Chapter 6 Entropy What is Entropy Entropy is





- Slides: 25

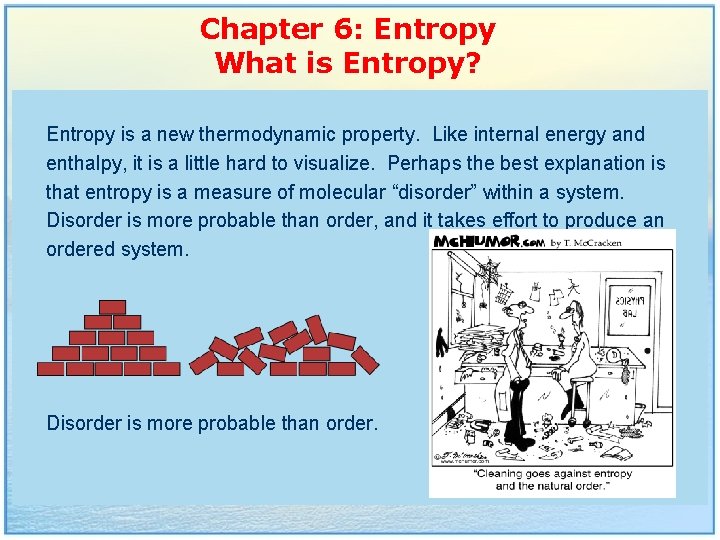
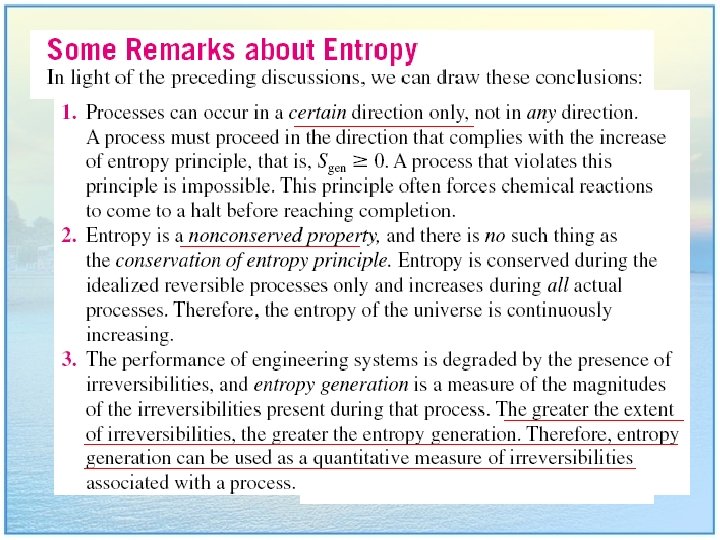
Chapter 6: Entropy What is Entropy? Entropy is a new thermodynamic property. Like internal energy and enthalpy, it is a little hard to visualize. Perhaps the best explanation is that entropy is a measure of molecular “disorder” within a system. Disorder is more probable than order, and it takes effort to produce an ordered system. Disorder is more probable than order.


What is a “Reversible and Irreversible” The word “reversible” simply means that something can be reversed, or to go in the opposite direction. In thermodynamics, the word means to reverse the process path. For example, if a process takes a system from State 1 to State 2, then if the process is “reversible” it can take the system from State 2 back to State 1 again. Unfortunately, this is seldom possible since nearly all thermodynamic processes are “irreversible”, meaning you can’t go back to where you started. This is because thermodynamic processes involve losses within the system due to internal friction, heat transfer, fluid viscosity, and so forth. • So, a “reversible” heat engine is a hypothetical engine that doesn’t really exist, but it does provides a good mathematical model with which to analyze and compare real (irreversible) heat engines.

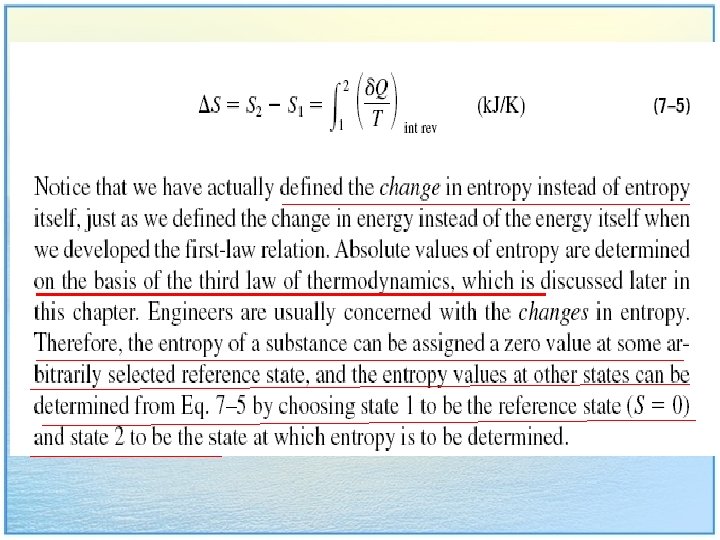
int rev stand for internally reversible


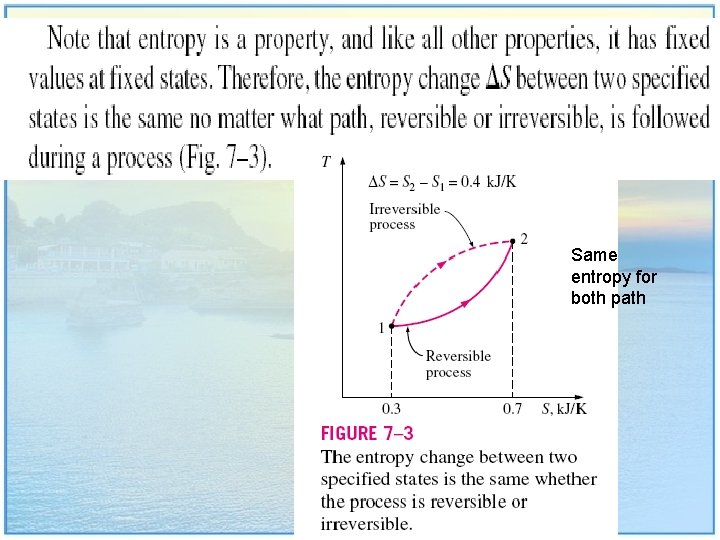
Same entropy for both path

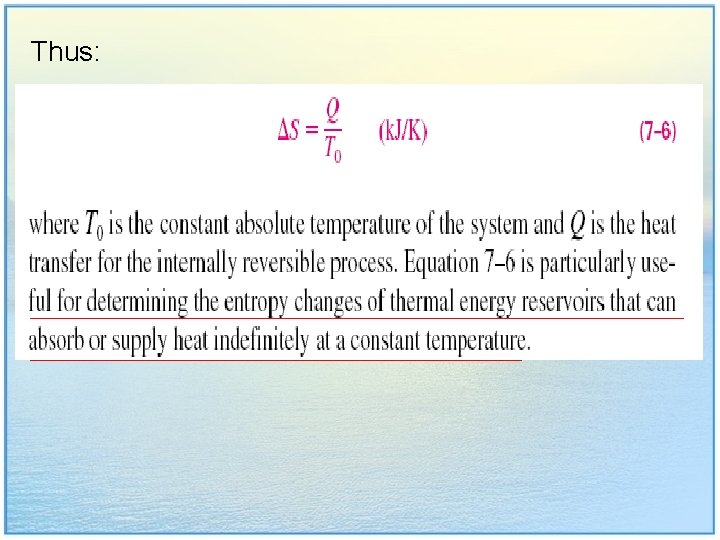
Thus:

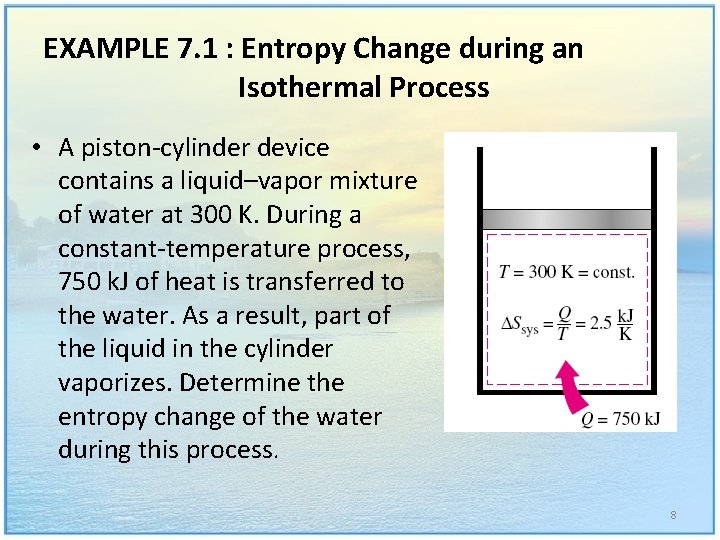
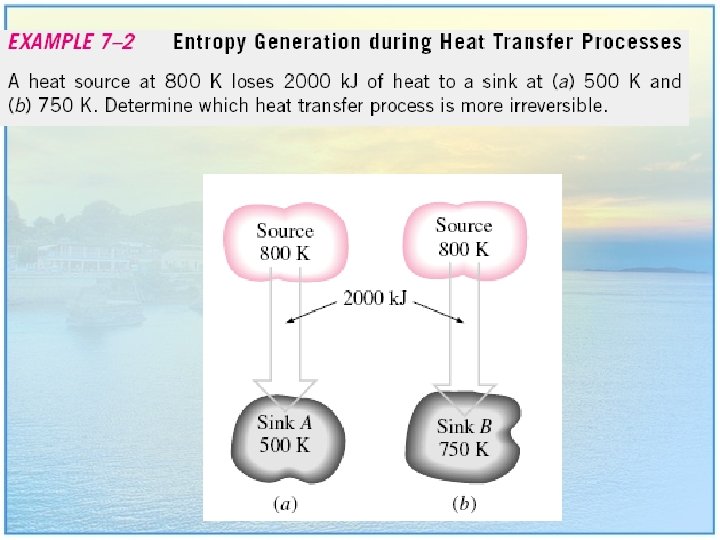
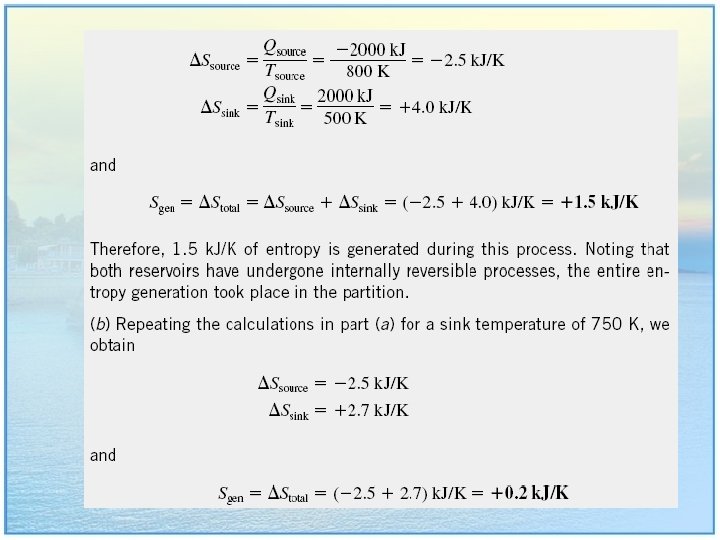
EXAMPLE 7. 1 : Entropy Change during an Isothermal Process • A piston-cylinder device contains a liquid–vapor mixture of water at 300 K. During a constant-temperature process, 750 k. J of heat is transferred to the water. As a result, part of the liquid in the cylinder vaporizes. Determine the entropy change of the water during this process. 8

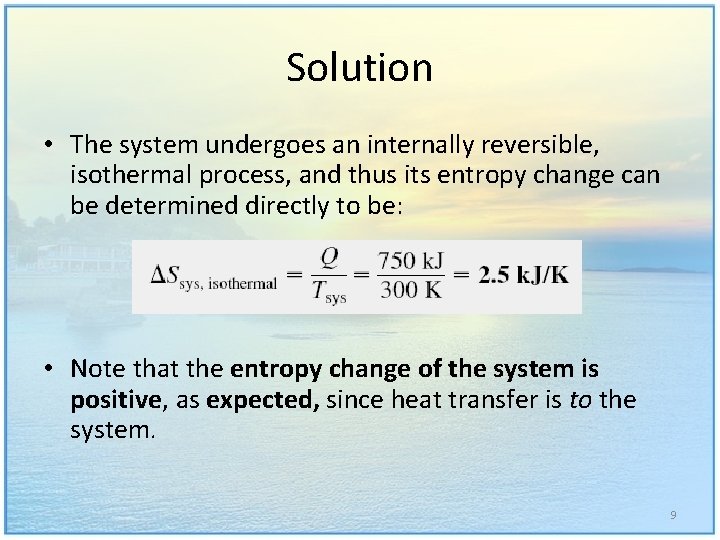
Solution • The system undergoes an internally reversible, isothermal process, and thus its entropy change can be determined directly to be: • Note that the entropy change of the system is positive, as expected, since heat transfer is to the system. 9

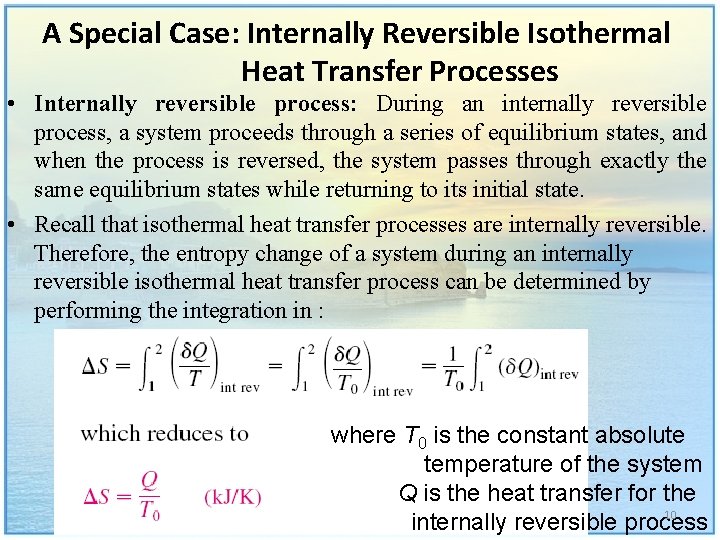
A Special Case: Internally Reversible Isothermal Heat Transfer Processes • Internally reversible process: During an internally reversible process, a system proceeds through a series of equilibrium states, and when the process is reversed, the system passes through exactly the same equilibrium states while returning to its initial state. • Recall that isothermal heat transfer processes are internally reversible. Therefore, the entropy change of a system during an internally reversible isothermal heat transfer process can be determined by performing the integration in : where T 0 is the constant absolute temperature of the system Q is the heat transfer for the 10 internally reversible process

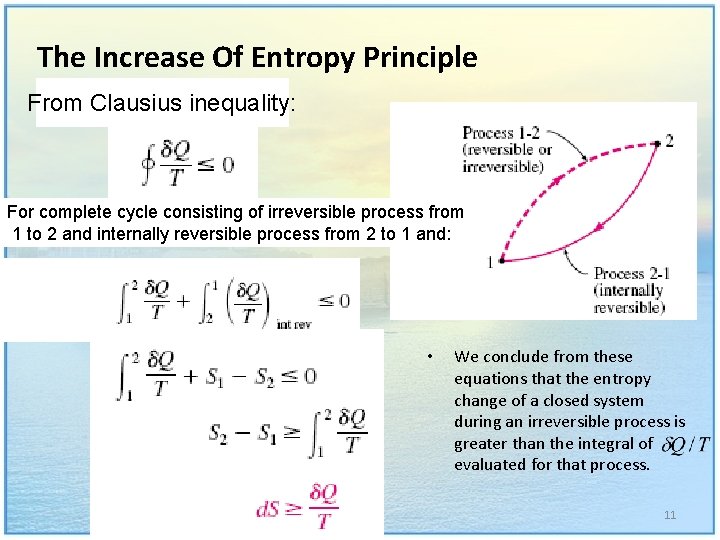
The Increase Of Entropy Principle From Clausius inequality: For complete cycle consisting of irreversible process from 1 to 2 and internally reversible process from 2 to 1 and: • We conclude from these equations that the entropy change of a closed system during an irreversible process is greater than the integral of evaluated for that process. 11

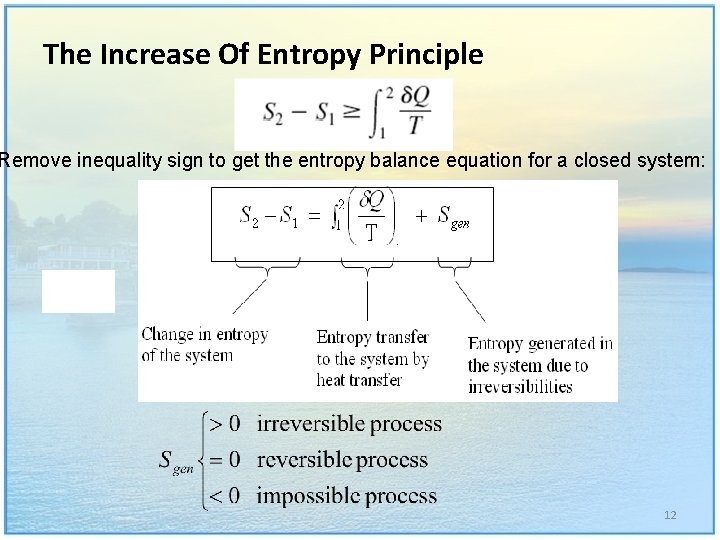
The Increase Of Entropy Principle Remove inequality sign to get the entropy balance equation for a closed system: 12

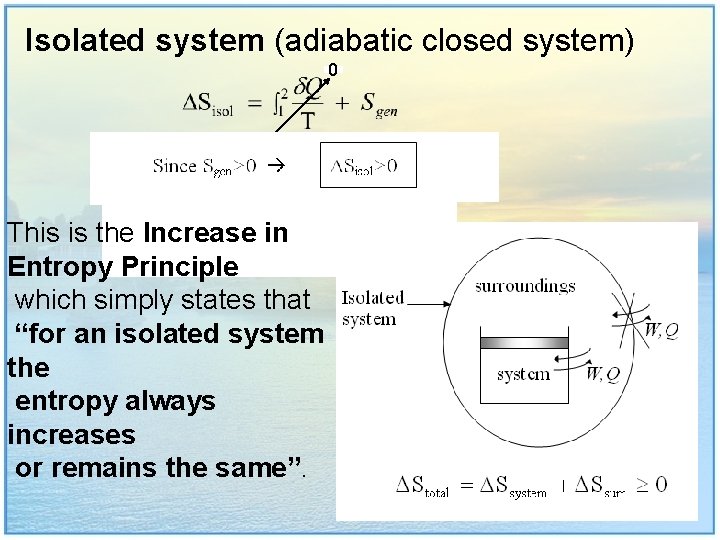
Isolated system (adiabatic closed system) 0 This is the Increase in Entropy Principle which simply states that “for an isolated system the entropy always increases or remains the same”. 13

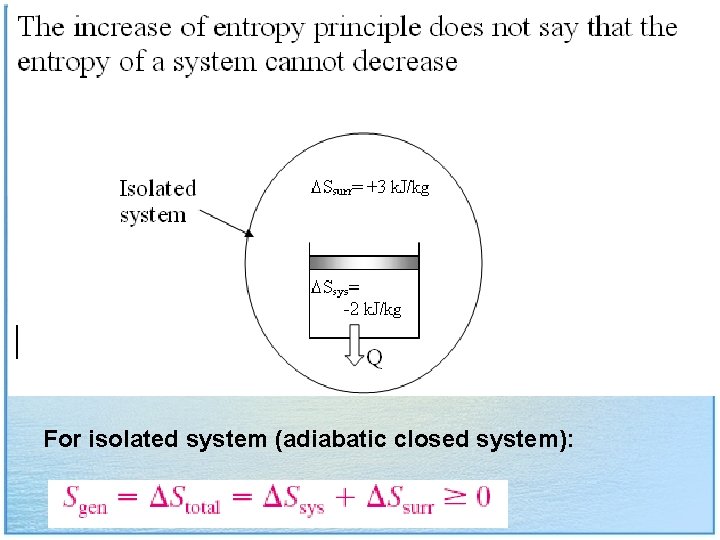
For isolated system (adiabatic closed system):





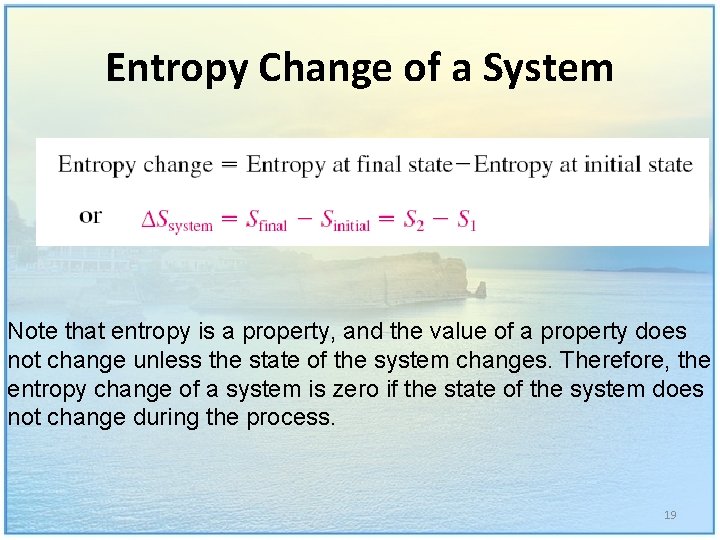
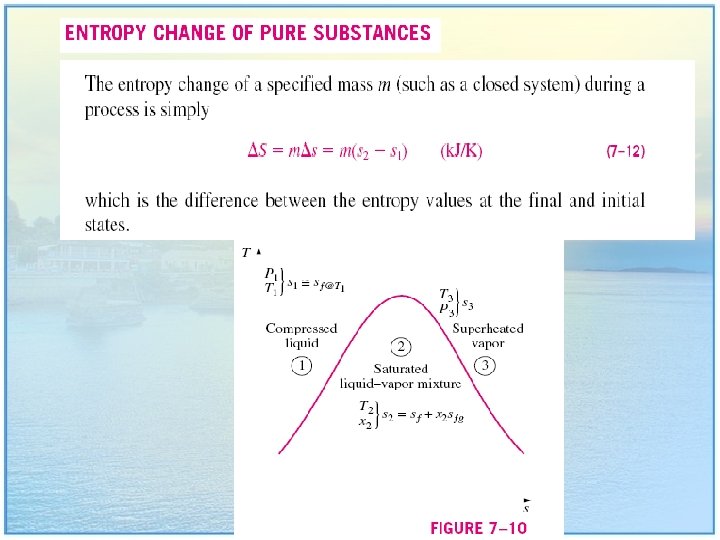
Entropy Change of a System Note that entropy is a property, and the value of a property does not change unless the state of the system changes. Therefore, the entropy change of a system is zero if the state of the system does not change during the process. 19


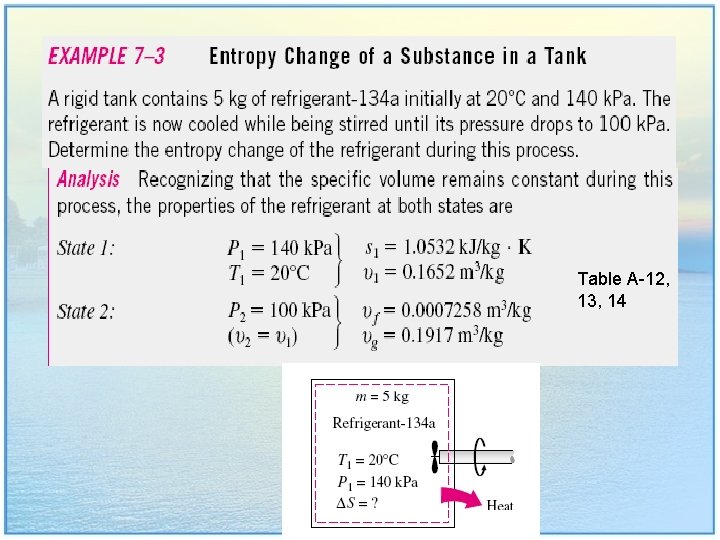
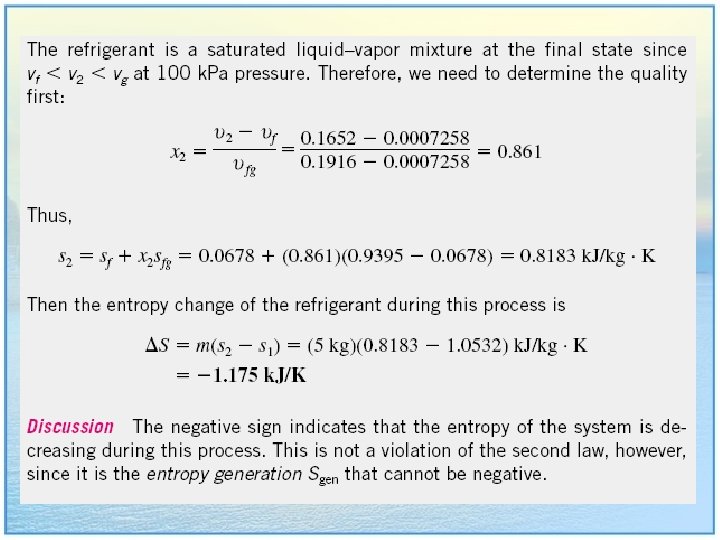
Table A-12, 13, 14


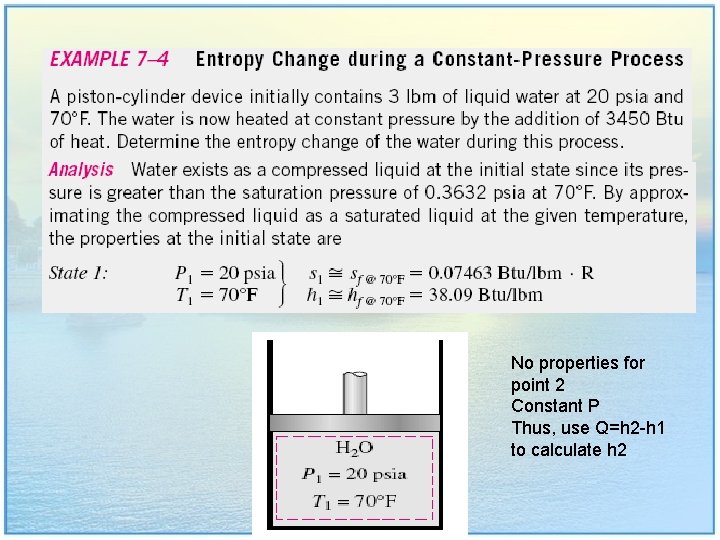
No properties for point 2 Constant P Thus, use Q=h 2 -h 1 to calculate h 2

