Entropy Change at Constant Volume The case of

- Slides: 13

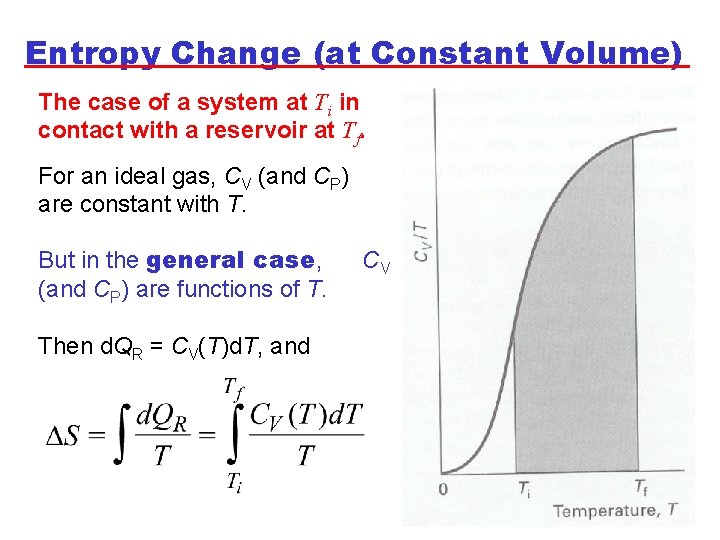
Entropy Change (at Constant Volume) The case of a system at Ti in contact with a reservoir at Tf. For an ideal gas, CV (and CP) are constant with T. But in the general case, (and CP) are functions of T. Then d. QR = CV(T)d. T, and CV

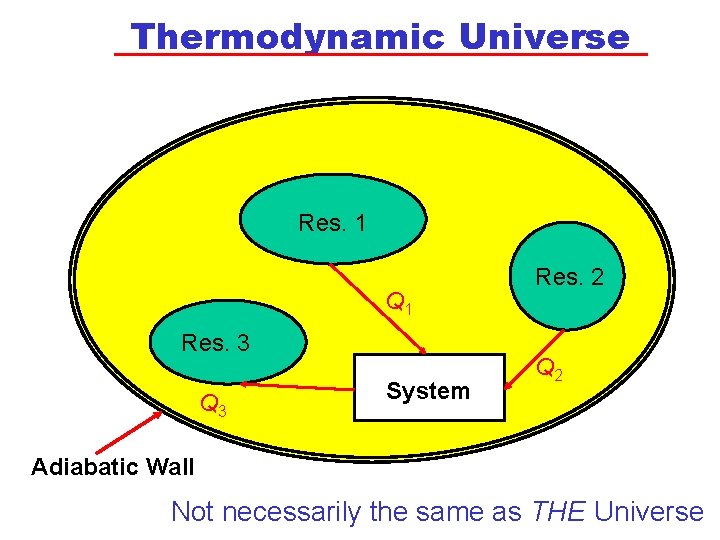
Thermodynamic Universe Res. 1 Q 1 Res. 3 Q 3 System Res. 2 Q 2 Adiabatic Wall Not necessarily the same as THE Universe

Entropy Change, DS, in a Thermodynamic Universe DSuniv = DSsyst + DSres 1 + DSres 2 + …. Entropy can decrease or increase within the various parts of thermodynamic universe. For an irreversible process within the universe, DSuniv 0. For a reversible process within the universe, DSuniv =0. Thus, S goes to a maximum within a thermodynamic universe (i. e. , a thermally-isolated system). (2 nd Law!) Implication: In a thermodynamic universe, a higher entropy state must follow a lower entropy state. Entropy gives us the direction of time!

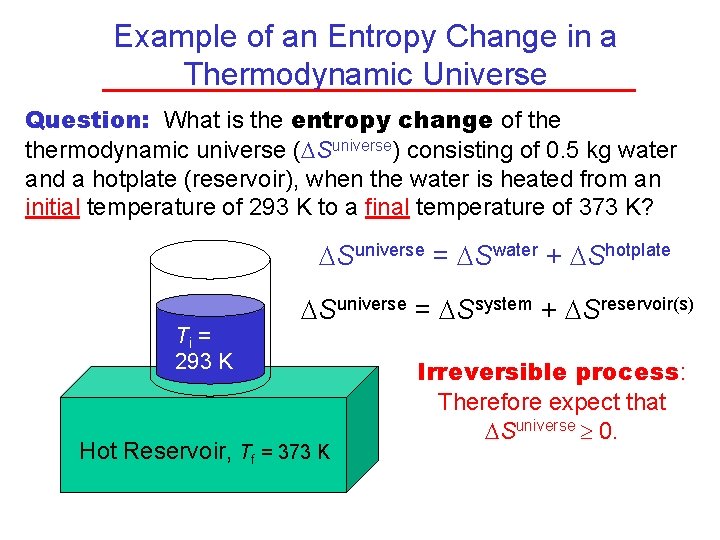
Example of an Entropy Change in a Thermodynamic Universe Question: What is the entropy change of thermodynamic universe (DSuniverse) consisting of 0. 5 kg water and a hotplate (reservoir), when the water is heated from an initial temperature of 293 K to a final temperature of 373 K? DSuniverse = DSwater + DShotplate Ti = 293 K DSuniverse = DSsystem + DSreservoir(s) Hot Reservoir, Tf = 373 K Irreversible process: Therefore expect that DSuniverse 0.

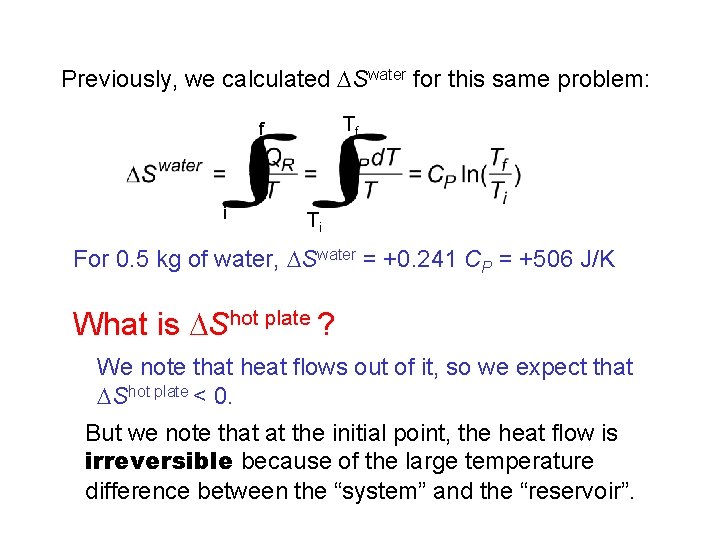
Previously, we calculated DSwater for this same problem: Tf f i Ti For 0. 5 kg of water, DSwater = +0. 241 CP = +506 J/K What is DShot plate ? We note that heat flows out of it, so we expect that DShot plate < 0. But we note that at the initial point, the heat flow is irreversible because of the large temperature difference between the “system” and the “reservoir”.

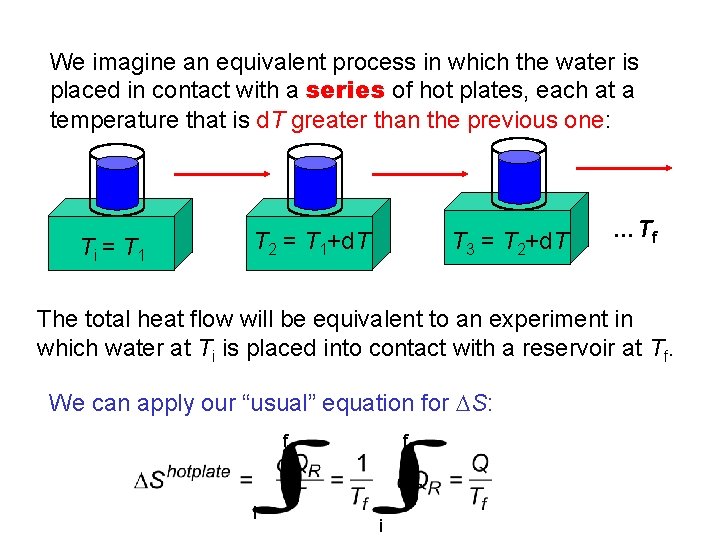
We imagine an equivalent process in which the water is placed in contact with a series of hot plates, each at a temperature that is d. T greater than the previous one: Ti = T 1 T 2 = T 1+d. T T 3 = T 2+d. T …Tf The total heat flow will be equivalent to an experiment in which water at Ti is placed into contact with a reservoir at Tf. We can apply our “usual” equation for DS: f i

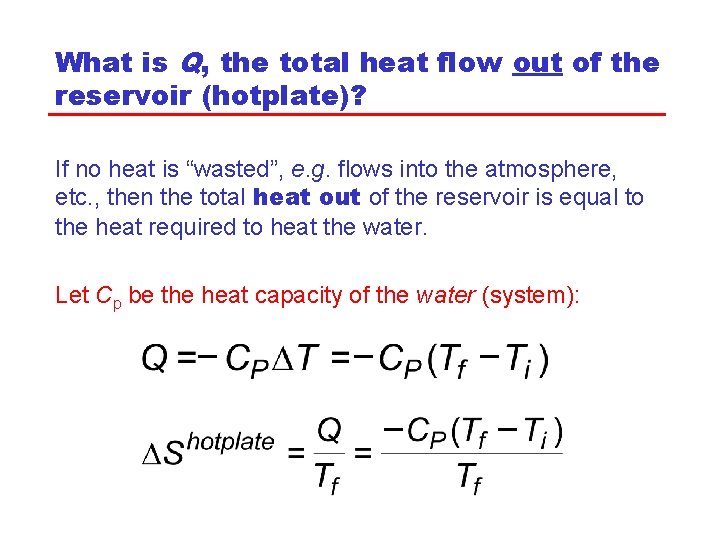
What is Q, the total heat flow out of the reservoir (hotplate)? If no heat is “wasted”, e. g. flows into the atmosphere, etc. , then the total heat out of the reservoir is equal to the heat required to heat the water. Let Cp be the heat capacity of the water (system):

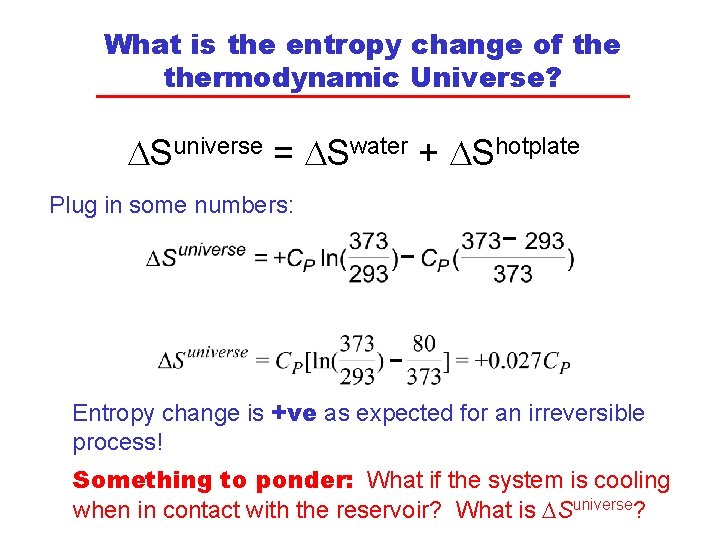
What is the entropy change of thermodynamic Universe? DSuniverse = DSwater + DShotplate Plug in some numbers: Entropy change is +ve as expected for an irreversible process! Something to ponder: What if the system is cooling when in contact with the reservoir? What is DSuniverse?

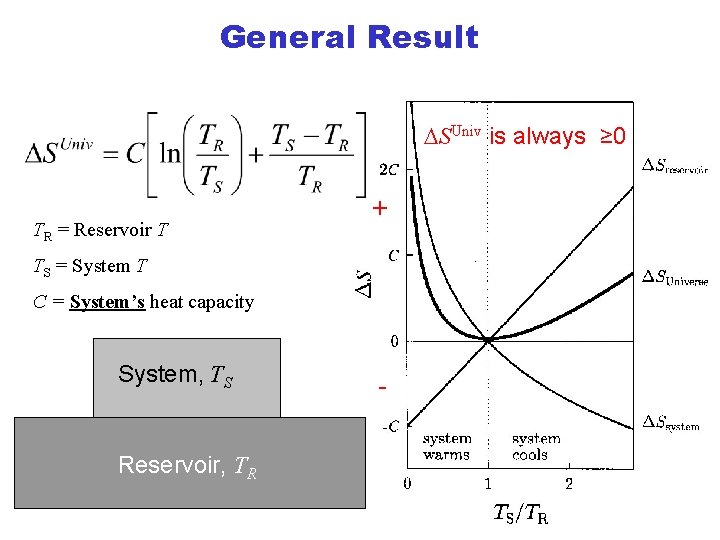
General Result DSUniv is always ≥ 0 TR = Reservoir T + TS = System T C = System’s heat capacity System, TS Reservoir, TR -

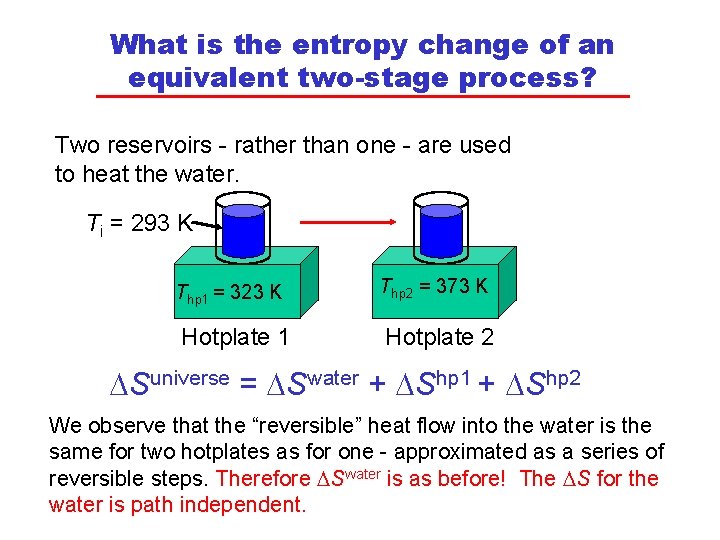
What is the entropy change of an equivalent two-stage process? Two reservoirs - rather than one - are used to heat the water. Ti = 293 K Thp 1 = 323 K Thp 2 = 373 K Hotplate 1 Hotplate 2 DSuniverse = DSwater + DShp 1 + DShp 2 We observe that the “reversible” heat flow into the water is the same for two hotplates as for one - approximated as a series of reversible steps. Therefore DSwater is as before! The DS for the water is path independent.

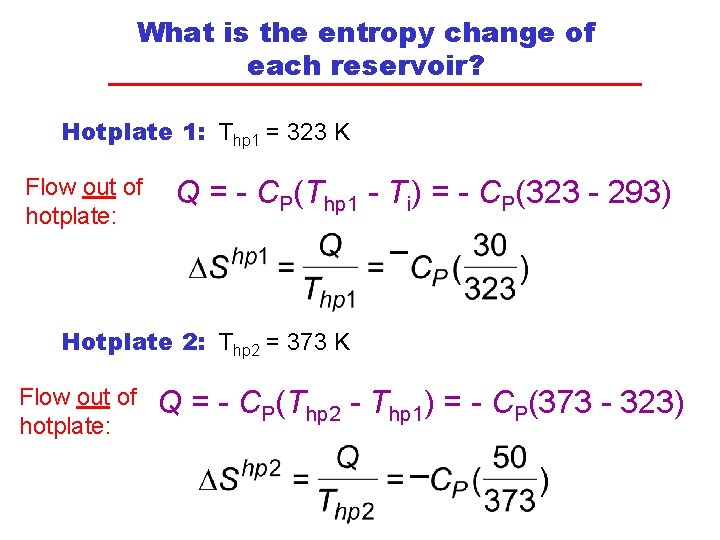
What is the entropy change of each reservoir? Hotplate 1: Thp 1 = 323 K Flow out of hotplate: Q = - CP(Thp 1 - Ti) = - CP(323 - 293) Hotplate 2: Thp 2 = 373 K Flow out of hotplate: Q = - CP(Thp 2 - Thp 1) = - CP(373 - 323)

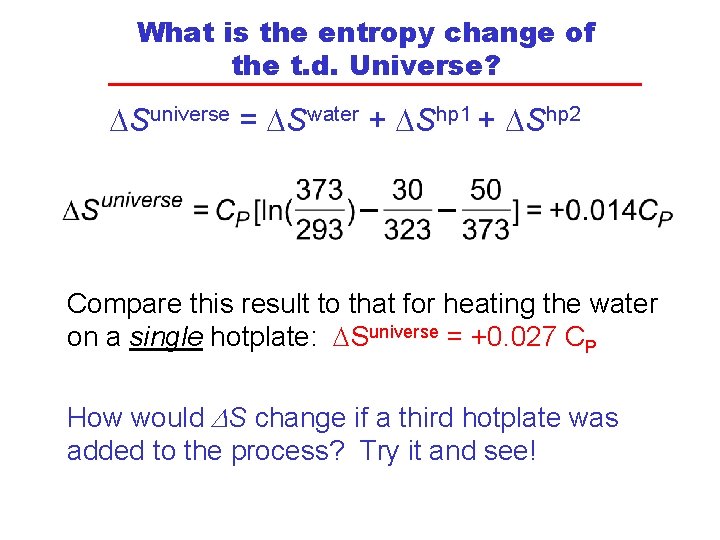
What is the entropy change of the t. d. Universe? DSuniverse = DSwater + DShp 1 + DShp 2 Compare this result to that for heating the water on a single hotplate: DSuniverse = +0. 027 CP How would DS change if a third hotplate was added to the process? Try it and see!

Entropy change of a reversible process As more and more hotplates are added, DSuniverse will decrease. Adding more and more hotplates is causing the process to approach a reversible process. As the number of hotplates increases towards , DSuniverse will approach 0, just as expected for a reversible process!