Heat Transfer and Entropy Changes Tro Chapter 18




- Slides: 9

Heat Transfer and Entropy Changes Tro Chapter 18 – Free Energy and Thermodynamics 18. 5 Heat Transfer and Changes in the Entropy of the Surroundings

Heat Exchange and DSsurroundings • When a system process is exothermic, it adds heat to the surroundings, increasing the entropy of the surroundings. • When a system process is endothermic, it takes heat from the surroundings, decreasing the entropy of the surroundings. • The amount the entropy of the surroundings changes depends on its original temperature. • The higher the original temperature, the less effect addition or removal of heat has.

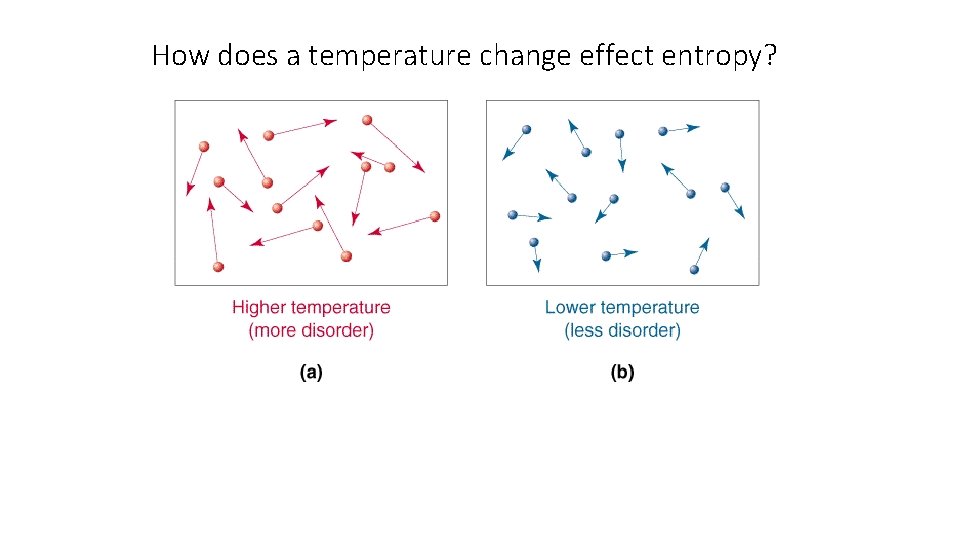
How does a temperature change effect entropy?

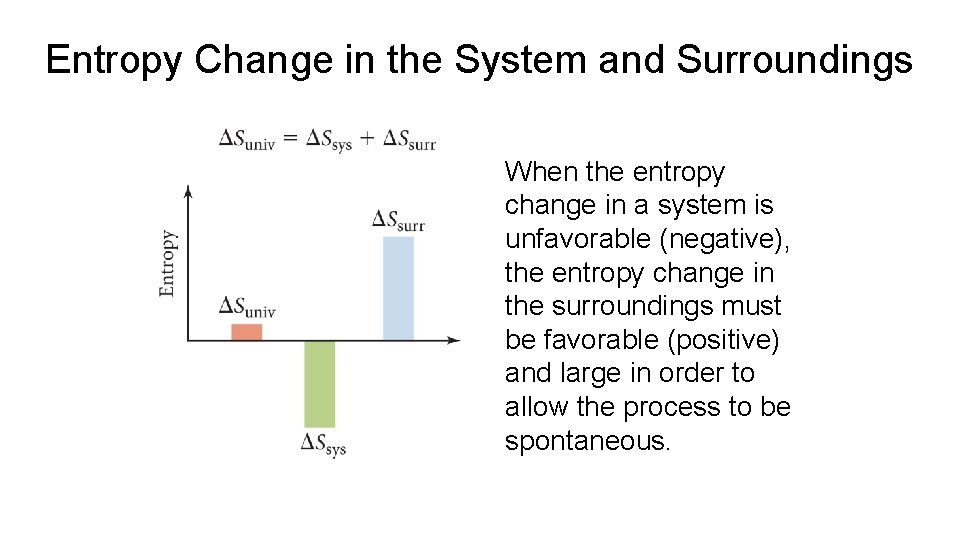
Entropy Change in the System and Surroundings When the entropy change in a system is unfavorable (negative), the entropy change in the surroundings must be favorable (positive) and large in order to allow the process to be spontaneous.


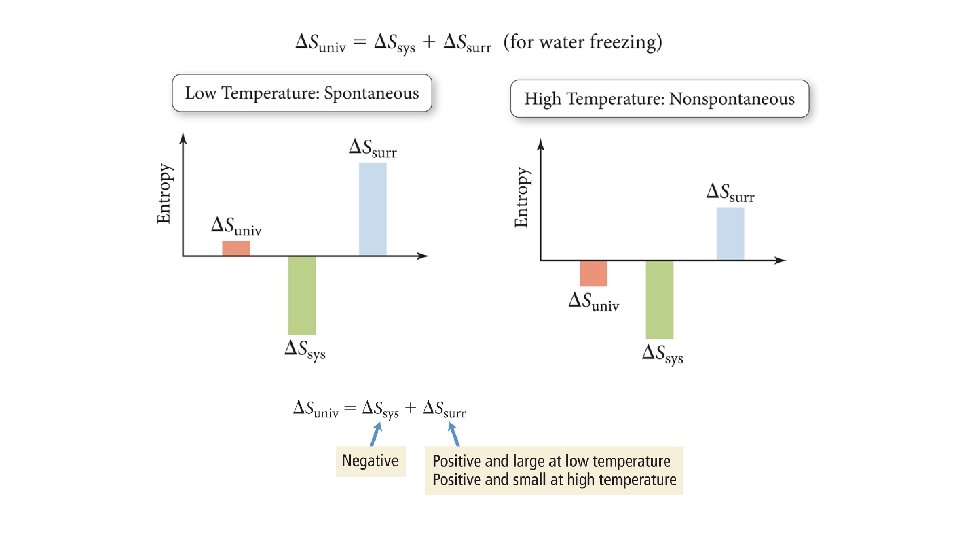
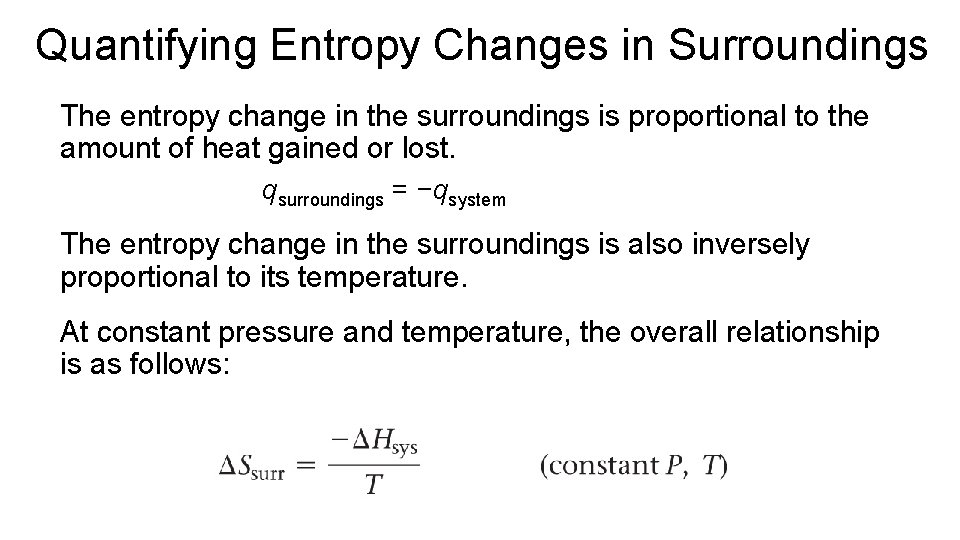
Quantifying Entropy Changes in Surroundings The entropy change in the surroundings is proportional to the amount of heat gained or lost. qsurroundings = −qsystem The entropy change in the surroundings is also inversely proportional to its temperature. At constant pressure and temperature, the overall relationship is as follows:

Entropy and Spontaneity ●Exothermic reaction are often spontaneous ●Reactions that lead to greater disorder are often spontaneous ●ΔStotal = Δ Ssystem + Δ Ssurroundings ●Δ Stotal > 0, spontaneous Δ Stotal < 0, nonspontaneous Δ Stotal = 0, at equilibrium

Given the following reaction at 298 K, 2 N 2(g) + O 2(g) 2 N 2 O(g) ΔHrxn = +163. 2 k. J • Calculate the entropy change in the surroundings when this reaction occurs at 298 K. • Determine the sign of the entropy change for the system. • Determine the sign of the entropy change for the universe. Is the reaction spontaneous?

Given the following reaction at 298 K, N 2(g) + 3 H 2(g) 2 NH 3(g) ΔHrxn = − 91. 8 k. J • Calculate the entropy change in the surroundings when this reaction occurs at 298 K. • Determine the sign of the entropy change for the system. • Determine the sign of the entropy change for the universe. Is the reaction spontaneous?