Entropy Change at Constant Volume For an ideal

- Slides: 11

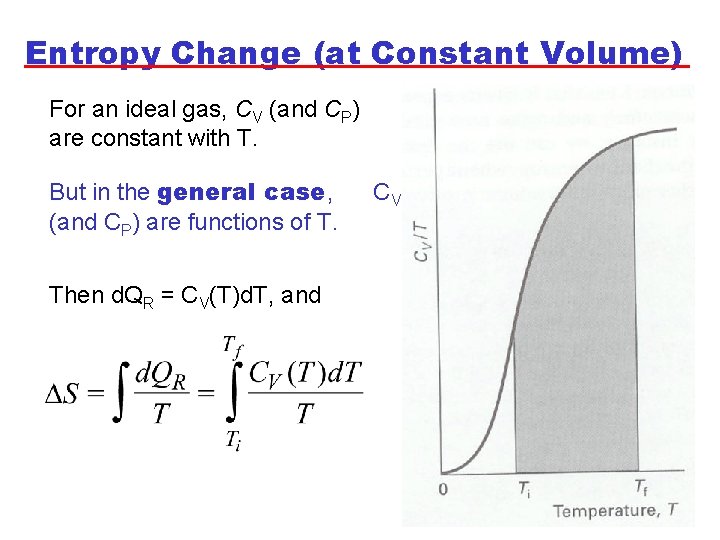
Entropy Change (at Constant Volume) For an ideal gas, CV (and CP) are constant with T. But in the general case, (and CP) are functions of T. Then d. QR = CV(T)d. T, and CV

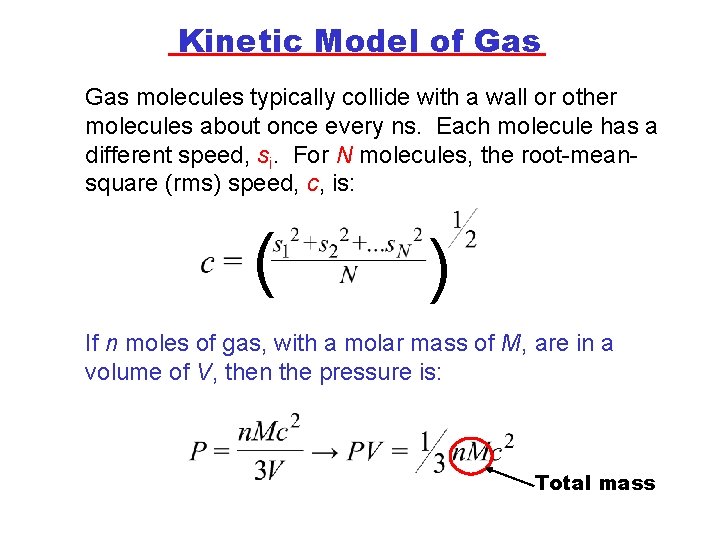
Kinetic Model of Gas molecules typically collide with a wall or other molecules about once every ns. Each molecule has a different speed, si. For N molecules, the root-meansquare (rms) speed, c, is: ( ) If n moles of gas, with a molar mass of M, are in a volume of V, then the pressure is: Total mass

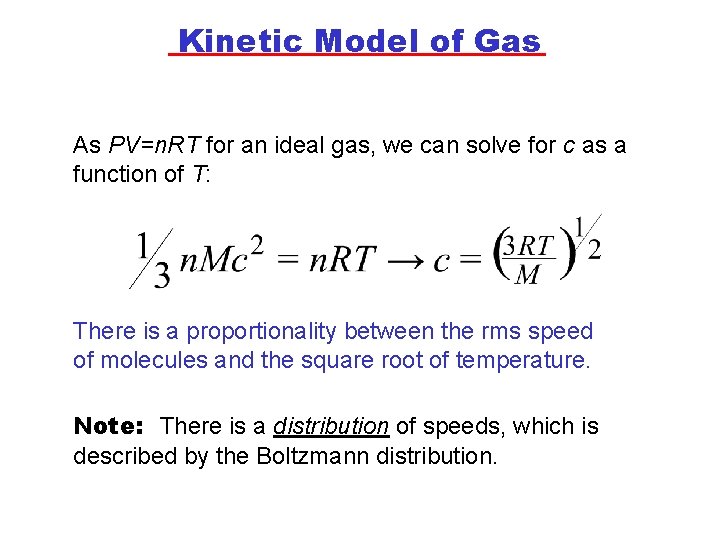
Kinetic Model of Gas As PV=n. RT for an ideal gas, we can solve for c as a function of T: There is a proportionality between the rms speed of molecules and the square root of temperature. Note: There is a distribution of speeds, which is described by the Boltzmann distribution.

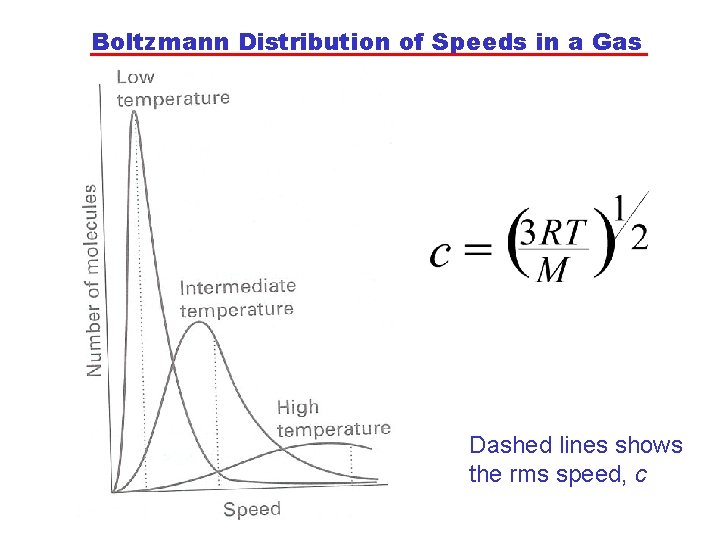
Boltzmann Distribution of Speeds in a Gas Dashed lines shows the rms speed, c

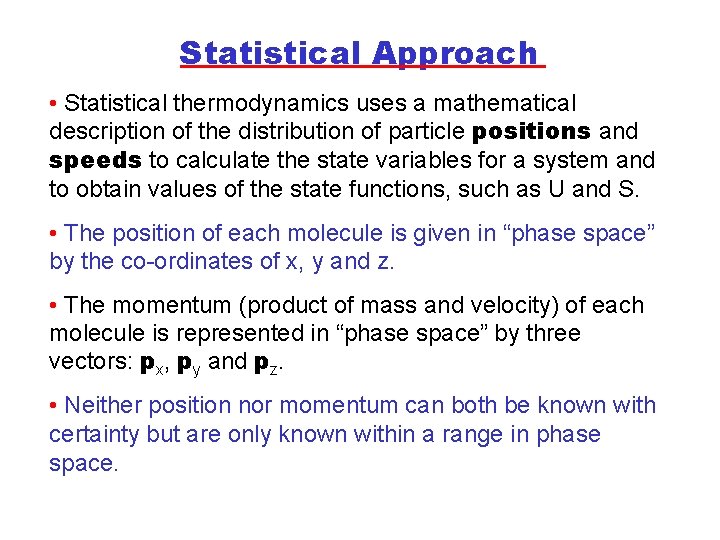
Statistical Approach • Statistical thermodynamics uses a mathematical description of the distribution of particle positions and speeds to calculate the state variables for a system and to obtain values of the state functions, such as U and S. • The position of each molecule is given in “phase space” by the co-ordinates of x, y and z. • The momentum (product of mass and velocity) of each molecule is represented in “phase space” by three vectors: px, py and pz. • Neither position nor momentum can both be known with certainty but are only known within a range in phase space.

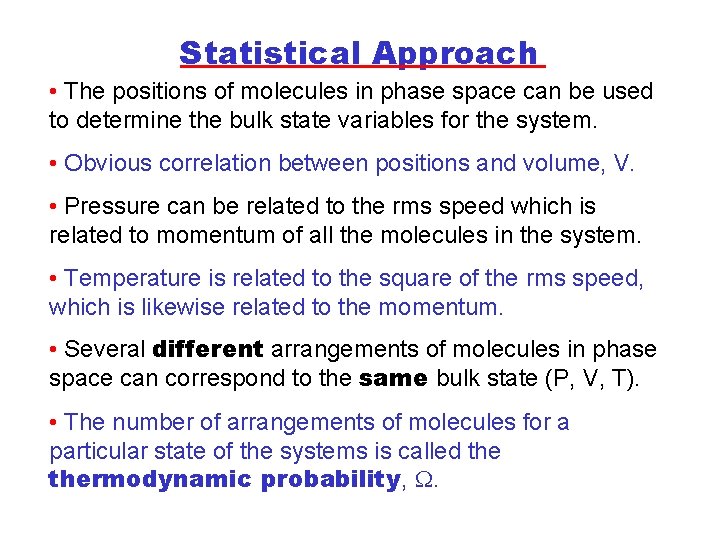
Statistical Approach • The positions of molecules in phase space can be used to determine the bulk state variables for the system. • Obvious correlation between positions and volume, V. • Pressure can be related to the rms speed which is related to momentum of all the molecules in the system. • Temperature is related to the square of the rms speed, which is likewise related to the momentum. • Several different arrangements of molecules in phase space can correspond to the same bulk state (P, V, T). • The number of arrangements of molecules for a particular state of the systems is called thermodynamic probability, .

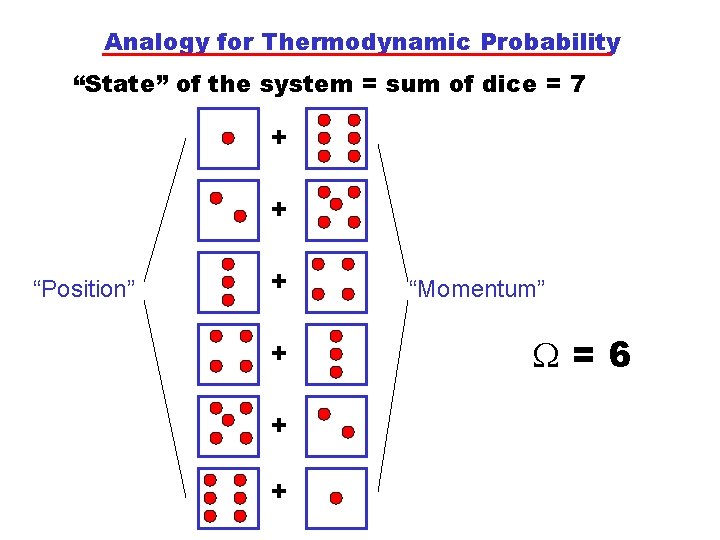
Analogy for Thermodynamic Probability “State” of the system = sum of dice = 7 + + “Position” + + “Momentum” =6

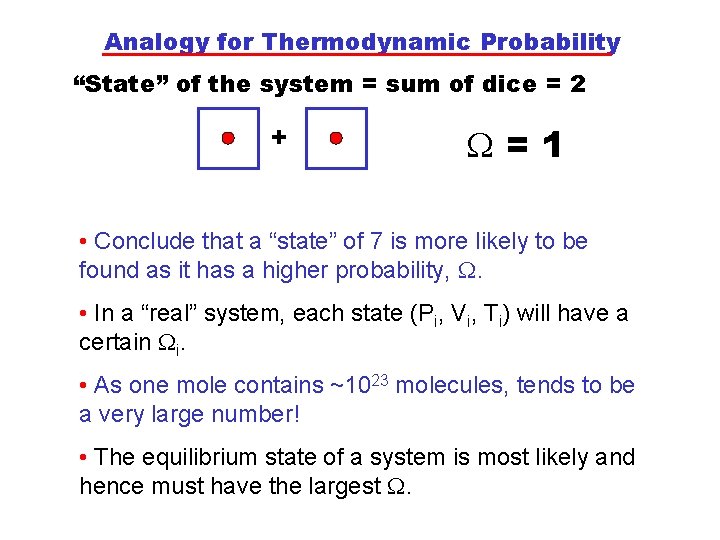
Analogy for Thermodynamic Probability “State” of the system = sum of dice = 2 + =1 • Conclude that a “state” of 7 is more likely to be found as it has a higher probability, . • In a “real” system, each state (Pi, Vi, Ti) will have a certain i. • As one mole contains ~1023 molecules, tends to be a very large number! • The equilibrium state of a system is most likely and hence must have the largest .

Relationship between and S • In an isolated system, or thermodynamic Universe, molecules will spontaneously move from a low state to a high state. • Thus the for the system approaches a maximum. • Previously we saw that in a thermally-isolated system (or t. d. Universe), S approaches a maximum. • CONCLUDE that there must be some proportionality between and S.

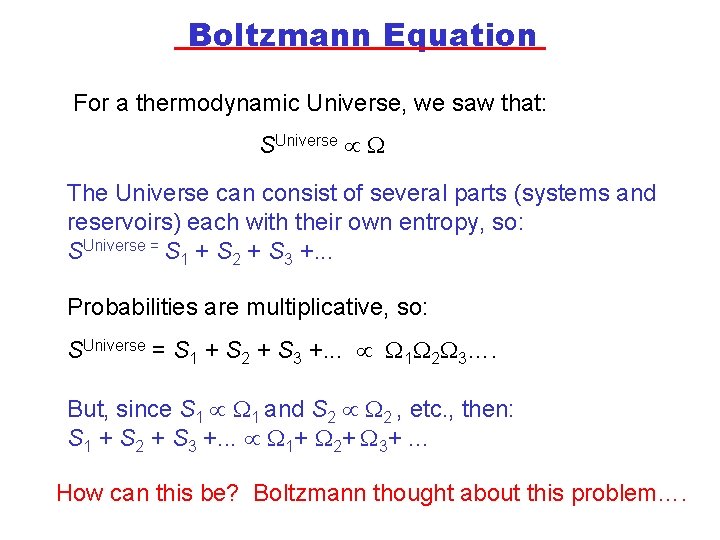
Boltzmann Equation For a thermodynamic Universe, we saw that: SUniverse The Universe can consist of several parts (systems and reservoirs) each with their own entropy, so: SUniverse = S 1 + S 2 + S 3 +. . . Probabilities are multiplicative, so: SUniverse = S 1 + S 2 + S 3 +. . . 1 2 3…. But, since S 1 1 and S 2 2 , etc. , then: S 1 + S 2 + S 3 +. . . 1+ 2+ 3+. . . How can this be? Boltzmann thought about this problem….

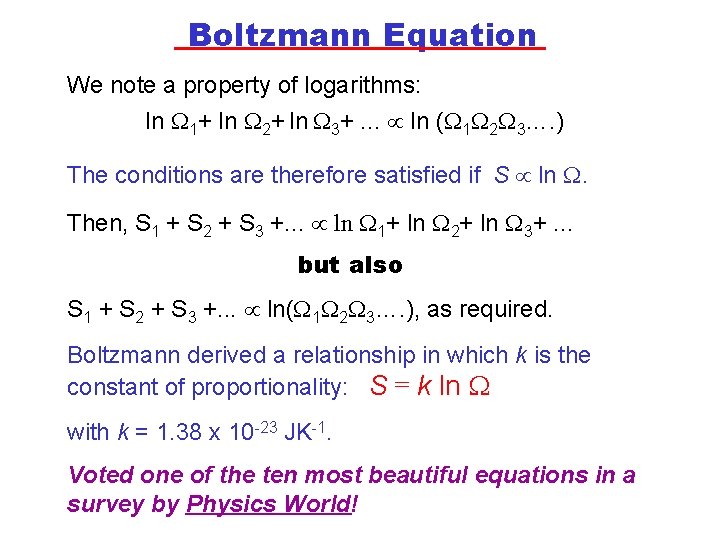
Boltzmann Equation We note a property of logarithms: ln 1+ ln 2+ ln 3+. . . ln ( 1 2 3…. ) The conditions are therefore satisfied if S ln . Then, S 1 + S 2 + S 3 +. . . ln 1+ ln 2+ ln 3+. . . but also S 1 + S 2 + S 3 +. . . ln( 1 2 3…. ), as required. Boltzmann derived a relationship in which k is the constant of proportionality: S = k ln with k = 1. 38 x 10 -23 JK-1. Voted one of the ten most beautiful equations in a survey by Physics World!