Entropy In classical thermodynamics the entropy is defined

- Slides: 4

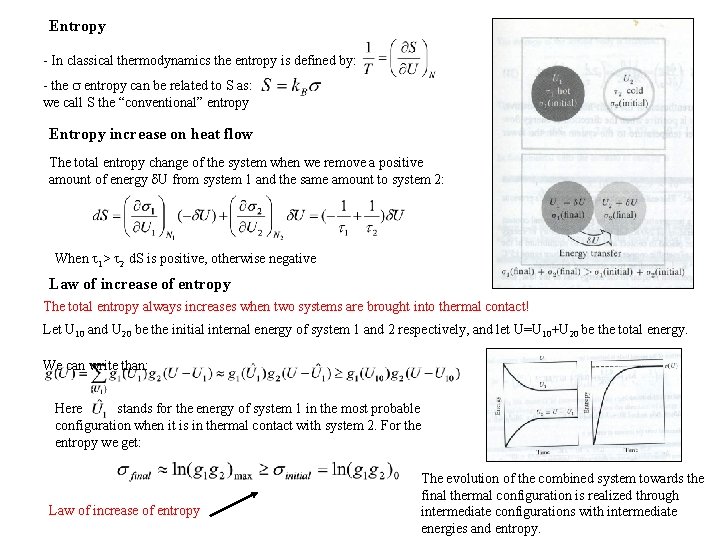
Entropy - In classical thermodynamics the entropy is defined by: - the entropy can be related to S as: we call S the “conventional” entropy Entropy increase on heat flow The total entropy change of the system when we remove a positive amount of energy U from system 1 and the same amount to system 2: When 1> 2 d. S is positive, otherwise negative Law of increase of entropy The total entropy always increases when two systems are brought into thermal contact! Let U 10 and U 20 be the initial internal energy of system 1 and 2 respectively, and let U=U 10+U 20 be the total energy. We can write than: Here stands for the energy of system 1 in the most probable configuration when it is in thermal contact with system 2. For the entropy we get: Law of increase of entropy The evolution of the combined system towards the final thermal configuration is realized through intermediate configurations with intermediate energies and entropy.


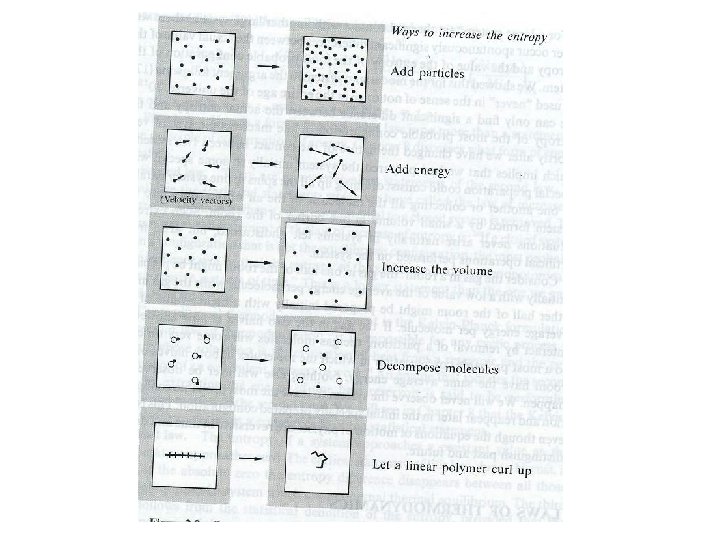
Laws of Thermodynamics - When thermodynamics is studied as a nonstatistical subject (phenomenology) --> four postulates - These postulates are theoretically based in our statistical formulation. Zeroth law: If two systems are in thermal equilibrium with a third system, they must be in thermal equilibrium with each other. Immediate consequence of the condition for thermal equilibrium First law: Heat is a form of energy and together with work can contribute to the change of the internal energy of the system --> practically the principle of energy conservation. Second law: the law of increase of entropy. If a closed system is in a configuration that is not the equilibrium configuration , the most probable consequence will be that the entropy of the system will increase monotonically in successive instants of time. Many other formulations are known…. Third law: The entropy of a system approaches a constant value (must come in flat with T) as the temperature approaches zero. This constant value might not necessarily be: 0 Entropy as a logarithm: two important consequences of the fact that entropy is defined as the logarithm of the number of accessible states, instead of as the number of accessible states itself: 1. The entropy becomes an extensive parameter 2. The entropy is insensitive to the precision with which the energy of a closed system is defined or fixed. Let D(U) be the number of accessible states per unit energy range. From here: Typically , as for the system of N spins, g(U) is of order 2 N, D(U) 2 N/N , where is the average energy per particle.

Problems: 1. Find why the solution of the Example: “Entropy increase on heat flow” (page 44 -45) is not totally correct. In fact it is quite a rough approximation…. 2. We pour a glass of water (m=0. 2 kg) at temperature Ti=600 C in a lake with water at temperature T 0=100 C. Calculate the change in the entropy of the Universe due to this process. The specific heat of water is: cw=4180 J/(Kg. K) 3. Solve problem 5 on page 53. 4. Solve problem 6 on page 54 Extra problem: A famous British duo Flanders and Swan have a nice song about the laws of thermodynamic. You can download it (I suggest to listen it, it is funny) in mp 3 format here, and you can get the verses here. Find the scientifically wrong statements in their song, and argue why it is wrong or not complete.