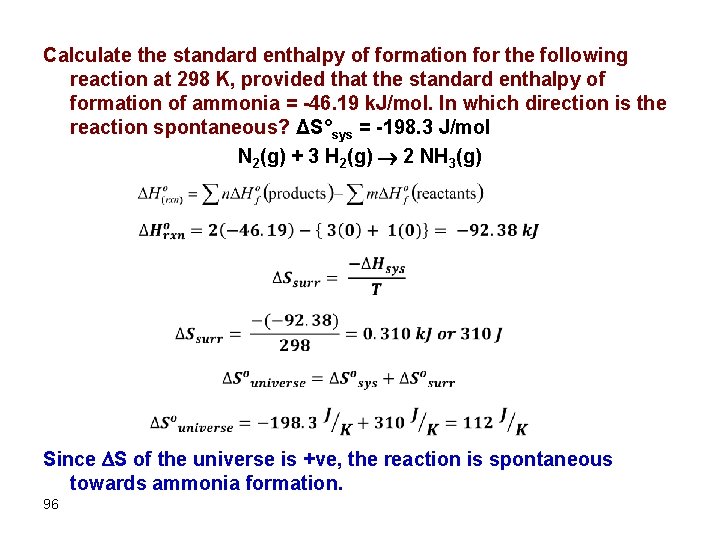
Calculate the standard enthalpy of formation for the following reaction at 298 K, provided that the standard enthalpy of formation of ammonia = -46. 19 k. J/mol. In which direction is the reaction spontaneous? ΔS°sys = -198. 3 J/mol N 2(g) + 3 H 2(g) 2 NH 3(g) Since S of the universe is +ve, the reaction is spontaneous towards ammonia formation. 96

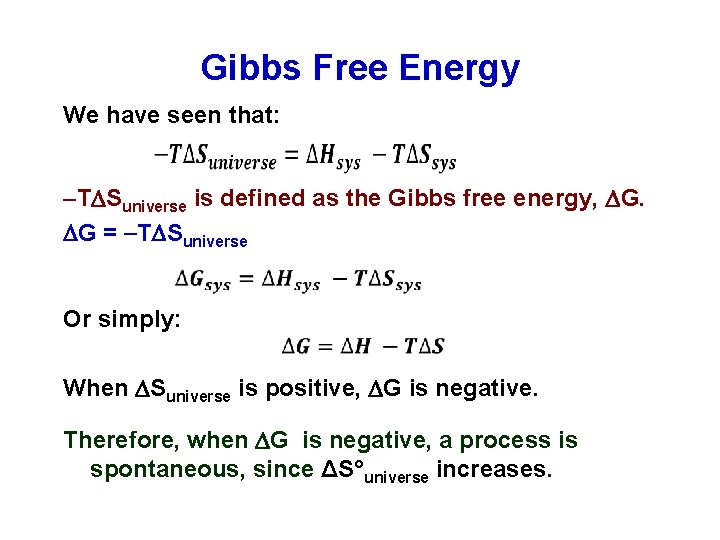
Gibbs Free Energy We have seen that: T Suniverse is defined as the Gibbs free energy, G. G = T Suniverse Or simply: When Suniverse is positive, G is negative. Therefore, when G is negative, a process is spontaneous, since ΔS°universe increases.

Gibbs Free Energy 1. If G is negative, the forward reaction is spontaneous. 2. If G is 0, the system is at equilibrium. 3. If G is positive, the reaction is spontaneous in the reverse direction.


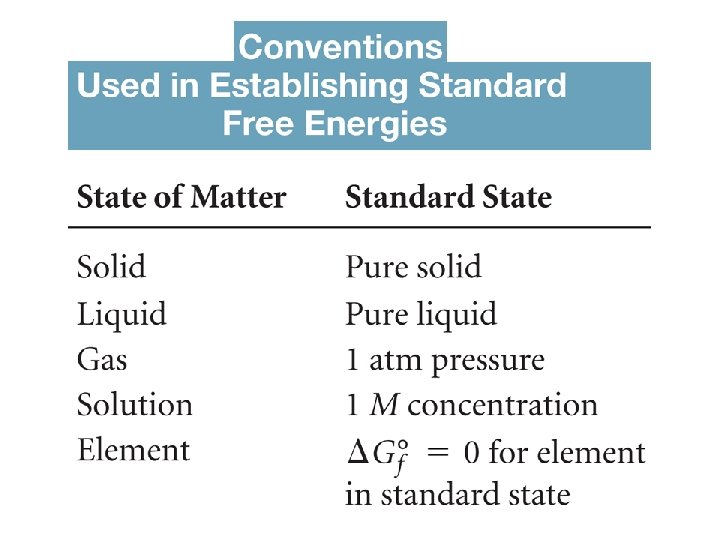
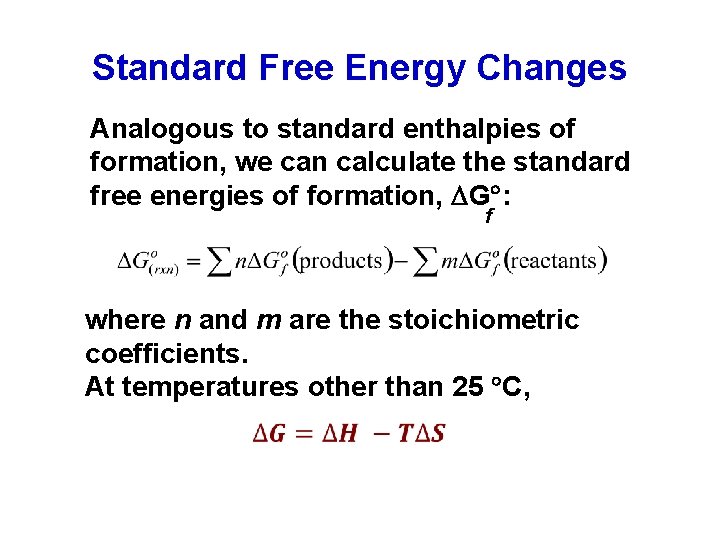
Standard Free Energy Changes Analogous to standard enthalpies of formation, we can calculate the standard free energies of formation, G : f where n and m are the stoichiometric coefficients. At temperatures other than 25 C,

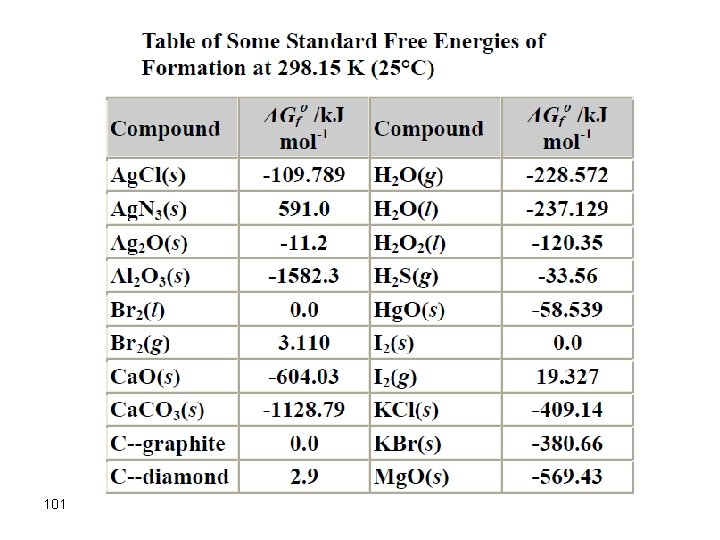
101

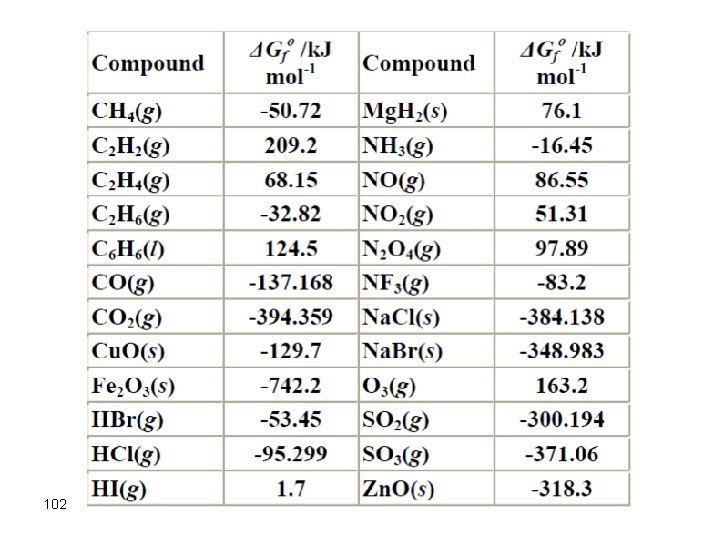
102

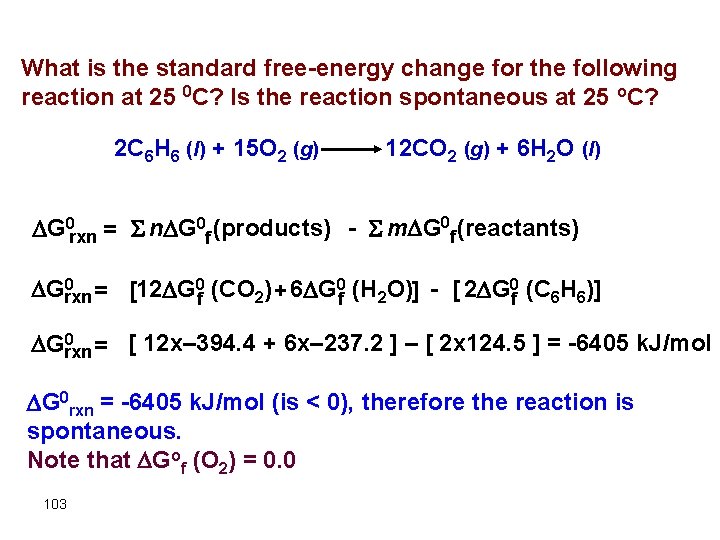
What is the standard free-energy change for the following reaction at 25 0 C? Is the reaction spontaneous at 25 o. C? 2 C 6 H 6 (l) + 15 O 2 (g) 12 CO 2 (g) + 6 H 2 O (l) G 0 rxn = S n G 0 f (products) - S m G 0 f (reactants) 0 Grxn = [12 G 0 f (CO 2) + 6 G 0 f (H 2 O)] - [ 2 G 0 f (C 6 H 6)] 0 Grxn = [ 12 x– 394. 4 + 6 x– 237. 2 ] – [ 2 x 124. 5 ] = -6405 k. J/mol G 0 rxn = -6405 k. J/mol (is < 0), therefore the reaction is spontaneous. Note that Gof (O 2) = 0. 0 103

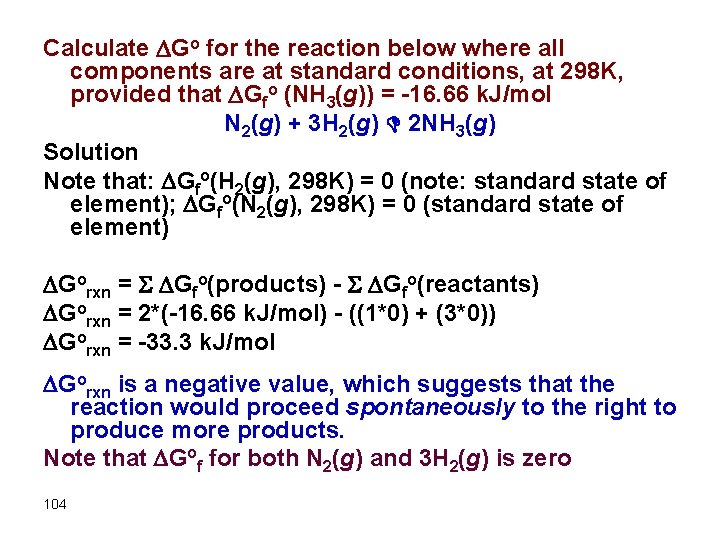
Calculate Go for the reaction below where all components are at standard conditions, at 298 K, provided that Gfo (NH 3(g)) = -16. 66 k. J/mol N 2(g) + 3 H 2(g) D 2 NH 3(g) Solution Note that: Gfo(H 2(g), 298 K) = 0 (note: standard state of element); Gfo(N 2(g), 298 K) = 0 (standard state of element) Gorxn = S Gfo(products) - S Gfo(reactants) Gorxn = 2*(-16. 66 k. J/mol) - ((1*0) + (3*0)) Gorxn = -33. 3 k. J/mol Gorxn is a negative value, which suggests that the reaction would proceed spontaneously to the right to produce more products. Note that Gof for both N 2(g) and 3 H 2(g) is zero 104

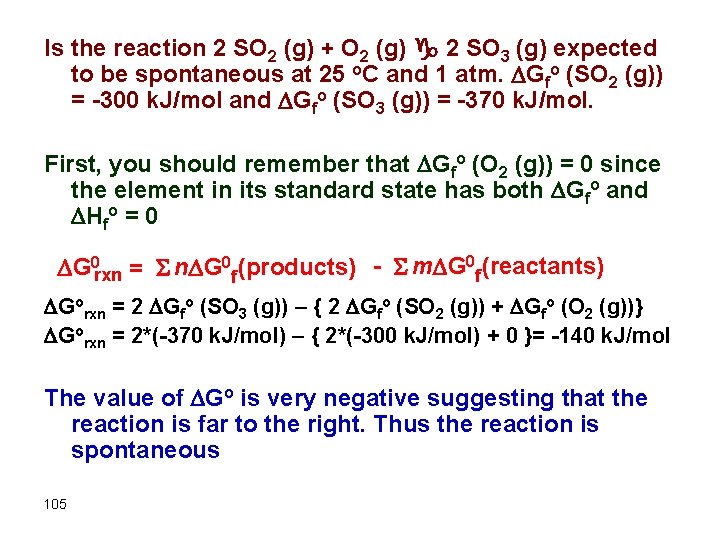
Is the reaction 2 SO 2 (g) + O 2 (g) g 2 SO 3 (g) expected to be spontaneous at 25 o. C and 1 atm. Gfo (SO 2 (g)) = -300 k. J/mol and Gfo (SO 3 (g)) = -370 k. J/mol. First, you should remember that Gfo (O 2 (g)) = 0 since the element in its standard state has both Gfo and Hfo = 0 G 0 rxn = S n G 0 f (products) - S m G 0 f (reactants) Gorxn = 2 Gfo (SO 3 (g)) – { 2 Gfo (SO 2 (g)) + Gfo (O 2 (g))} Gorxn = 2*(-370 k. J/mol) – { 2*(-300 k. J/mol) + 0 }= -140 k. J/mol The value of Go is very negative suggesting that the reaction is far to the right. Thus the reaction is spontaneous 105

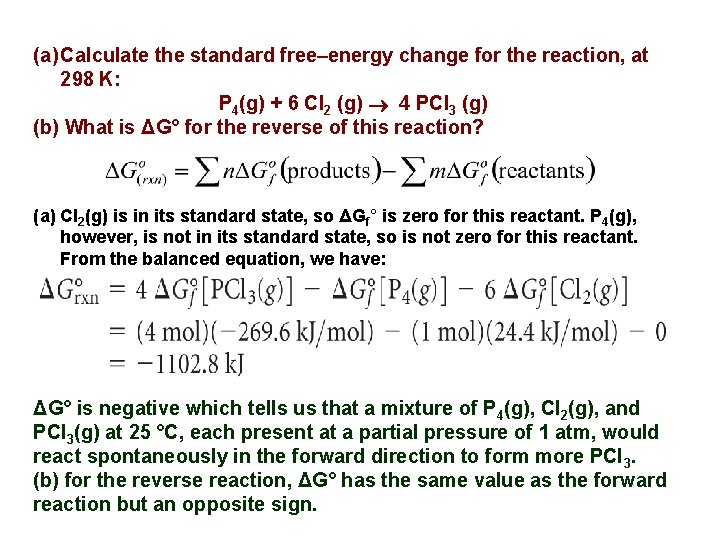
(a) Calculate the standard free–energy change for the reaction, at 298 K: P 4(g) + 6 Cl 2 (g) 4 PCl 3 (g) (b) What is ΔG° for the reverse of this reaction? (a) Cl 2(g) is in its standard state, so ΔGf° is zero for this reactant. P 4(g), however, is not in its standard state, so is not zero for this reactant. From the balanced equation, we have: ΔG° is negative which tells us that a mixture of P 4(g), Cl 2(g), and PCl 3(g) at 25 °C, each present at a partial pressure of 1 atm, would react spontaneously in the forward direction to form more PCl 3. (b) for the reverse reaction, ΔG° has the same value as the forward reaction but an opposite sign.

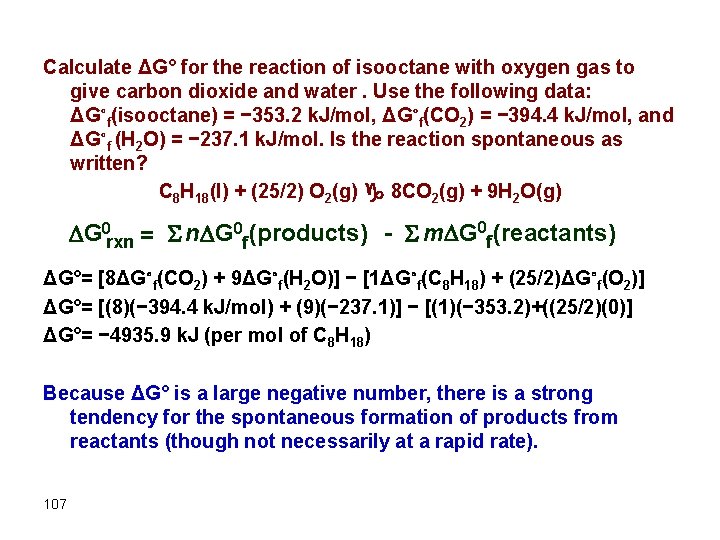
Calculate ΔG° for the reaction of isooctane with oxygen gas to give carbon dioxide and water. Use the following data: ΔG∘f(isooctane) = − 353. 2 k. J/mol, ΔG∘f(CO 2) = − 394. 4 k. J/mol, and ΔG∘f (H 2 O) = − 237. 1 k. J/mol. Is the reaction spontaneous as written? C 8 H 18(l) + (25/2) O 2(g) g 8 CO 2(g) + 9 H 2 O(g) G 0 rxn = S n G 0 f (products) - S m G 0 f (reactants) ΔG°= [8ΔG∘f(CO 2) + 9ΔG∘f(H 2 O)] − [1ΔG∘f(C 8 H 18) + (25/2)ΔG∘f(O 2)] ΔG°= [(8)(− 394. 4 k. J/mol) + (9)(− 237. 1)] − [(1)(− 353. 2)+((25/2)(0)] ΔG°= − 4935. 9 k. J (per mol of C 8 H 18) Because ΔG° is a large negative number, there is a strong tendency for the spontaneous formation of products from reactants (though not necessarily at a rapid rate). 107

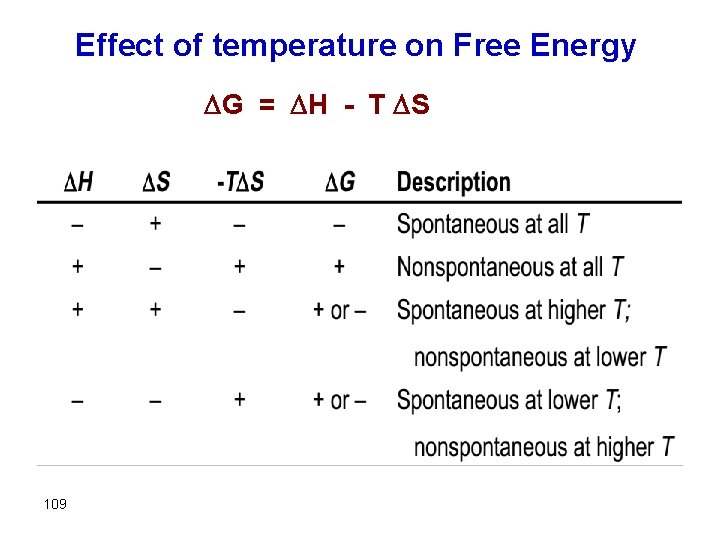
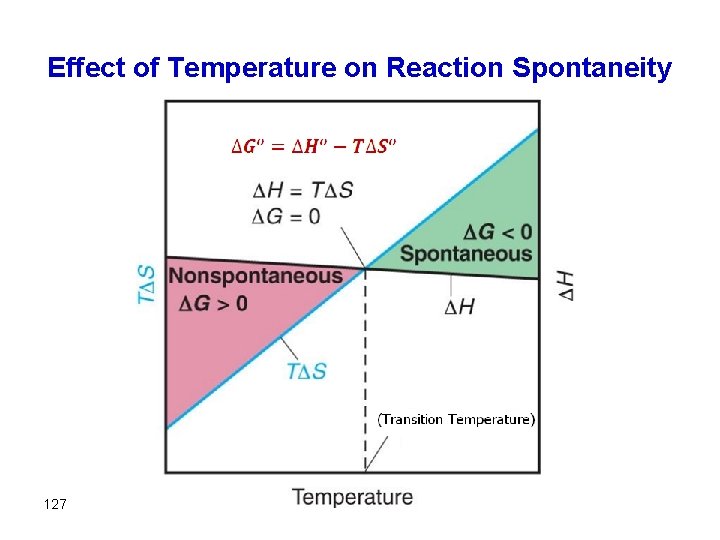
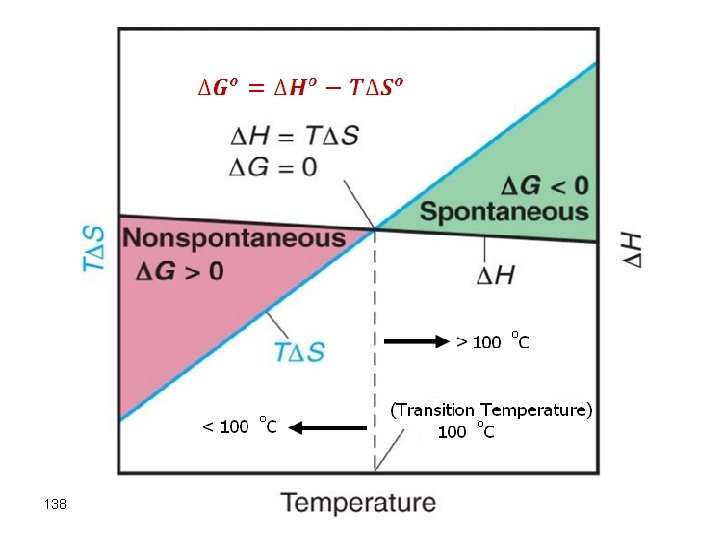
Free Energy and Temperature There are two parts in the free energy equation: ΔG° = ΔH°−TΔS° H — the enthalpy term T S — the entropy term The temperature dependence of free energy then comes from the entropy term.

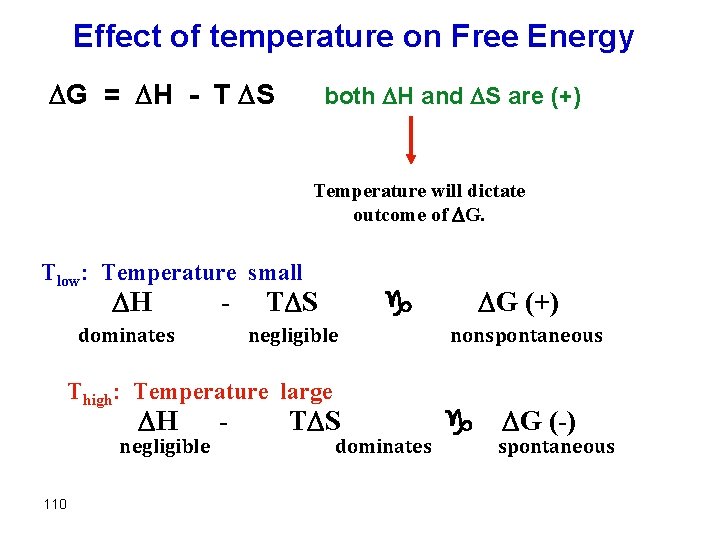
Effect of temperature on Free Energy G = H - T S 109

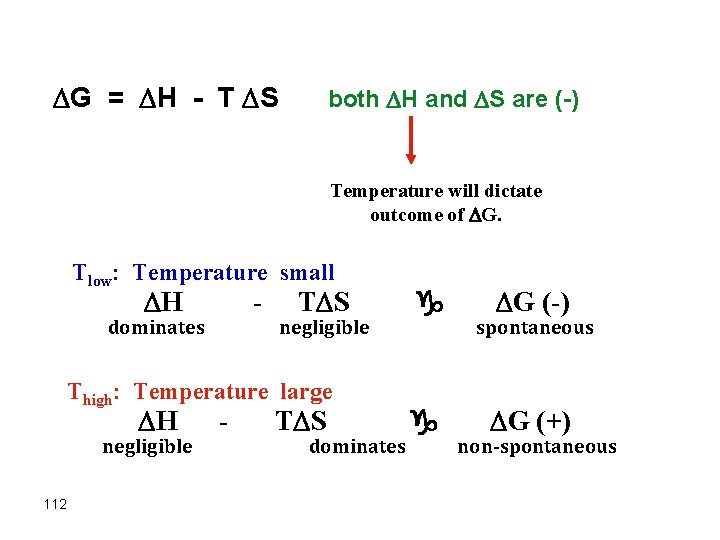
Effect of temperature on Free Energy G = H - T S both H and S are (+) Temperature will dictate outcome of G. Tlow: Temperature small H - dominates negligible Thigh: Temperature large H negligible 110 - G (+) g T S dominates nonspontaneous g G (-) spontaneous

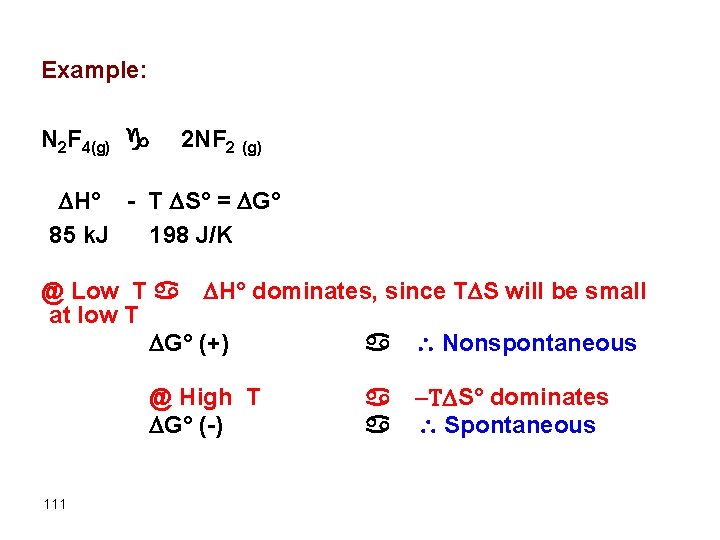
Example: N 2 F 4(g) g 2 NF 2 (g) H° - T S° = G° 85 k. J 198 J/K @ Low T a H° dominates, since T S will be small at low T G° (+) a Nonspontaneous @ High T G° (-) 111 a T S° dominates a Spontaneous

G = H - T S both H and S are (-) Temperature will dictate outcome of G. Tlow: Temperature small H - dominates T S negligible Thigh: Temperature large H negligible 112 - T S dominates g g G (-) spontaneous G (+) non-spontaneous

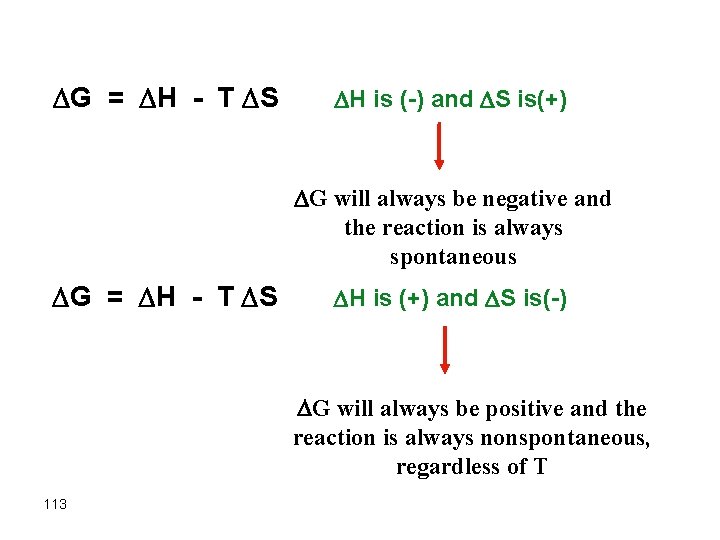
G = H - T S H is (-) and S is(+) G will always be negative and the reaction is always spontaneous G = H - T S H is (+) and S is(-) G will always be positive and the reaction is always nonspontaneous, regardless of T 113

Can you predict the sign of H and S for each case? . 114

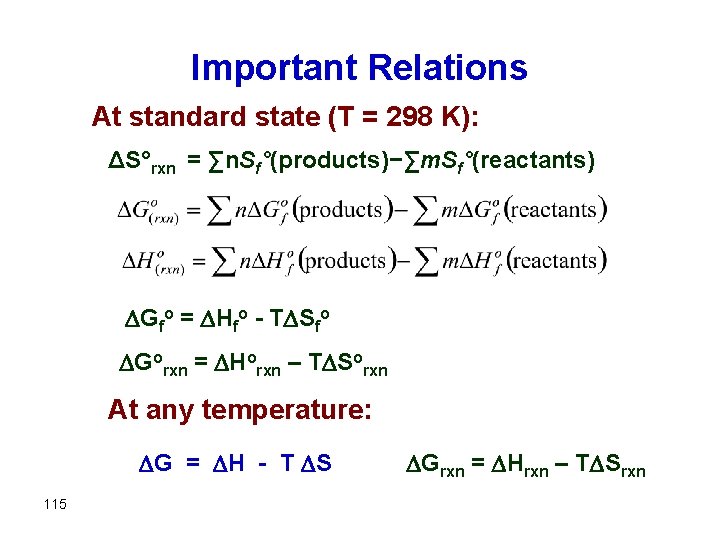
Important Relations At standard state (T = 298 K): ΔS°rxn = ∑n. Sf°(products)−∑m. Sf°(reactants) Gfo = Hfo - T Sfo Gorxn = Horxn – T Sorxn At any temperature: G = H - T S 115 Grxn = Hrxn – T Srxn

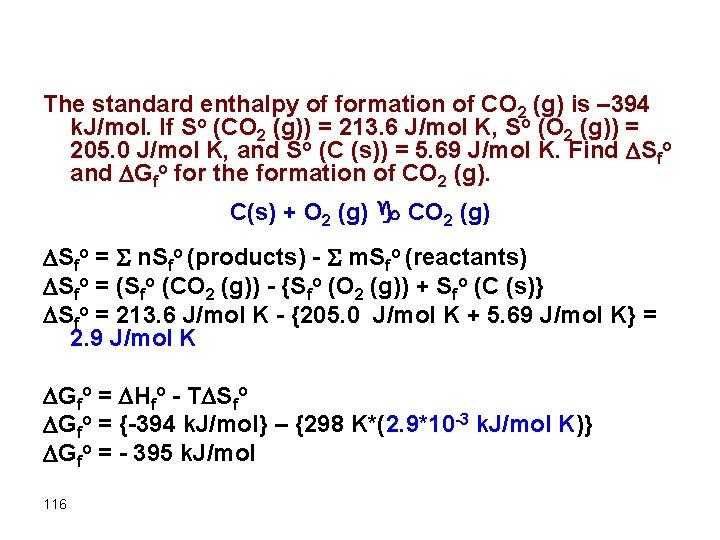
The standard enthalpy of formation of CO 2 (g) is – 394 k. J/mol. If So (CO 2 (g)) = 213. 6 J/mol K, So (O 2 (g)) = 205. 0 J/mol K, and So (C (s)) = 5. 69 J/mol K. Find Sfo and Gfo for the formation of CO 2 (g). C(s) + O 2 (g) g CO 2 (g) Sfo = S n. Sfo (products) - S m. Sfo (reactants) Sfo = (Sfo (CO 2 (g)) - {Sfo (O 2 (g)) + Sfo (C (s)} Sfo = 213. 6 J/mol K - {205. 0 J/mol K + 5. 69 J/mol K} = 2. 9 J/mol K Gfo = Hfo - T Sfo Gfo = {-394 k. J/mol} – {298 K*(2. 9*10 -3 k. J/mol K)} Gfo = - 395 k. J/mol 116

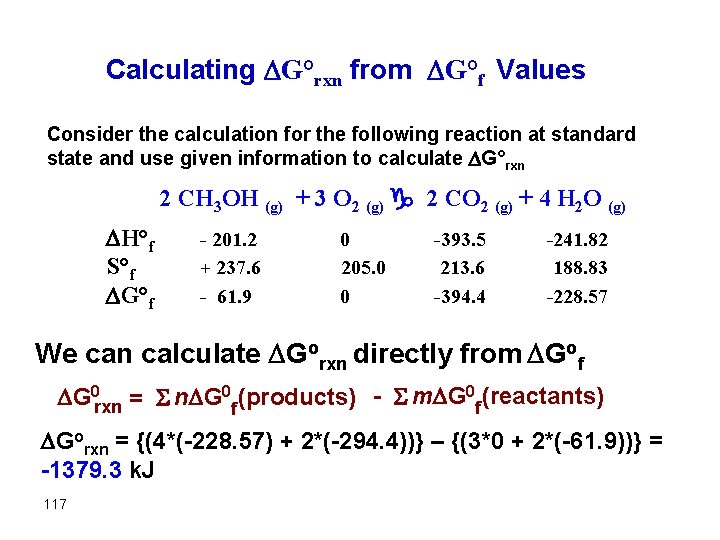
Calculating G°rxn from G°f Values Consider the calculation for the following reaction at standard state and use given information to calculate G°rxn 2 CH 3 OH (g) + 3 O 2 (g) g 2 CO 2 (g) + 4 H 2 O (g) H°f S°f G°f - 201. 2 + 237. 6 - 61. 9 0 205. 0 0 -393. 5 213. 6 -394. 4 -241. 82 188. 83 -228. 57 We can calculate Gorxn directly from Gof G 0 rxn = S n G 0 f (products) - S m G 0 f (reactants) Gorxn = {(4*(-228. 57) + 2*(-294. 4))} – {(3*0 + 2*(-61. 9))} = -1379. 3 k. J 117

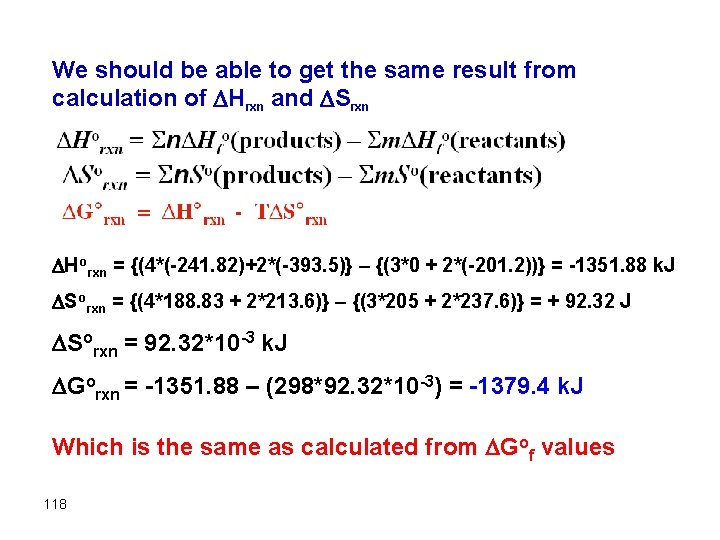
We should be able to get the same result from calculation of Hrxn and Srxn Horxn = {(4*(-241. 82)+2*(-393. 5)} – {(3*0 + 2*(-201. 2))} = -1351. 88 k. J Sorxn = {(4*188. 83 + 2*213. 6)} – {(3*205 + 2*237. 6)} = + 92. 32 J Sorxn = 92. 32*10 -3 k. J Gorxn = -1351. 88 – (298*92. 32*10 -3) = -1379. 4 k. J Which is the same as calculated from Gof values 118

Calculate the standard free–energy change for the formation of NO(g) from N 2(g) and O 2(g) at 298 K: N 2(g) + O 2(g) 2 NO(g) given that ΔH° = 180. 7 k. J and ΔS° = 24. 7 J/K. Is the reaction spontaneous under these conditions? Because ΔG° is positive, the reaction is not spontaneous under standard conditions, (298 K).

Calculate the standard free-energy change (ΔG°) at 25°C for the reaction H 2(g)+O 2(g)⇌H 2 O 2(l). At 25°C, if the standard enthalpy change (ΔH°) is − 187. 78 k. J/mol, and ΔS°= - 226. 3 J/K per mol of H 2 O 2, is the reaction spontaneous as written? . ΔG° = ΔH°−TΔS° ΔG° =− 187. 78 k. J − (298. 15 K)[− 0. 226. 3 k. J] ΔG° = − 187. 78 + 67. 47 = − 120. 31 k. J/mol The negative value of ΔG° indicates that the reaction is spontaneous as written. The enthalpy term dominates, indicating that the strength of the bonds formed in the product more than compensates for the unfavorable ΔS° term and for the energy needed to break bonds in the reactants. 120

In the following reaction at 298 K: C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(l) ΔH° = – 2220 k. J (a) Predict whether ΔG° for this reaction is more negative or less negative than ΔH°. (b) Use data from Appendix C to calculate ΔG° for the reaction at 298 K. Is your prediction from part (a) correct? ΔG° = ΔH° – TΔS° a. To determine whether ΔG° is more negative or less negative than ΔH°, we need to determine the sign of the term TΔS°. Because T is the absolute temperature, 298 K, it is always a positive number. We can predict the sign of ΔS° by looking at the reaction, where it is clear that ΔS° is negative, and the term (– TΔS°) should be +ve. This means that ΔG° should be less negative than ΔH°. b. (b) As we predicted, ΔGo is less negative than ΔH° because of the decrease in entropy during the reaction.

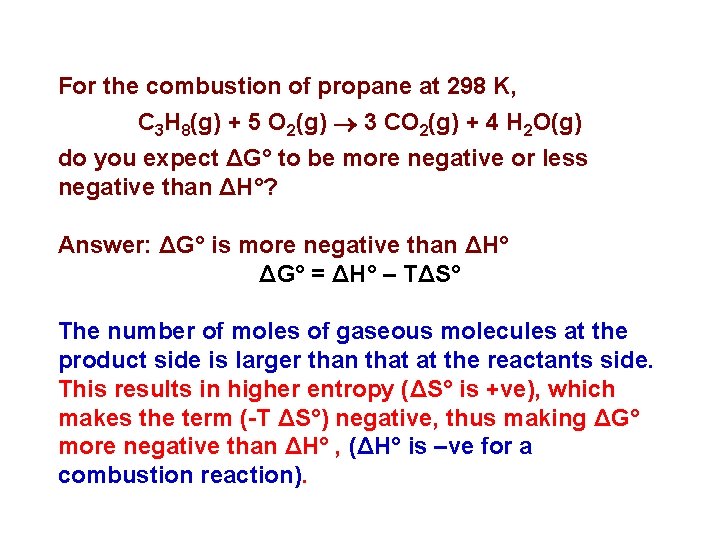
For the combustion of propane at 298 K, C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(g) do you expect ΔG° to be more negative or less negative than ΔH°? Answer: ΔG° is more negative than ΔH° ΔG° = ΔH° – TΔS° The number of moles of gaseous molecules at the product side is larger than that at the reactants side. This results in higher entropy (ΔS° is +ve), which makes the term (-T ΔS°) negative, thus making ΔG° more negative than ΔH° , (ΔH° is –ve for a combustion reaction).

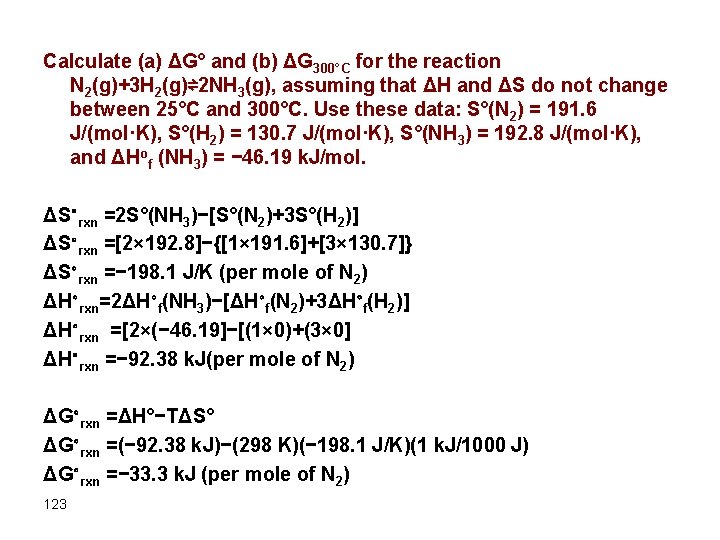
Calculate (a) ΔG° and (b) ΔG 300°C for the reaction N 2(g)+3 H 2(g)⇌2 NH 3(g), assuming that ΔH and ΔS do not change between 25°C and 300°C. Use these data: S°(N 2) = 191. 6 J/(mol·K), S°(H 2) = 130. 7 J/(mol·K), S°(NH 3) = 192. 8 J/(mol·K), and ΔHof (NH 3) = − 46. 19 k. J/mol. ΔS∘rxn =2 S°(NH 3)−[S°(N 2)+3 S°(H 2)] ΔS∘rxn =[2× 192. 8]−{[1× 191. 6]+[3× 130. 7]} ΔS∘rxn =− 198. 1 J/K (per mole of N 2) ΔH∘rxn=2ΔH∘f(NH 3)−[ΔH∘f(N 2)+3ΔH∘f(H 2)] ΔH∘rxn =[2×(− 46. 19]−[(1× 0)+(3× 0] ΔH∘rxn =− 92. 38 k. J(per mole of N 2) ΔG∘rxn =ΔH°−TΔS° ΔG∘rxn =(− 92. 38 k. J)−(298 K)(− 198. 1 J/K)(1 k. J/1000 J) ΔG∘rxn =− 33. 3 k. J (per mole of N 2) 123

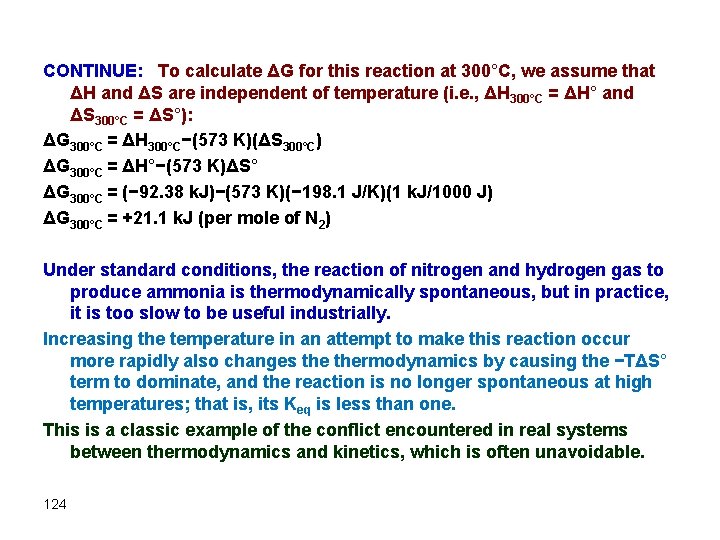
CONTINUE: To calculate ΔG for this reaction at 300°C, we assume that ΔH and ΔS are independent of temperature (i. e. , ΔH 300°C = ΔH° and ΔS 300°C = ΔS°): ΔG 300°C = ΔH 300°C−(573 K)(ΔS 300°C) ΔG 300°C = ΔH°−(573 K)ΔS° ΔG 300°C = (− 92. 38 k. J)−(573 K)(− 198. 1 J/K)(1 k. J/1000 J) ΔG 300°C = +21. 1 k. J (per mole of N 2) Under standard conditions, the reaction of nitrogen and hydrogen gas to produce ammonia is thermodynamically spontaneous, but in practice, it is too slow to be useful industrially. Increasing the temperature in an attempt to make this reaction occur more rapidly also changes thermodynamics by causing the −TΔS° term to dominate, and the reaction is no longer spontaneous at high temperatures; that is, its Keq is less than one. This is a classic example of the conflict encountered in real systems between thermodynamics and kinetics, which is often unavoidable. 124

Chemical Thermodynamics - 3 Lecture 26 125

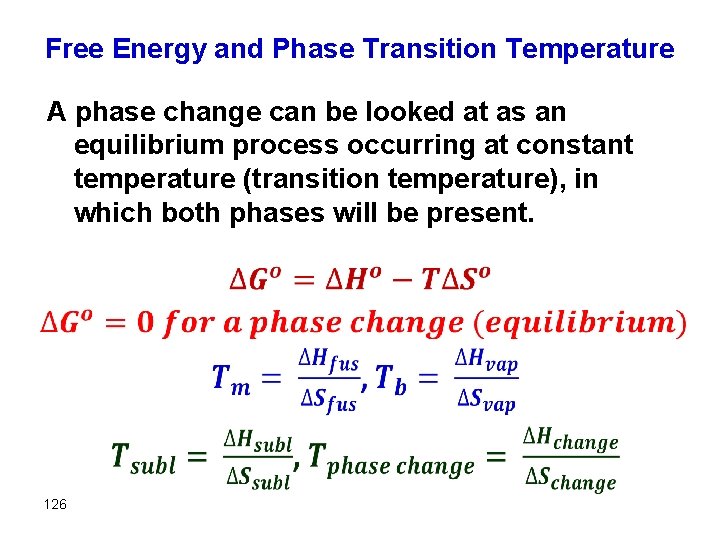
Free Energy and Phase Transition Temperature A phase change can be looked at as an equilibrium process occurring at constant temperature (transition temperature), in which both phases will be present. 126

Effect of Temperature on Reaction Spontaneity 127

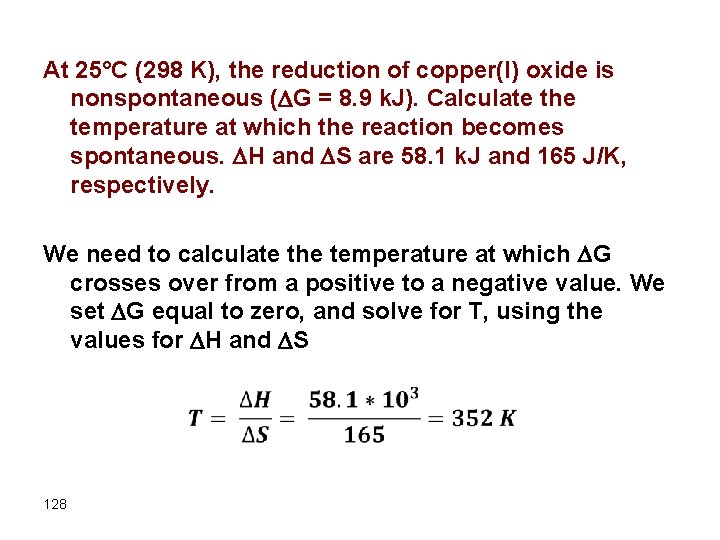
At 25°C (298 K), the reduction of copper(I) oxide is nonspontaneous ( G = 8. 9 k. J). Calculate the temperature at which the reaction becomes spontaneous. H and S are 58. 1 k. J and 165 J/K, respectively. We need to calculate the temperature at which G crosses over from a positive to a negative value. We set G equal to zero, and solve for T, using the values for H and S 128

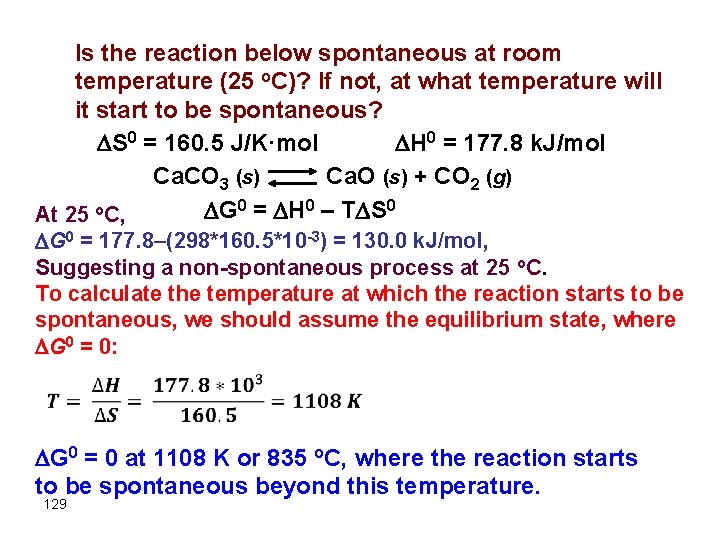
Is the reaction below spontaneous at room temperature (25 o. C)? If not, at what temperature will it start to be spontaneous? S 0 = 160. 5 J/K·mol H 0 = 177. 8 k. J/mol Ca. CO 3 (s) Ca. O (s) + CO 2 (g) G 0 = H 0 – T S 0 At 25 o. C, G 0 = 177. 8–(298*160. 5*10 -3) = 130. 0 k. J/mol, Suggesting a non-spontaneous process at 25 o. C. To calculate the temperature at which the reaction starts to be spontaneous, we should assume the equilibrium state, where G 0 = 0: G 0 = 0 at 1108 K or 835 o. C, where the reaction starts to be spontaneous beyond this temperature. 129

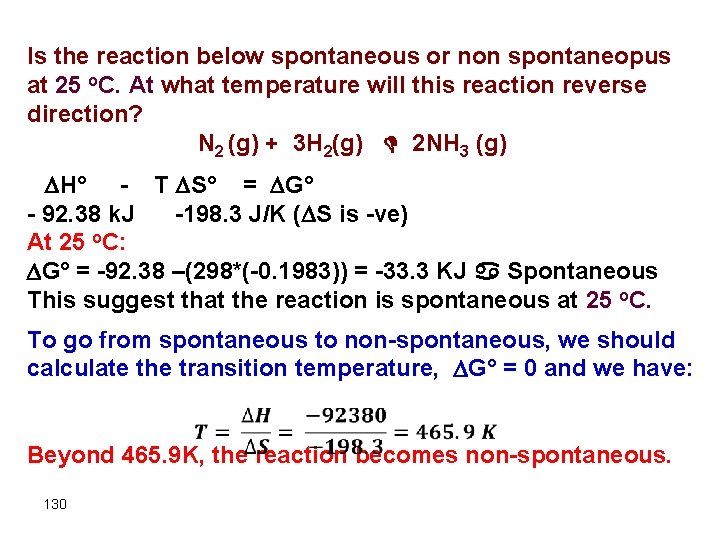
Is the reaction below spontaneous or non spontaneopus at 25 o. C. At what temperature will this reaction reverse direction? N 2 (g) + 3 H 2(g) D 2 NH 3 (g) H° - T S° = G° - 92. 38 k. J -198. 3 J/K ( S is -ve) At 25 o. C: G° = -92. 38 –(298*(-0. 1983)) = -33. 3 KJ a Spontaneous This suggest that the reaction is spontaneous at 25 o. C. To go from spontaneous to non-spontaneous, we should calculate the transition temperature, G° = 0 and we have: Beyond 465. 9 K, the reaction becomes non-spontaneous. 130

Relating ΔG to a Phase Change at Equilibrium (a) Write the chemical equation that defines the normal boiling point of liquid carbon tetrachloride, CCl 4(l). (b) What is the value of ΔG° for the equilibrium in part (a)? ΔG° = ΔH° – TΔS° (a) The chemical equation is the change of state from liquid to gas. CCl 4(l)DCCl 4(g) (b) At equilibrium, ΔG = 0. In any normal boiling–point equilibrium, both liquid and vapor are in their standard state of pure liquid and vapor at 1 atm. Consequently, Q = 1, ln Q = 0, and ΔG = ΔG° for this process. We conclude that ΔG° = 0 for the equilibrium representing the normal boiling point of any liquid. (We would also find that ΔG° = 0 for the equilibria relevant to normal melting points and normal sublimation points. )

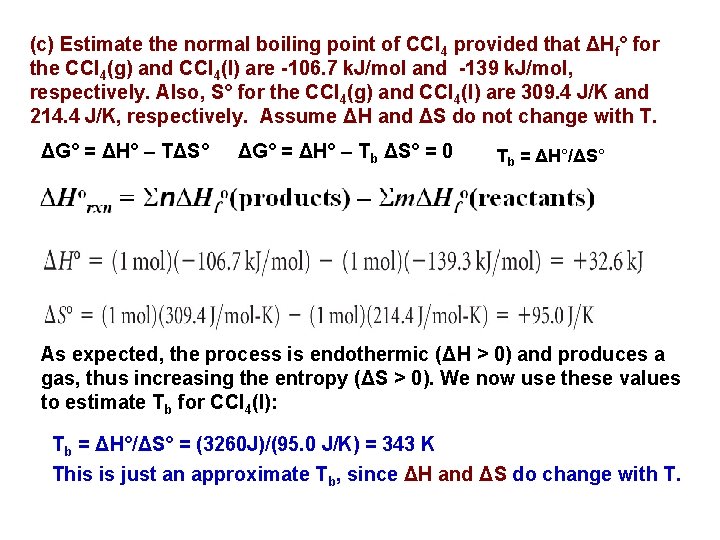
(c) Estimate the normal boiling point of CCl 4 provided that ΔHf° for the CCl 4(g) and CCl 4(l) are -106. 7 k. J/mol and -139 k. J/mol, respectively. Also, S° for the CCl 4(g) and CCl 4(l) are 309. 4 J/K and 214. 4 J/K, respectively. Assume ΔH and ΔS do not change with T. ΔG° = ΔH° – TΔS° ΔG° = ΔH° – Tb ΔS° = 0 Tb = ΔH°/ΔS° As expected, the process is endothermic (ΔH > 0) and produces a gas, thus increasing the entropy (ΔS > 0). We now use these values to estimate Tb for CCl 4(l): Tb = ΔH°/ΔS° = (3260 J)/(95. 0 J/K) = 343 K This is just an approximate Tb, since ΔH and ΔS do change with T.

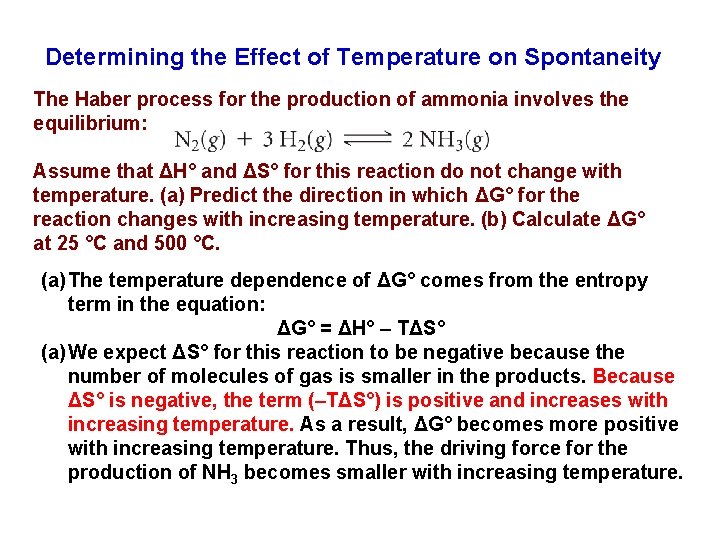
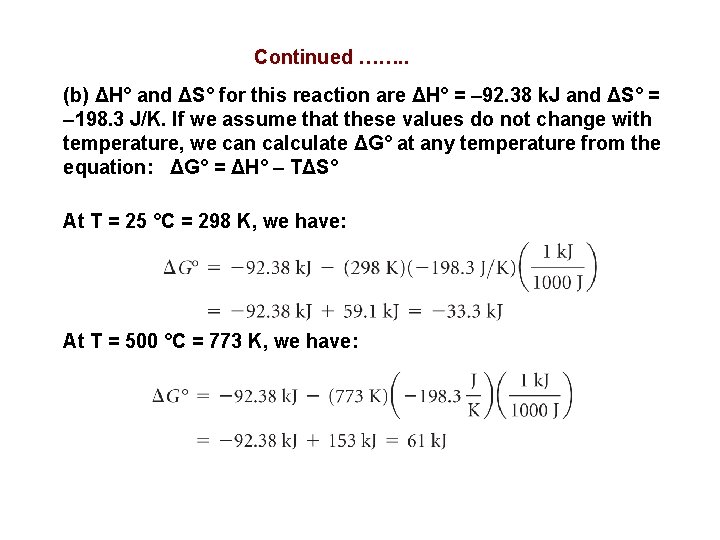
Determining the Effect of Temperature on Spontaneity The Haber process for the production of ammonia involves the equilibrium: Assume that ΔH° and ΔS° for this reaction do not change with temperature. (a) Predict the direction in which ΔG° for the reaction changes with increasing temperature. (b) Calculate ΔG° at 25 °C and 500 °C. (a) The temperature dependence of ΔG° comes from the entropy term in the equation: ΔG° = ΔH° – TΔS° (a) We expect ΔS° for this reaction to be negative because the number of molecules of gas is smaller in the products. Because ΔS° is negative, the term (–TΔS°) is positive and increases with increasing temperature. As a result, ΔG° becomes more positive with increasing temperature. Thus, the driving force for the production of NH 3 becomes smaller with increasing temperature.

Continued ……. . (b) ΔH° and ΔS° for this reaction are ΔH° = – 92. 38 k. J and ΔS° = – 198. 3 J/K. If we assume that these values do not change with temperature, we can calculate ΔG° at any temperature from the equation: ΔG° = ΔH° – TΔS° At T = 25 °C = 298 K, we have: At T = 500 °C = 773 K, we have:

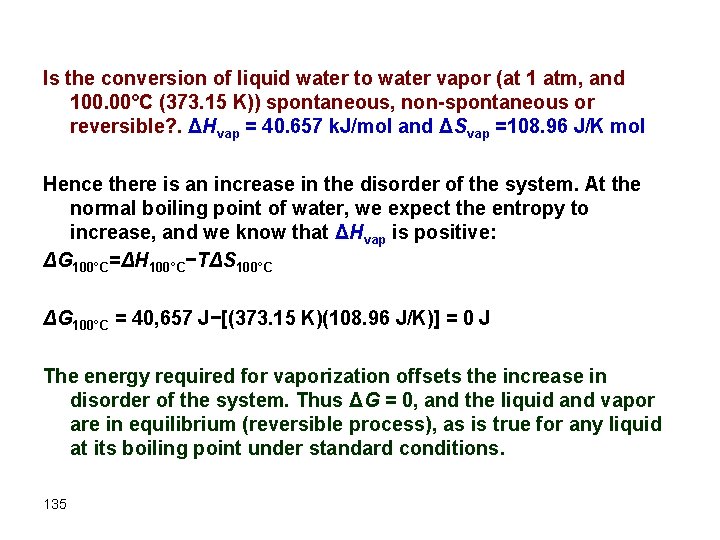
Is the conversion of liquid water to water vapor (at 1 atm, and 100. 00°C (373. 15 K)) spontaneous, non-spontaneous or reversible? . ΔHvap = 40. 657 k. J/mol and ΔSvap =108. 96 J/K mol Hence there is an increase in the disorder of the system. At the normal boiling point of water, we expect the entropy to increase, and we know that ΔHvap is positive: ΔG 100°C=ΔH 100°C−TΔS 100°C ΔG 100°C = 40, 657 J−[(373. 15 K)(108. 96 J/K)] = 0 J The energy required for vaporization offsets the increase in disorder of the system. Thus ΔG = 0, and the liquid and vapor are in equilibrium (reversible process), as is true for any liquid at its boiling point under standard conditions. 135

Is heating 1 mol of liquid water to 110°C will convert it to water vapor spontaneously or not, assuming that ΔH and ΔS do not change significantly with temperature? . ΔHvap = 40. 657 k. J/mol and ΔSvap =108. 96 J/K mol) ΔG 110°C = ΔH −TΔS ΔG 110°C = 40, 657 J−[(383. 15 K)(108. 96 J/K)] = − 1091 J At 110°C, ΔG < 0, and vaporization is predicted to occur spontaneously and irreversibly. Is heating 1 mol of liquid water to 90°C will convert it to water vapor spontaneously or not, assuming that ΔH and ΔS do not change significantly with temperature? . ΔG 90°C = ΔH −TΔS ΔG 90°C = 40, 657 J−[(363. 15 K)(108. 96 J/K)] = 1088 J At 90°C, ΔG > 0, and water does not spontaneously convert to water vapor. 136

At what temperature will liquid water be at equilibrium with water vapor? . Inserting the values of ΔH and ΔS into the definition of ΔG, setting ΔG = 0 (for a reversible equilibrium process), and solving for T: ΔG = ΔH −TΔS, since ΔG = 0 ΔH =TΔS 40, 657 J = T * (108. 96 J/K)=373. 15 K Thus ΔG = 0 at T = 373. 15 K (the normal boiling point) and 1 atm, which indicates that liquid water and water vapor are in equilibrium. At temperatures greater than 373. 15 K, ΔG is negative, and water evaporates spontaneously and irreversibly. Below 373. 15 K, ΔG is positive, and water does not evaporate spontaneously. Instead, water vapor at a temperature less than 373. 15 K and 1 atm will spontaneously and irreversibly 137 condense to liquid water.

138

Free Energy and Equilibrium Under any conditions, standard or nonstandard, the free energy change can be found from the following relation: G = G + RT ln Q Where Q is the reaction quotient, representing the ratio of products to reactants concentrations (each raised to the power equals the number of moles in the balanced chemical equation) (Under standard conditions, all concentrations are 1 M, so Q = 1 and ln Q = 0; the last term drops out and we have: G = G ).


141

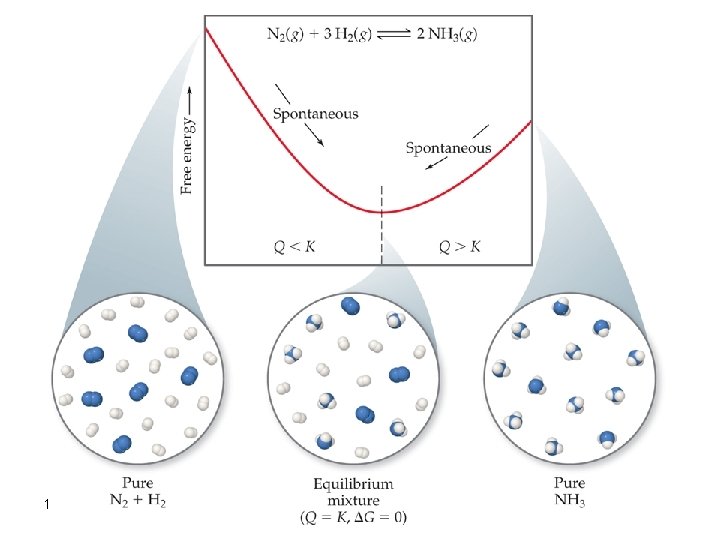
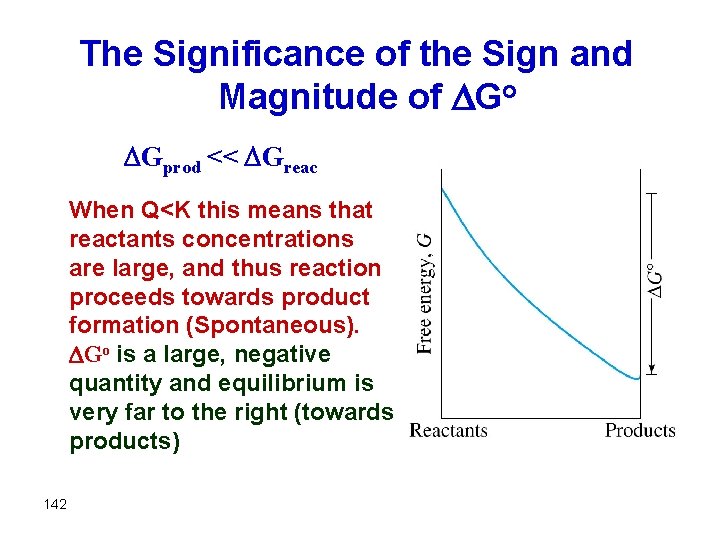
The Significance of the Sign and Magnitude of Go Gprod << Greac When Q<K this means that reactants concentrations are large, and thus reaction proceeds towards product formation (Spontaneous). Go is a large, negative quantity and equilibrium is very far to the right (towards products) 142

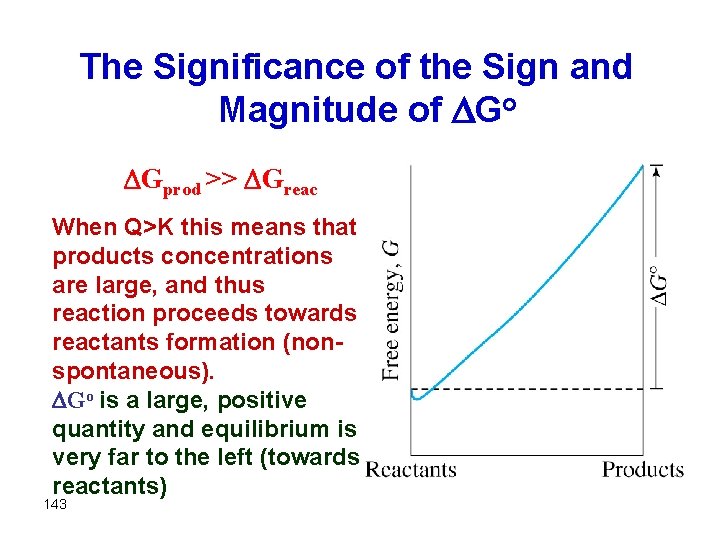
The Significance of the Sign and Magnitude of Go Gprod >> Greac When Q>K this means that products concentrations are large, and thus reaction proceeds towards reactants formation (nonspontaneous). Go is a large, positive quantity and equilibrium is very far to the left (towards reactants) 143


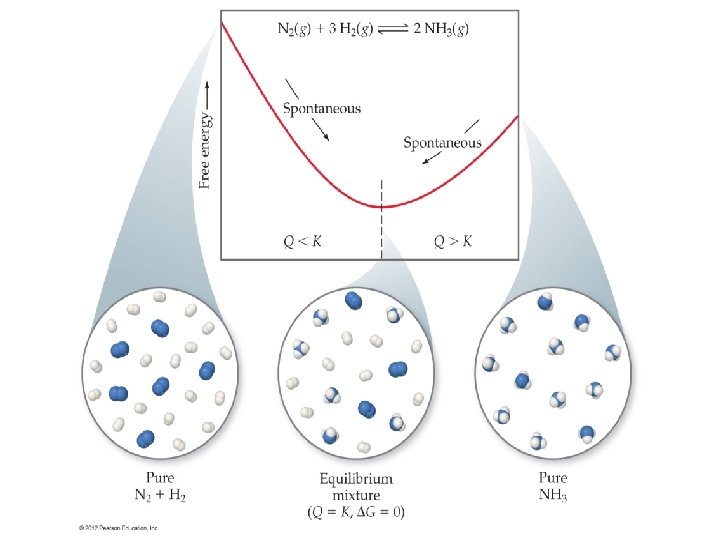
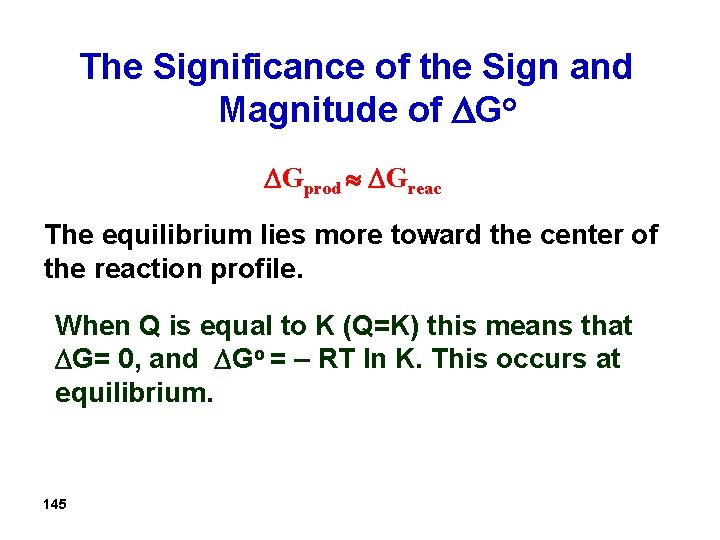
The Significance of the Sign and Magnitude of Go Gprod Greac The equilibrium lies more toward the center of the reaction profile. When Q is equal to K (Q=K) this means that G= 0, and Go = – RT ln K. This occurs at equilibrium. 145


147

148

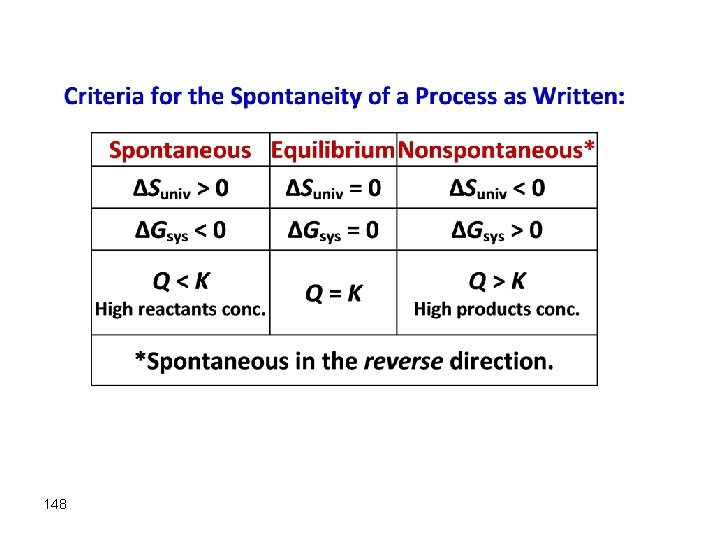
What is the criteria for spontaneity a. In terms of entropy and b. In terms of free energy? 1. 2. 3. 4. (a) The entropy of the universe increases and (b) the free energy of a system decreases at constant temperature. (a) The entropy of the system decreases and (b) the free energy of the universe increases at constant temperature. (a) The entropy of the system increases and (b) the free energy of the universe decreases at constant temperature. (a) The entropy of the universe decreases and (b) the free energy of a system increases at constant temperature.

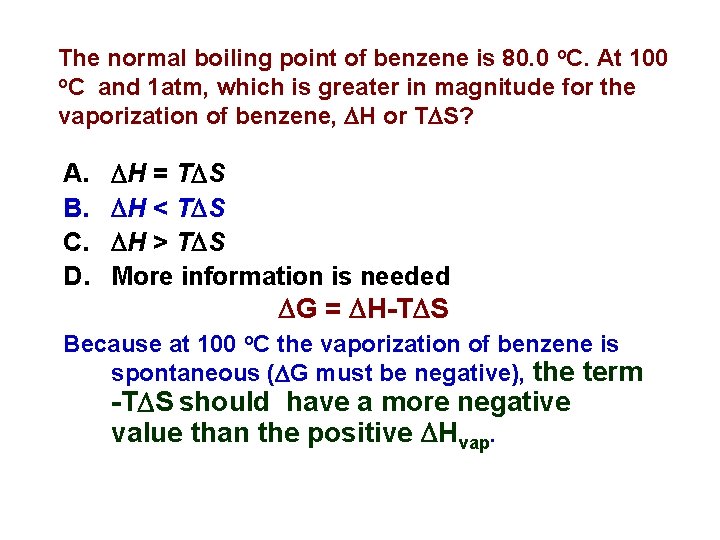
The normal boiling point of benzene is 80. 0 o. C. At 100 o. C and 1 atm, which is greater in magnitude for the vaporization of benzene, H or T S? A. B. C. D. H = T S H < T S H > T S More information is needed G = H-T S Because at 100 o. C the vaporization of benzene is spontaneous ( G must be negative), the term -T S should have a more negative value than the positive Hvap.

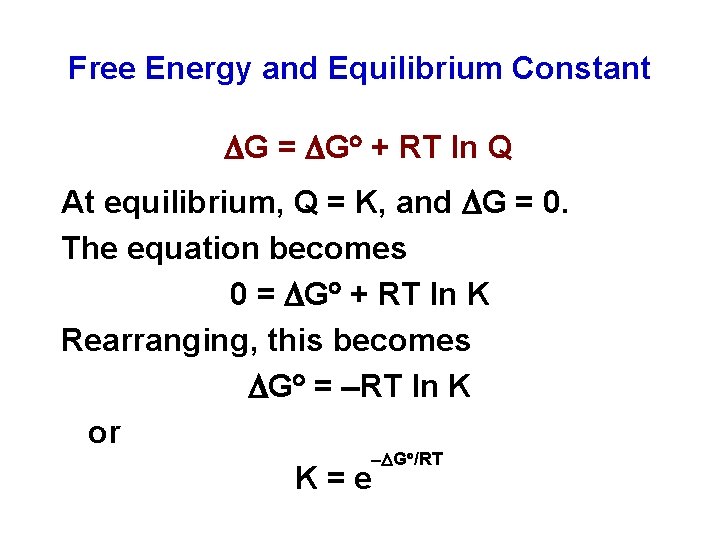
Free Energy and Equilibrium Constant G = G + RT ln Q At equilibrium, Q = K, and G = 0. The equation becomes 0 = G + RT ln K Rearranging, this becomes G = RT ln K or G /RT K = e

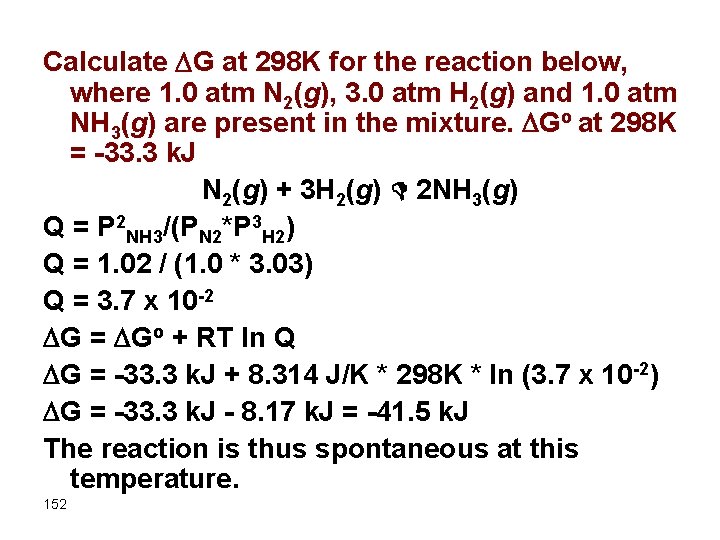
Calculate G at 298 K for the reaction below, where 1. 0 atm N 2(g), 3. 0 atm H 2(g) and 1. 0 atm NH 3(g) are present in the mixture. Go at 298 K = -33. 3 k. J N 2(g) + 3 H 2(g) D 2 NH 3(g) Q = P 2 NH 3/(PN 2*P 3 H 2) Q = 1. 02 / (1. 0 * 3. 03) Q = 3. 7 x 10 -2 G = Go + RT ln Q G = -33. 3 k. J + 8. 314 J/K * 298 K * ln (3. 7 x 10 -2) G = -33. 3 k. J - 8. 17 k. J = -41. 5 k. J The reaction is thus spontaneous at this temperature. 152

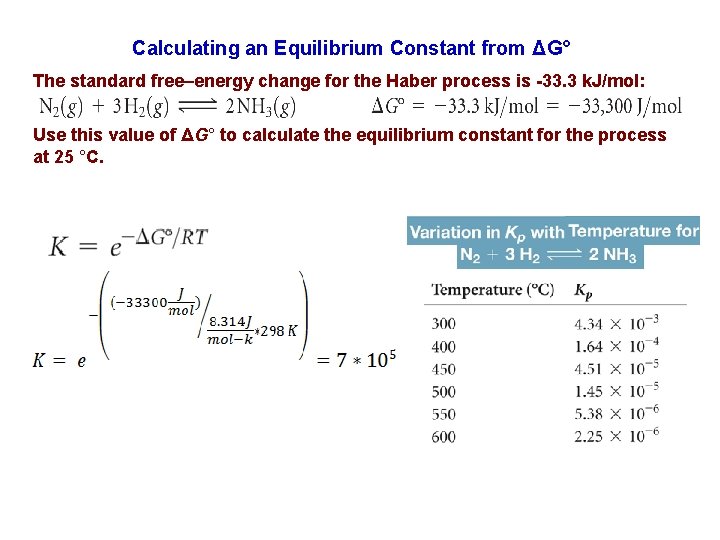
Calculate the equilibrium constant, Kp, at 25 o. C, for the reaction: 2 H 2 O (l) D 2 H 2 (g) + O 2 (g) Where Gof (H 2 O(l)) = -237. 2 k/mol, Gof (H 2(g)) = 0. 0 k/mol Gof (O 2(g)) = 0. 0 k/mol Gorxn = {(2*0. 0 + 1*0. 0)}–(2*(-237. 2))= +474. 4 k. J Gorxn = -RTln. Kp 474. 4*103 J/mol = - 8. 314 J/K mol*298 K ln Kp Kp = 7*10 -84 153

Calculating an Equilibrium Constant from ΔG° The standard free–energy change for the Haber process is -33. 3 k. J/mol: Use this value of ΔG° to calculate the equilibrium constant for the process at 25 °C.

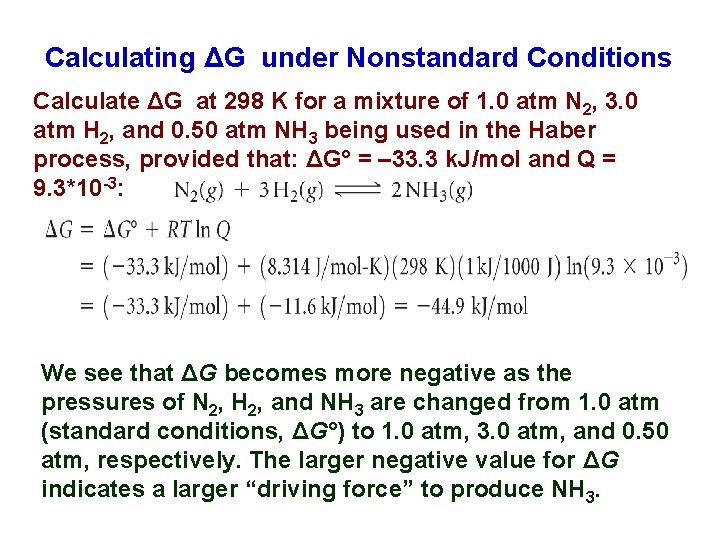
Calculating ΔG under Nonstandard Conditions Calculate ΔG at 298 K for a mixture of 1. 0 atm N 2, 3. 0 atm H 2, and 0. 50 atm NH 3 being used in the Haber process, provided that: ΔG° = – 33. 3 k. J/mol and Q = 9. 3*10 -3: We see that ΔG becomes more negative as the pressures of N 2, H 2, and NH 3 are changed from 1. 0 atm (standard conditions, ΔG°) to 1. 0 atm, 3. 0 atm, and 0. 50 atm, respectively. The larger negative value for ΔG indicates a larger “driving force” to produce NH 3.

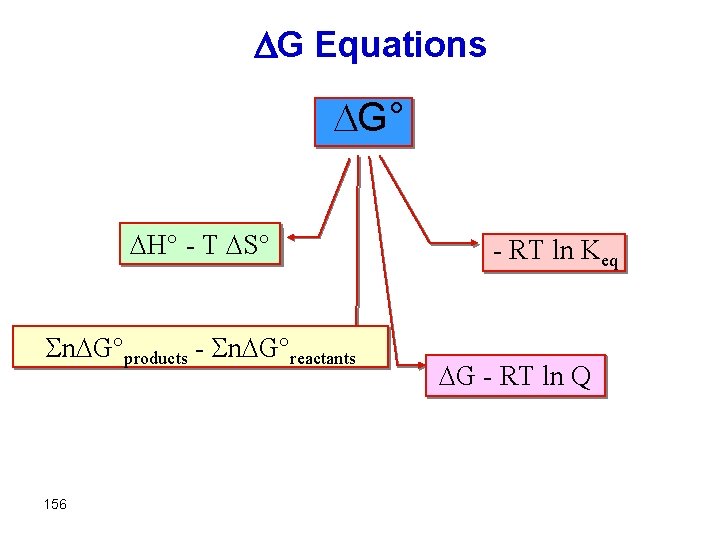
G Equations DG° DH° - T DS° Sn. DG°products - Sn. DG°reactants 156 - RT ln Keq DG - RT ln Q

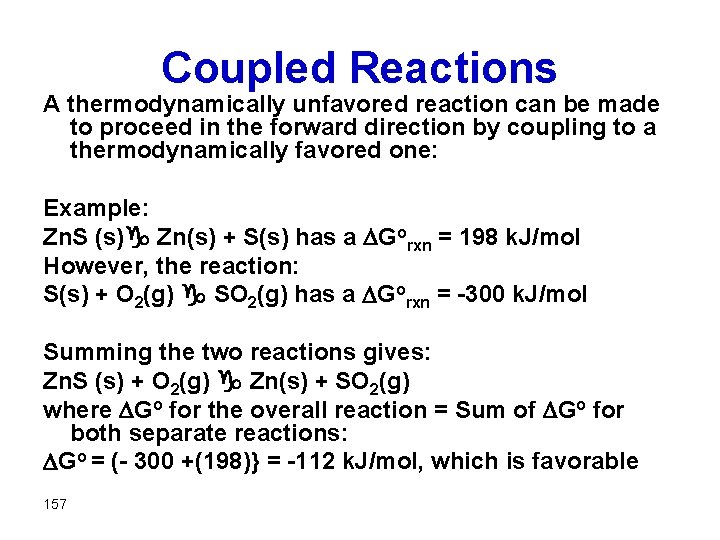
Coupled Reactions A thermodynamically unfavored reaction can be made to proceed in the forward direction by coupling to a thermodynamically favored one: Example: Zn. S (s)g Zn(s) + S(s) has a Gorxn = 198 k. J/mol However, the reaction: S(s) + O 2(g) g SO 2(g) has a Gorxn = -300 k. J/mol Summing the two reactions gives: Zn. S (s) + O 2(g) g Zn(s) + SO 2(g) where Go for the overall reaction = Sum of Go for both separate reactions: Go = (- 300 +(198)} = -112 k. J/mol, which is favorable 157

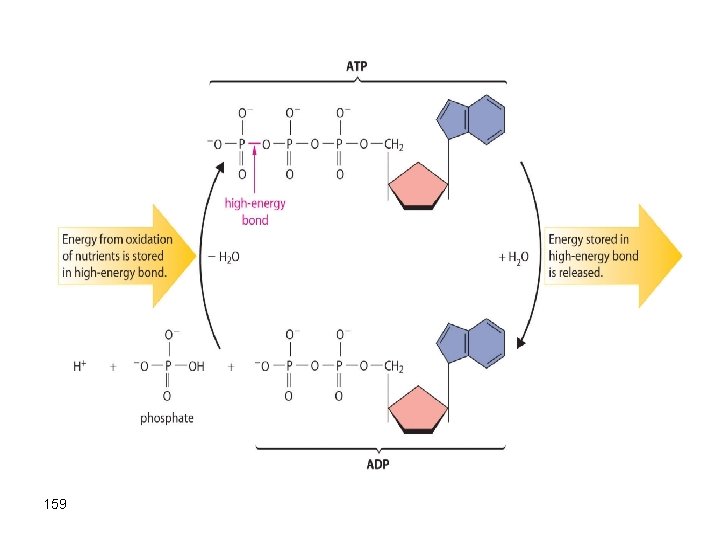
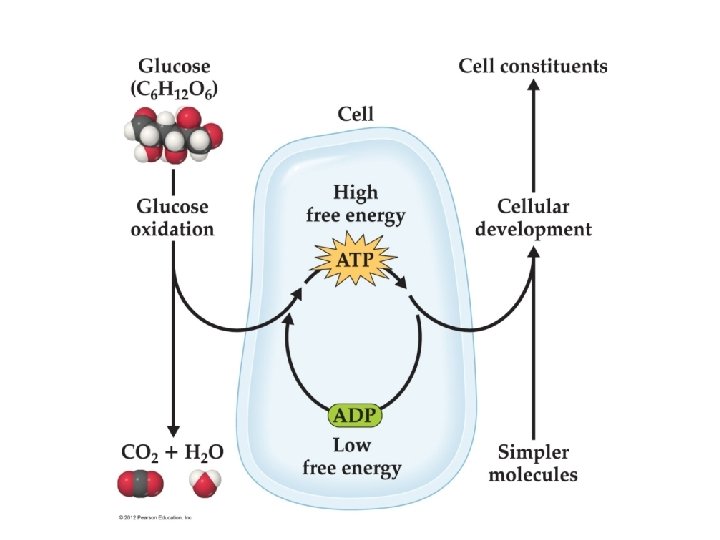
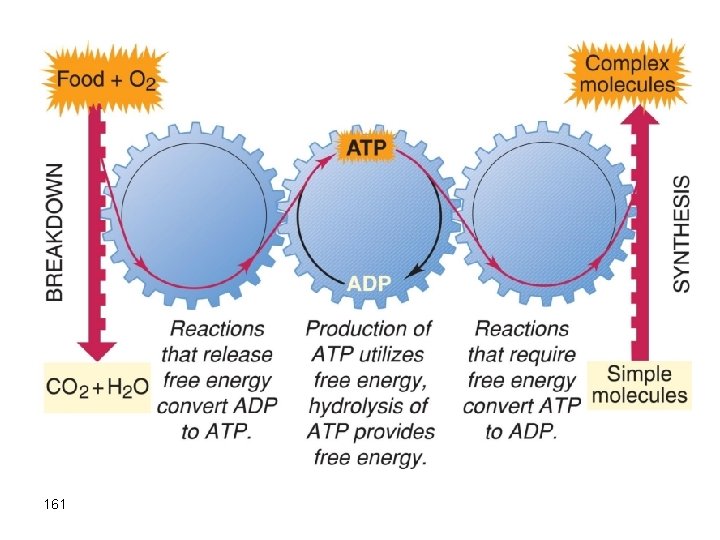
Chemistry and Life In the body, glucose is oxidized to CO 2 and water, and the energy released convert ADP (a low free energy molecule) into ATP (a high free energy molecule). ATP uses the free energy as an energy source for the conversion of simple molecules into more complicated building blocks needed for our well being. 158

159


161

This is the End of the Course Material Best Wishes 162
 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Chemical engineering thermodynamics 8th solution chapter 3
Chemical engineering thermodynamics 8th solution chapter 3 Introduction to chemical engineering thermodynamics
Introduction to chemical engineering thermodynamics Thermodynamics for chemical engineering
Thermodynamics for chemical engineering Chemical engineering thermodynamics 8th solution chapter 10
Chemical engineering thermodynamics 8th solution chapter 10 Equilibrium thermodynamics
Equilibrium thermodynamics 熱力
熱力 Chapter 6
Chapter 6 Chapter 6
Chapter 6 Trinitrogen monosulfide formula
Trinitrogen monosulfide formula Chapter 7 chemical formulas and chemical compounds test
Chapter 7 chemical formulas and chemical compounds test Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Isolated system
Isolated system Perpetual motion machine
Perpetual motion machine Internal energy formula
Internal energy formula Availability in thermodynamics
Availability in thermodynamics Steady flow energy equation thermodynamics
Steady flow energy equation thermodynamics Rigid tank thermodynamics
Rigid tank thermodynamics First law of td
First law of td 1st law of thermodynamics
1st law of thermodynamics Hot air balloon thermodynamics
Hot air balloon thermodynamics Joules experiment
Joules experiment Predicting spontaneity
Predicting spontaneity Third law of thermodynamics is depend on
Third law of thermodynamics is depend on Thermodynamics study guide
Thermodynamics study guide First law of thermodynamics for ideal gas
First law of thermodynamics for ideal gas Residual properties thermodynamics
Residual properties thermodynamics Work done in adiabatic process
Work done in adiabatic process Enthalpy of atomisation
Enthalpy of atomisation Heat transfer cengel
Heat transfer cengel Irreversible process in thermodynamics
Irreversible process in thermodynamics Free energy
Free energy Maxwell relation
Maxwell relation Rerational
Rerational Zeroth law of thermo
Zeroth law of thermo Control mass system
Control mass system Postech mse
Postech mse Thermodynamics ap chemistry
Thermodynamics ap chemistry Property tables thermodynamics
Property tables thermodynamics What is brayton cycle in thermodynamics
What is brayton cycle in thermodynamics Basic concepts of thermodynamics
Basic concepts of thermodynamics Heat transfer in polytropic process
Heat transfer in polytropic process Gibbs free energy
Gibbs free energy Archimedes labs competitors
Archimedes labs competitors Taxonomy is the branch of science that deals with –
Taxonomy is the branch of science that deals with – An iron bar of mass 869 g cools from
An iron bar of mass 869 g cools from Refrigerant cycle
Refrigerant cycle Thermodynamics and statistical mechanics
Thermodynamics and statistical mechanics Frist law of thermodynamics
Frist law of thermodynamics Zeroth law of thermodynamics
Zeroth law of thermodynamics 11th chemistry thermodynamics lec 10
11th chemistry thermodynamics lec 10 Laws of thermodynamics simple
Laws of thermodynamics simple 2nd law of thermodynamics
2nd law of thermodynamics Reversible and irreversible processes in thermodynamics
Reversible and irreversible processes in thermodynamics Isobaric process formula
Isobaric process formula Thermodynamics
Thermodynamics What is brayton cycle in thermodynamics
What is brayton cycle in thermodynamics Non spontaneous process
Non spontaneous process Thermodynamics deals with
Thermodynamics deals with Why do we study thermodynamics
Why do we study thermodynamics Zeroth law of thermodynamics
Zeroth law of thermodynamics What is steady flow process in thermodynamics
What is steady flow process in thermodynamics Lesson 10 thermodynamics unit review
Lesson 10 thermodynamics unit review Thermodynamics powerpoint
Thermodynamics powerpoint Thermal formula
Thermal formula Thermodynamics chapter 4
Thermodynamics chapter 4 What is flow work in thermodynamics
What is flow work in thermodynamics Air conditioner thermodynamics
Air conditioner thermodynamics Maxwell relations thermodynamics
Maxwell relations thermodynamics Residual properties in thermodynamics
Residual properties in thermodynamics Classical thermodynamics
Classical thermodynamics Postulates of thermodynamics
Postulates of thermodynamics Thermodynamics
Thermodynamics Kelvin statement
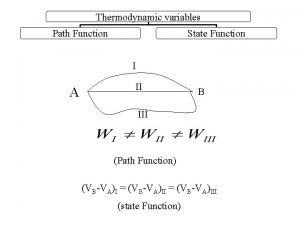
Kelvin statement State function and path function in thermodynamics
State function and path function in thermodynamics Introduction and basic concepts of thermodynamics
Introduction and basic concepts of thermodynamics Third law of thermodynamics derivation
Third law of thermodynamics derivation Thermodynamics
Thermodynamics Delta h delta s
Delta h delta s 11th chemistry thermodynamics lec 13
11th chemistry thermodynamics lec 13 Lee kesler equation
Lee kesler equation Internal energy formula thermodynamics
Internal energy formula thermodynamics What is a polytropic process in thermodynamics
What is a polytropic process in thermodynamics State function in thermodynamics
State function in thermodynamics 1st law of thermodynamics
1st law of thermodynamics First law of thermodynamics control mass
First law of thermodynamics control mass Thermodynamics equations
Thermodynamics equations Thermodynamics chapter 3
Thermodynamics chapter 3 Thermodynamics introduction and basic concepts
Thermodynamics introduction and basic concepts Thermodynamics intensive and extensive properties
Thermodynamics intensive and extensive properties Entropy thermodynamics
Entropy thermodynamics Euler equation thermodynamics
Euler equation thermodynamics Statistical thermodynamics
Statistical thermodynamics Open system
Open system Statistical thermodynamics is a study of
Statistical thermodynamics is a study of Microstates in thermodynamics
Microstates in thermodynamics Homogeneous system in thermodynamics
Homogeneous system in thermodynamics Tds equation in thermodynamics
Tds equation in thermodynamics Define carnot engine
Define carnot engine Thermodynamics an engineering approach
Thermodynamics an engineering approach Second law of thermodynamics
Second law of thermodynamics Refrigeration unit
Refrigeration unit Thermodynamics webquest
Thermodynamics webquest Table a-6 thermodynamics
Table a-6 thermodynamics Entropy in thermodynamics
Entropy in thermodynamics Open vs closed system
Open vs closed system State second law of thermodynamics
State second law of thermodynamics Thermodynamics
Thermodynamics What is brayton cycle in thermodynamics
What is brayton cycle in thermodynamics Ideal solution thermodynamics
Ideal solution thermodynamics Dq=cdt
Dq=cdt Thermodynamics
Thermodynamics Thermodynamics 1 formula sheet
Thermodynamics 1 formula sheet Thermodynamics
Thermodynamics Thermodynamics cartoon
Thermodynamics cartoon çengel
çengel First law of thermodynamics in cyclic process
First law of thermodynamics in cyclic process Exact
Exact Microstates in thermodynamics
Microstates in thermodynamics Third law of thermodynamics
Third law of thermodynamics Thermodynamics
Thermodynamics S=sf+xsfg
S=sf+xsfg Law of thermodynamics
Law of thermodynamics Exergy formula
Exergy formula Nozzle and diffuser
Nozzle and diffuser Second law of thermodynamics
Second law of thermodynamics Thermodynamics equations
Thermodynamics equations Thermodynamics
Thermodynamics Laws thermodynamics
Laws thermodynamics Quasi static process
Quasi static process Thermodynamics and statistical mechanics
Thermodynamics and statistical mechanics Thermodynamics is concerned with
Thermodynamics is concerned with Thermodynamics enthalpy of reaction and hess's law
Thermodynamics enthalpy of reaction and hess's law Properties of pure substances thermodynamics
Properties of pure substances thermodynamics State the second law of thermodynamics
State the second law of thermodynamics 2nd law of thermodynamics
2nd law of thermodynamics Thermodynamics
Thermodynamics Thermodynamic potentials
Thermodynamic potentials Pros and cons of oil energy
Pros and cons of oil energy Feedwater
Feedwater Microstates in thermodynamics
Microstates in thermodynamics Carnot cycle hurricane
Carnot cycle hurricane Thermodynamics an engineering approach
Thermodynamics an engineering approach Clausius inequality in thermodynamics
Clausius inequality in thermodynamics Ap chemistry thermodynamics
Ap chemistry thermodynamics Energy transfer rubbing hands together
Energy transfer rubbing hands together Thermodynamics cartoons
Thermodynamics cartoons Thermodynamics cengel and boles
Thermodynamics cengel and boles Endothermic reaction examples
Endothermic reaction examples Thermodynamics chapter 4
Thermodynamics chapter 4 Internal energy
Internal energy First law of thermodynamics sign convention
First law of thermodynamics sign convention Thermodynamics
Thermodynamics What is thermodynamic equilibrium
What is thermodynamic equilibrium Sssf thermodynamics
Sssf thermodynamics Transient analysis thermodynamics
Transient analysis thermodynamics Endothermic equation
Endothermic equation Tatiana erukhimova
Tatiana erukhimova Residual properties in thermodynamics
Residual properties in thermodynamics Q in thermodynamics
Q in thermodynamics