Chapter 17 Spontaneity Entropy and Free Energy Chapter








- Slides: 36

Chapter 17 Spontaneity, Entropy, and Free Energy

Chapter 17 Table of Contents 17. 1 17. 2 17. 3 17. 4 17. 5 17. 6 17. 7 17. 8 17. 9 Spontaneous Processes and Entropy and the Second Law of Thermodynamics The Effect of Temperature on Spontaneity Free Energy Entropy Changes in Chemical Reactions Free Energy and Chemical Reactions The Dependence of Free Energy on Pressure Free Energy and Equilibrium Free Energy and Work Copyright © Cengage Learning. All rights reserved 2

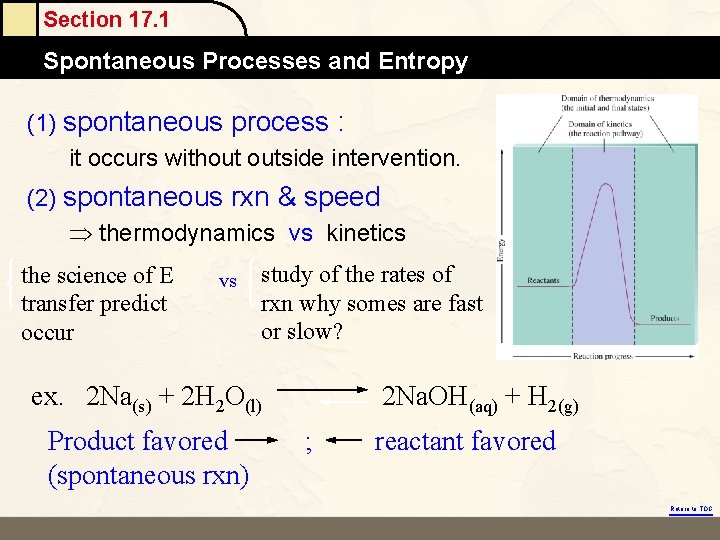
Section 17. 1 Spontaneous Processes and Entropy (1) spontaneous process : it occurs without outside intervention. (2) spontaneous rxn & speed thermodynamics vs kinetics the science of E transfer predict occur vs study of the rates of rxn why somes are fast or slow? ex. 2 Na(s) + 2 H 2 O(l) Product favored (spontaneous rxn) 2 Na. OH(aq) + H 2(g) ; reactant favored Return to TOC

Section 17. 1 Spontaneous Processes and Entropy Return to TOC Copyright © Cengage Learning. All rights reserved 4

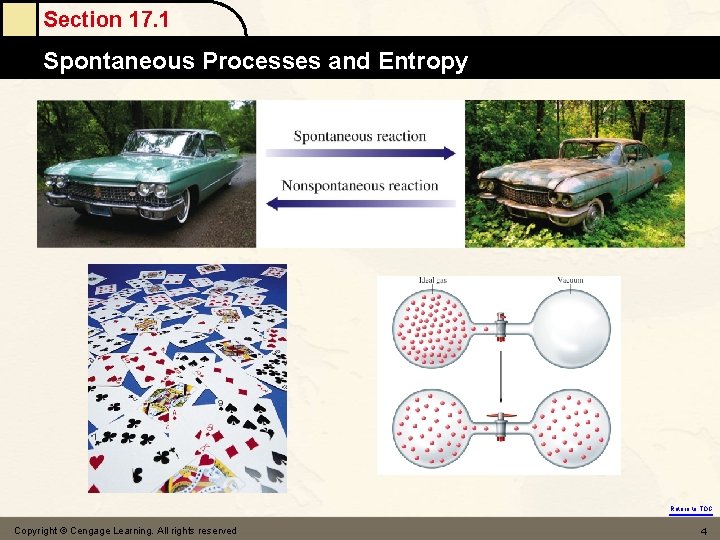
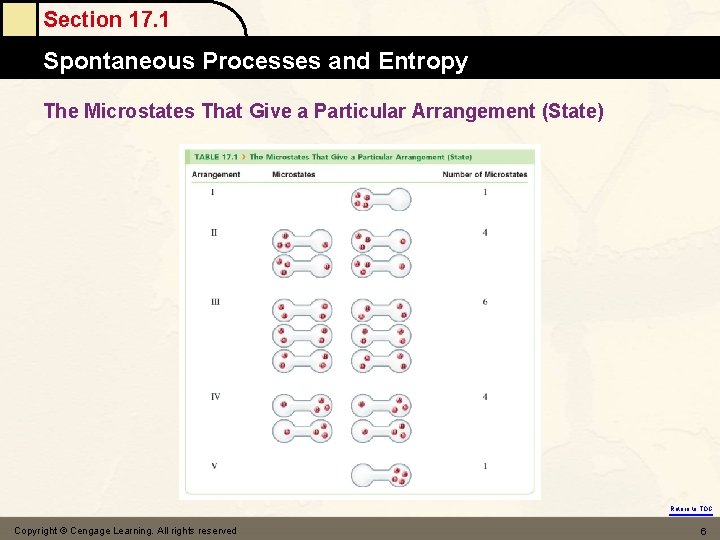
Section 17. 1 Spontaneous Processes and Entropy (3) why product - favored ? H < 0 energy ? (exothermic) How about H 2 O(s) → H 2 O(l) H > 0 at 0℃ (4) something else ! Entropy ( S ) S = the driving force for a spontaneous process is an increase in the entropy of the universe. = a measure of randomness or disorder of a system the great the randomness, the greater S. = the number of arrangements probability the higher probability, the higher S Return to TOC

Section 17. 1 Spontaneous Processes and Entropy The Microstates That Give a Particular Arrangement (State) Return to TOC Copyright © Cengage Learning. All rights reserved 6

Section 17. 1 Spontaneous Processes and Entropy (5) S 0 H, E? ( S = 0 for a perfect crystal at 0 K) (6) S = Sf - Si (7) generalization : a) S(g) >> S(l) > S(s) b) S : more complex molecules > simpler (ex) : CH 3 CH 2 CH 3 > CH 4 c) S : ionic solid (weaker force) > ionic solid (stronger force) (ex) : Na. F(s) > Mg. O(s) d) liq and solid dissolve in a solvent S > 0 gas dissolve in a solvent S < 0 Return to TOC

Section 17. 1 Spontaneous Processes and Entropy Concept Check Predict the sign of S for each of the following, and explain: + a) The evaporation of alcohol – b) The freezing of water – c) Compressing an ideal gas at constant temperature + d) Heating an ideal gas at constant pressure + e) Dissolving Na. Cl in water Return to TOC Copyright © Cengage Learning. All rights reserved 8

Section 17. 2 Atomic Masses Entropy and the Second Law of Thermodynamics In any spontaneous process, there is always an increase in the entropy of the universe Suniv. > 0 for spontaneous rxn Suniv. = Ssys. + Ssurr. System: rxn R P Suniv < 0 → rxn ← Suniv = 0 rxn at equilibrium Suniv. > 0 rxn Return to TOC

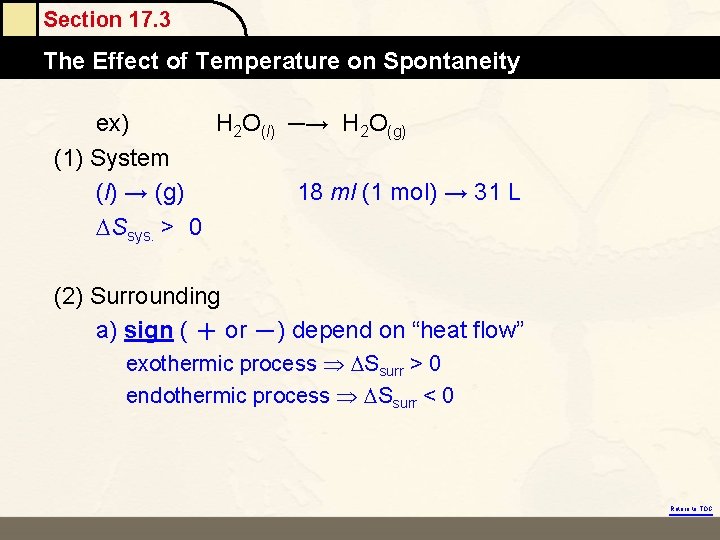
Section 17. 3 The Effect Mole of Temperature on Spontaneity ex) H 2 O(l) ─→ H 2 O(g) (1) System (l) → (g) 18 ml (1 mol) → 31 L Ssys. > 0 (2) Surrounding a) sign ( + or -) depend on “heat flow” exothermic process Ssurr > 0 endothermic process Ssurr < 0 Return to TOC

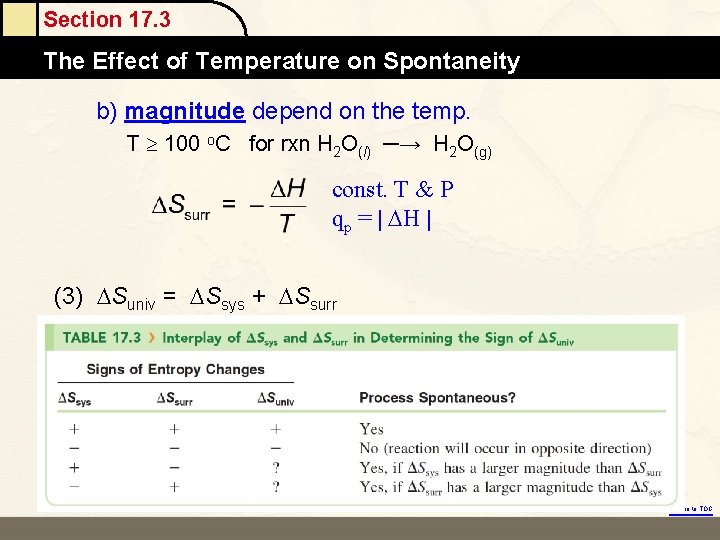
Section 17. 3 The Effect Mole of Temperature on Spontaneity b) magnitude depend on the temp. T 100 o. C for rxn H 2 O(l) ─→ H 2 O(g) const. T & P qp = | H | (3) Suniv = Ssys + Ssurr Return to TOC

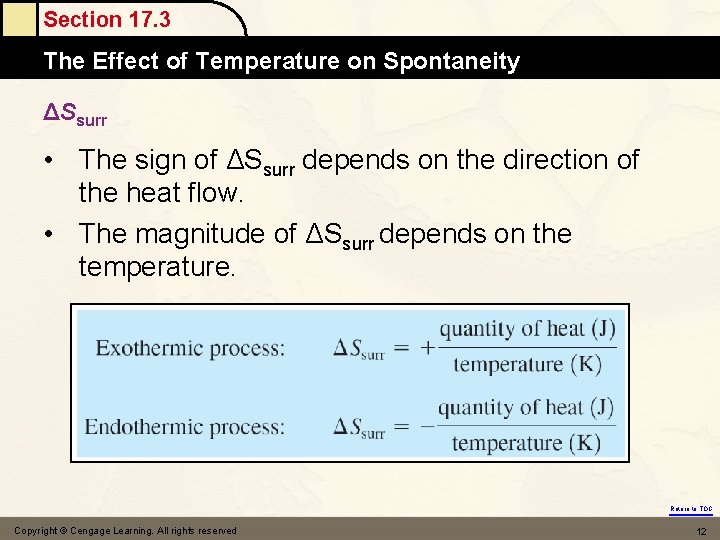
Section 17. 3 The Effect Mole of Temperature on Spontaneity ΔSsurr • The sign of ΔSsurr depends on the direction of the heat flow. • The magnitude of ΔSsurr depends on the temperature. Return to TOC Copyright © Cengage Learning. All rights reserved 12

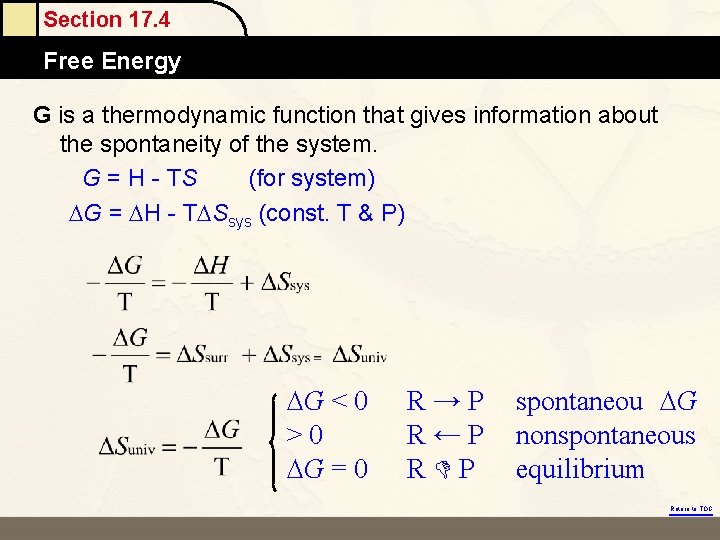
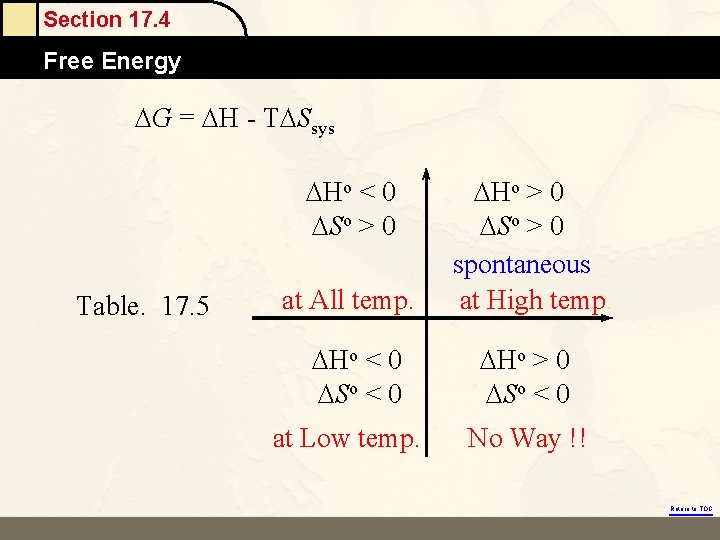
Section 17. 4 Free Energy G is a thermodynamic function that gives information about the spontaneity of the system. G = H - TS (for system) G = H - T Ssys (const. T & P) G < 0 >0 G = 0 R→P R←P R P spontaneou G nonspontaneous equilibrium Return to TOC

Section 17. 4 Free Energy G = H - T Ssys Ho < 0 So > 0 Table. 17. 5 at All temp. Ho > 0 So > 0 spontaneous at High temp. Ho < 0 So < 0 Ho > 0 So < 0 at Low temp. No Way !! Return to TOC

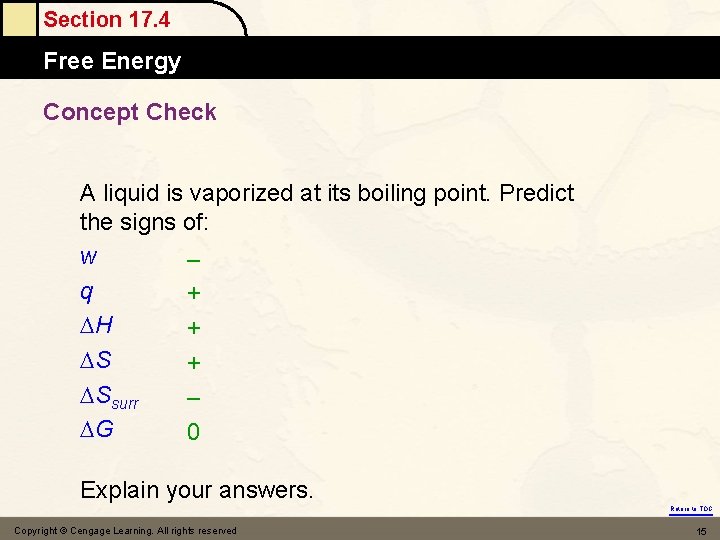
Section 17. 4 Free Energy Concept Check A liquid is vaporized at its boiling point. Predict the signs of: w – q + H + Ssurr – G 0 Explain your answers. Return to TOC Copyright © Cengage Learning. All rights reserved 15

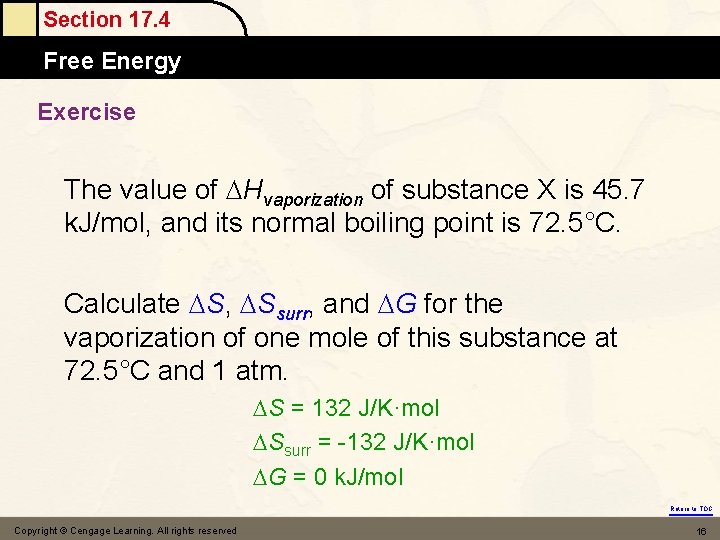
Section 17. 4 Free Energy Exercise The value of Hvaporization of substance X is 45. 7 k. J/mol, and its normal boiling point is 72. 5°C. Calculate S, Ssurr, and G for the vaporization of one mole of this substance at 72. 5°C and 1 atm. S = 132 J/K·mol Ssurr = -132 J/K·mol G = 0 k. J/mol Return to TOC Copyright © Cengage Learning. All rights reserved 16

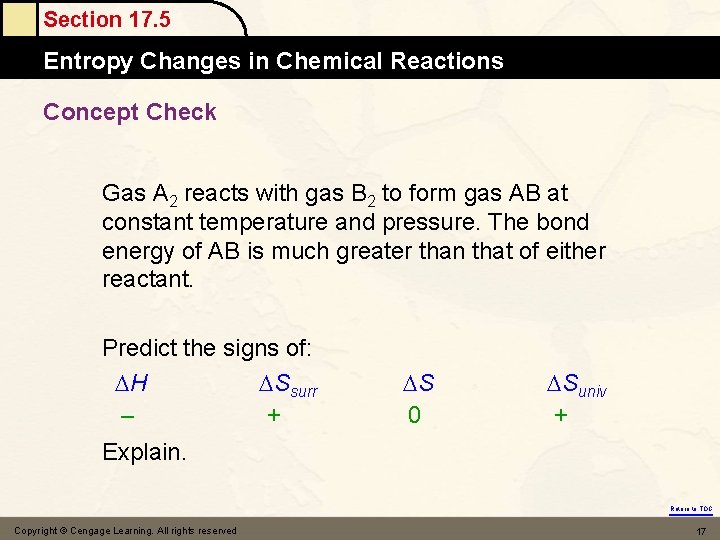
Section 17. 5 Entropy Changes in Chemical Reactions Concept Check Gas A 2 reacts with gas B 2 to form gas AB at constant temperature and pressure. The bond energy of AB is much greater than that of either reactant. Predict the signs of: H Ssurr – + S 0 Suniv + Explain. Return to TOC Copyright © Cengage Learning. All rights reserved 17

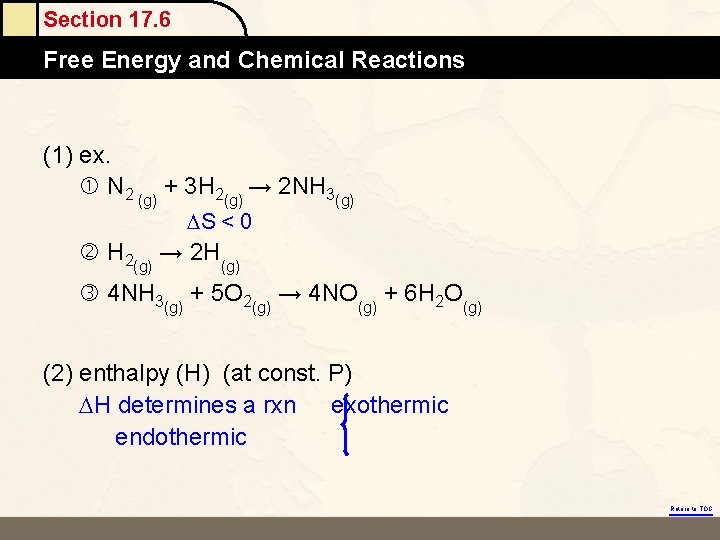
Section 17. 6 Free Energy and Chemical Reactions (1) ex. N 2 (g) + 3 H 2(g) → 2 NH 3(g) S < 0 H 2(g) → 2 H(g) 4 NH 3(g) + 5 O 2(g) → 4 NO(g) + 6 H 2 O(g) (2) enthalpy (H) (at const. P) H determines a rxn exothermic endothermic Return to TOC

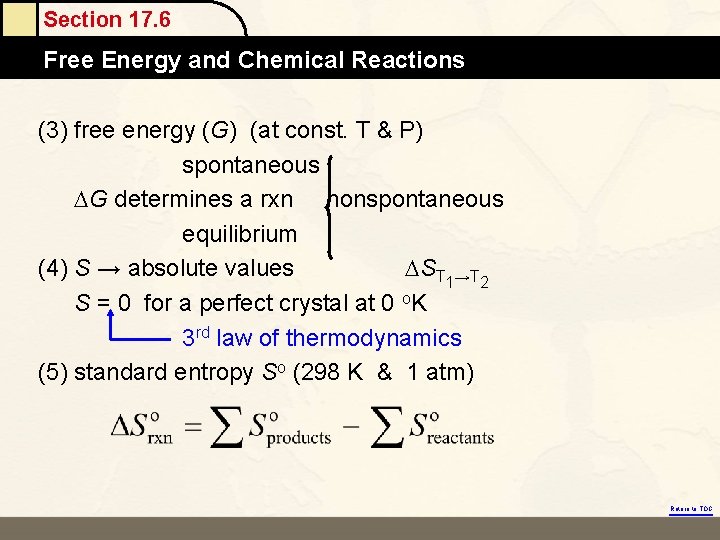
Section 17. 6 Free Energy and Chemical Reactions (3) free energy (G) (at const. T & P) spontaneous G determines a rxn nonspontaneous equilibrium (4) S → absolute values ST 1→T 2 S = 0 for a perfect crystal at 0 o. K 3 rd law of thermodynamics (5) standard entropy So (298 K & 1 atm) Return to TOC

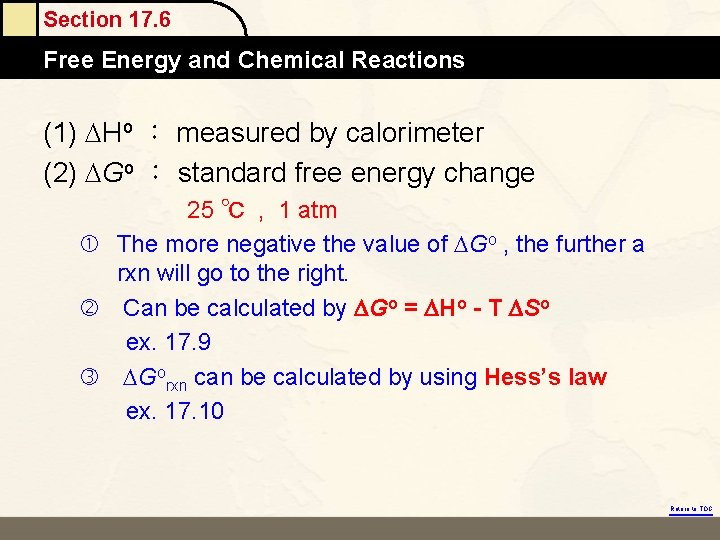
Section 17. 6 Free Energy and Chemical Reactions (1) Ho : measured by calorimeter (2) Go : standard free energy change 25 ℃ , 1 atm The more negative the value of Go , the further a rxn will go to the right. Can be calculated by DGo = DHo - T DSo ex. 17. 9 Gorxn can be calculated by using Hess’s law ex. 17. 10 Return to TOC

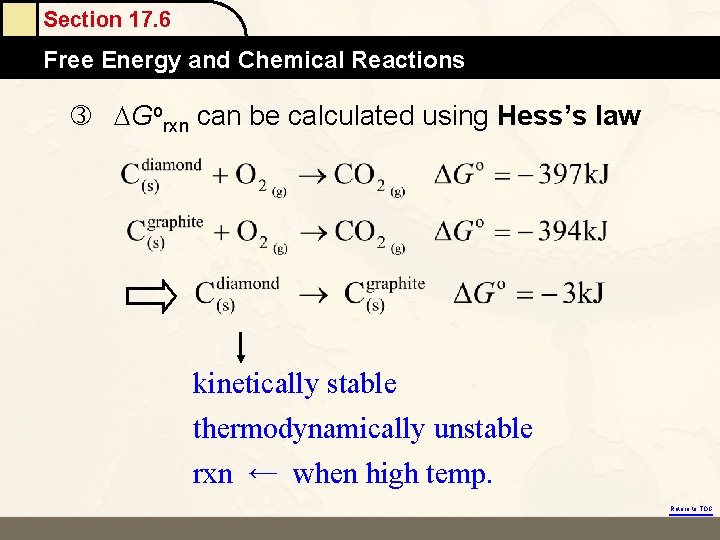
Section 17. 6 Free Energy and Chemical Reactions Gorxn can be calculated using Hess’s law kinetically stable thermodynamically unstable rxn ← when high temp. Return to TOC

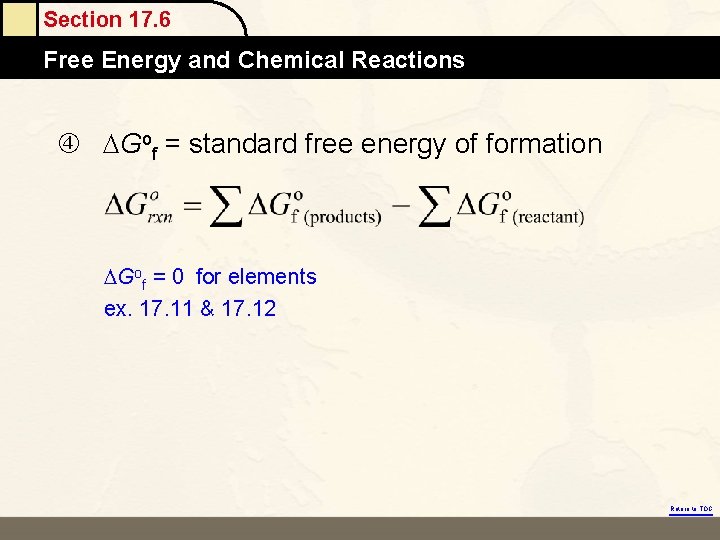
Section 17. 6 Free Energy and Chemical Reactions Gof = standard free energy of formation Gof = 0 for elements ex. 17. 11 & 17. 12 Return to TOC

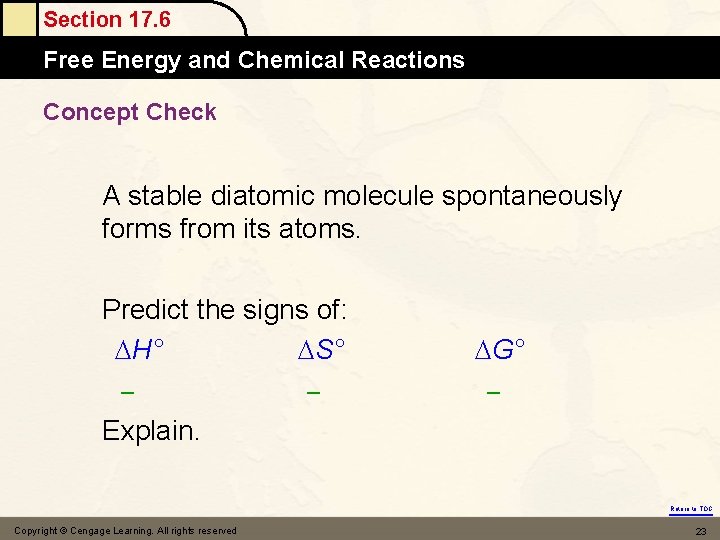
Section 17. 6 Free Energy and Chemical Reactions Concept Check A stable diatomic molecule spontaneously forms from its atoms. Predict the signs of: H° S° – – G° – Explain. Return to TOC Copyright © Cengage Learning. All rights reserved 23

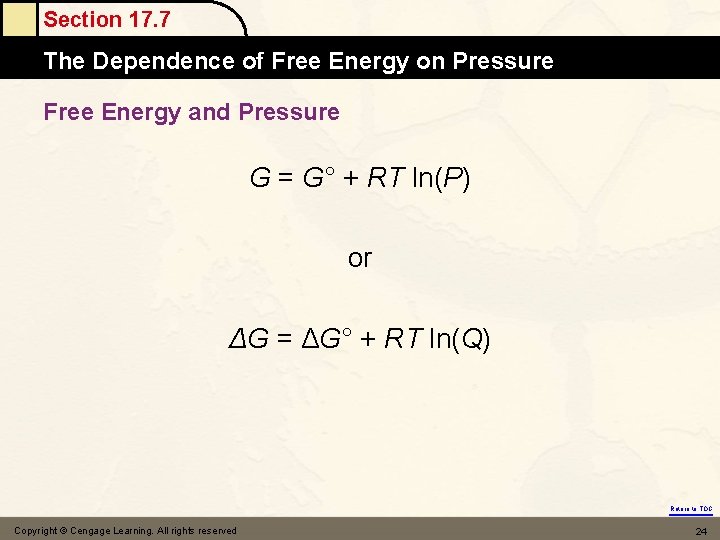
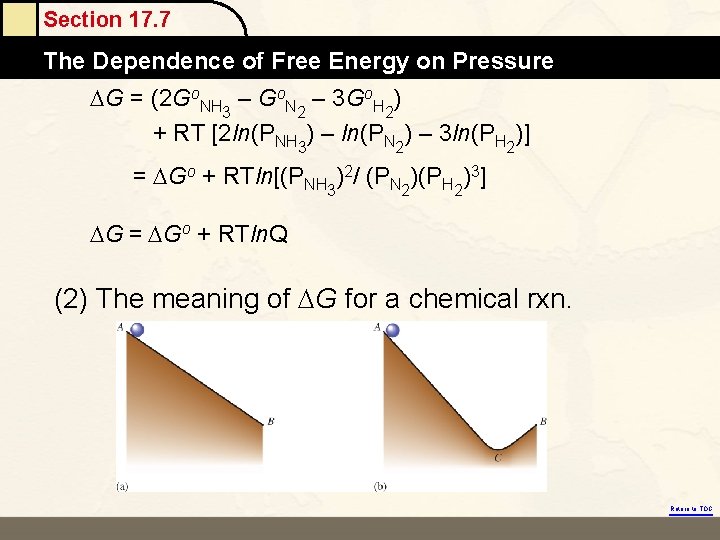
Section 17. 7 The Dependence of Free Energy on Pressure Free Energy and Pressure G = G° + RT ln(P) or ΔG = ΔG° + RT ln(Q) Return to TOC Copyright © Cengage Learning. All rights reserved 24

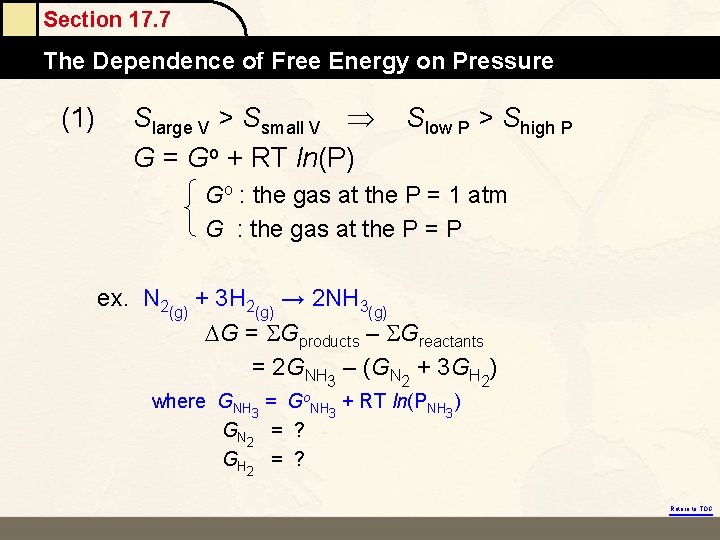
Section 17. 7 The Dependence of Free Energy on Pressure (1) Slarge V > Ssmall V G = Go + RT ln(P) Slow P > Shigh P Go : the gas at the P = 1 atm G : the gas at the P = P ex. N 2(g) + 3 H 2(g) → 2 NH 3(g) G = Gproducts – Greactants = 2 GNH 3 – (GN 2 + 3 GH 2) where GNH 3 = Go. NH 3 + RT ln(PNH 3) GN 2 = ? GH 2 = ? Return to TOC

Section 17. 7 The Dependence of Free Energy on Pressure G = (2 Go. NH 3 – Go. N 2 – 3 Go. H 2) + RT [2 ln(PNH 3) – ln(PN 2) – 3 ln(PH 2)] = Go + RTln[(PNH 3)2/ (PN 2)(PH 2)3] G = Go + RTln. Q (2) The meaning of G for a chemical rxn. Return to TOC

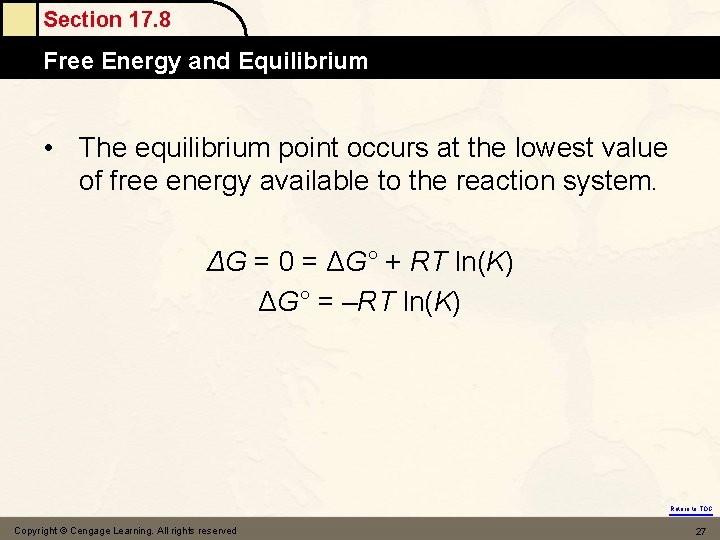
Section 17. 8 Free Energy and Equilibrium • The equilibrium point occurs at the lowest value of free energy available to the reaction system. ΔG = 0 = ΔG° + RT ln(K) ΔG° = –RT ln(K) Return to TOC Copyright © Cengage Learning. All rights reserved 27

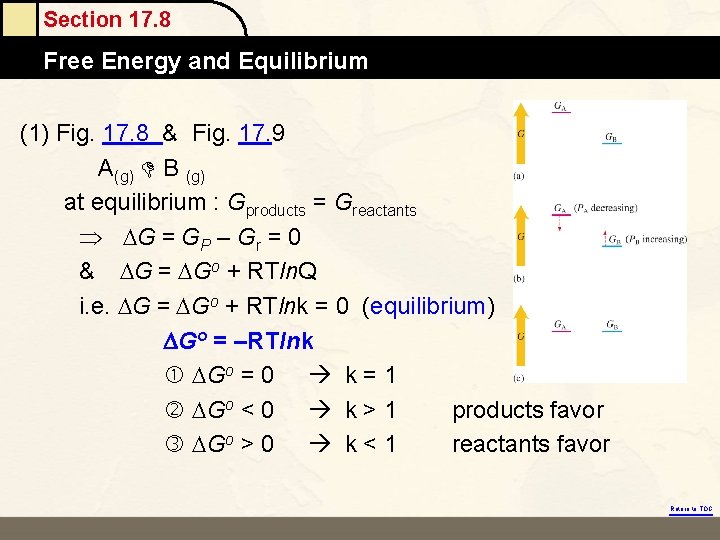
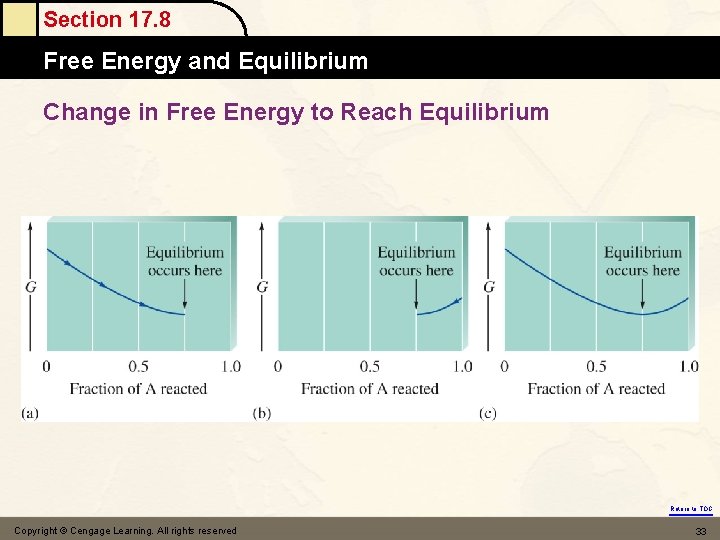
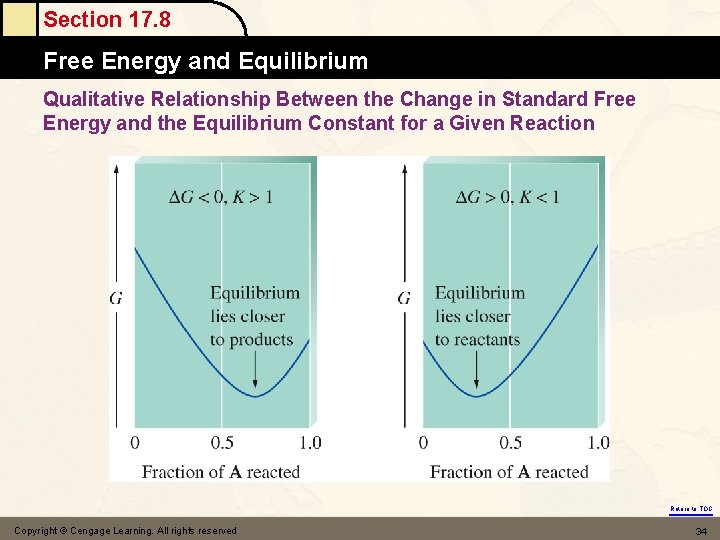
Section 17. 8 Free Energy and Equilibrium (1) Fig. 17. 8 & Fig. 17. 9 A(g) B (g) at equilibrium : Gproducts = Greactants G = GP – Gr = 0 & G = Go + RTln. Q i. e. G = Go + RTlnk = 0 (equilibrium) DGo = –RTlnk Go = 0 k = 1 Go < 0 k > 1 products favor Go > 0 k < 1 reactants favor Return to TOC

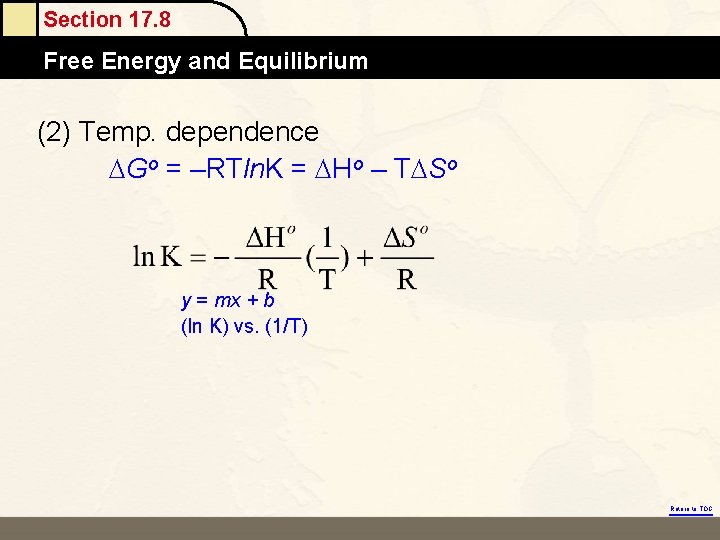
Section 17. 8 Free Energy and Equilibrium (2) Temp. dependence Go = –RTln. K = Ho – T So y = mx + b (ln K) vs. (1/T) Return to TOC

Section 17. 8 Free Energy and Equilibrium ex. 17. 14: N 2 + 3 H 2 2 NH 3 (g) (g) Go = – 33. 3 k. J at 25 o. C (a) PNH 3 = 1. 00, PN 2 = 1. 47 atm, PH 2 = 1. 00 10 -2 atm predict the rxn direction to reach equilibrium? Solution : G = Go + RTln. Q Return to TOC

Section 17. 8 Free Energy and Equilibrium ex. 17. 15: Calculate k for the follow rxn at 25 o. C 4 Fe (s) + 3 O 2(g) 2 Fe 2 O 3(s) Hof (k. J/mol) 0 0 -826 Sof (J/kmol) 27 205 90 Solution : G = Go + RTln. Q Go = Ho – T So Return to TOC

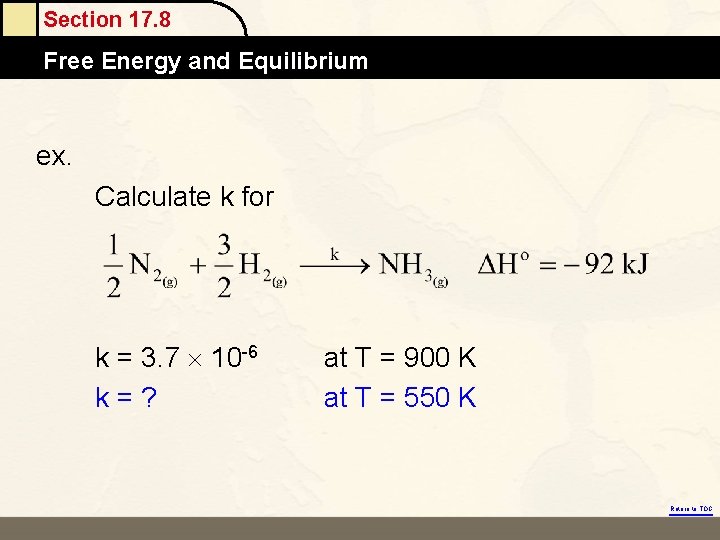
Section 17. 8 Free Energy and Equilibrium ex. Calculate k for k = 3. 7 10 -6 k=? at T = 900 K at T = 550 K Return to TOC

Section 17. 8 Free Energy and Equilibrium Change in Free Energy to Reach Equilibrium Return to TOC Copyright © Cengage Learning. All rights reserved 33

Section 17. 8 Free Energy and Equilibrium Qualitative Relationship Between the Change in Standard Free Energy and the Equilibrium Constant for a Given Reaction Return to TOC Copyright © Cengage Learning. All rights reserved 34

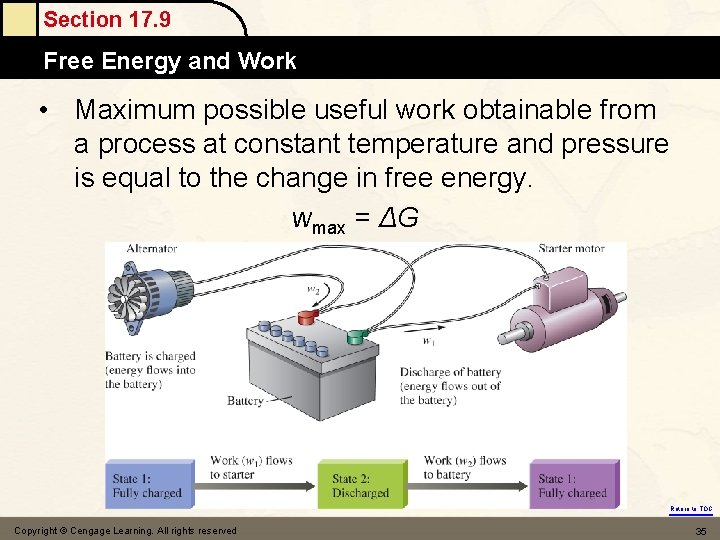
Section 17. 9 Free Energy and Work • Maximum possible useful work obtainable from a process at constant temperature and pressure is equal to the change in free energy. wmax = ΔG Return to TOC Copyright © Cengage Learning. All rights reserved 35

Section 17. 9 Free Energy and Work • Achieving the maximum work available from a spontaneous process can occur only via a hypothetical pathway. Any real pathway wastes energy. • All real processes are irreversible. • First law: You can’t win, you can only break even. • Second law: You can’t break even. Return to TOC Copyright © Cengage Learning. All rights reserved 36