THE INCREASE OF ENTROPY PRINCIPLE Consider a cycle
- Slides: 7

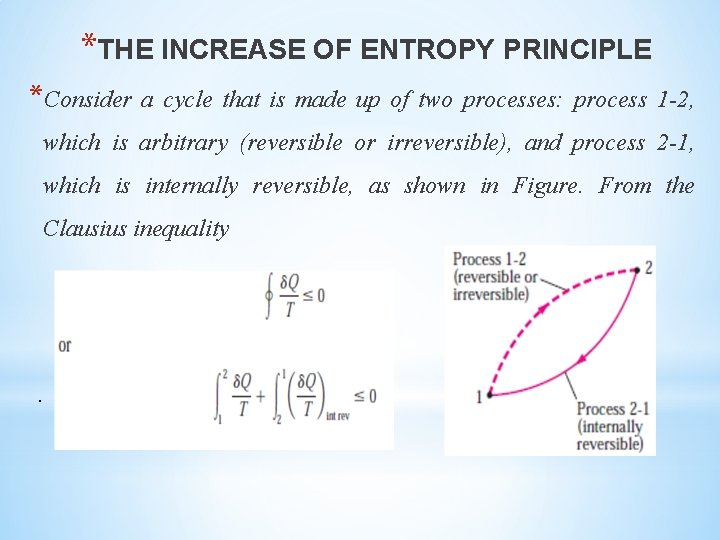
*THE INCREASE OF ENTROPY PRINCIPLE *Consider a cycle that is made up of two processes: process 1 -2, which is arbitrary (reversible or irreversible), and process 2 -1, which is internally reversible, as shown in Figure. From the Clausius inequality .

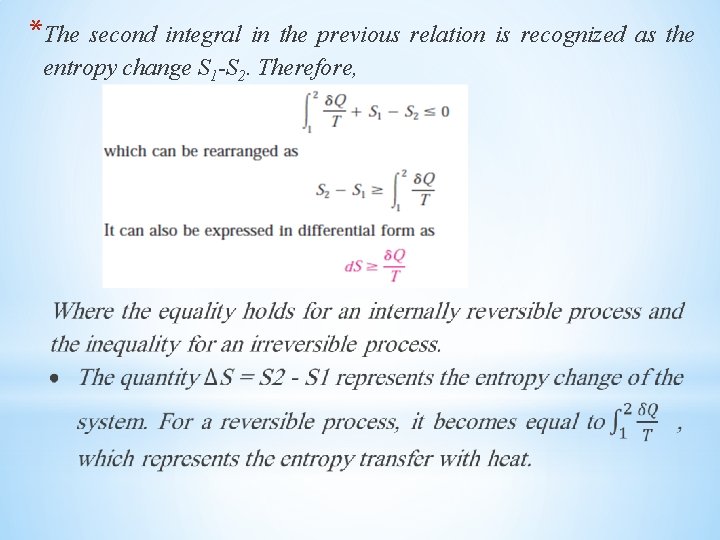
*The second integral in the previous relation is recognized as the entropy change S 1 -S 2. Therefore,

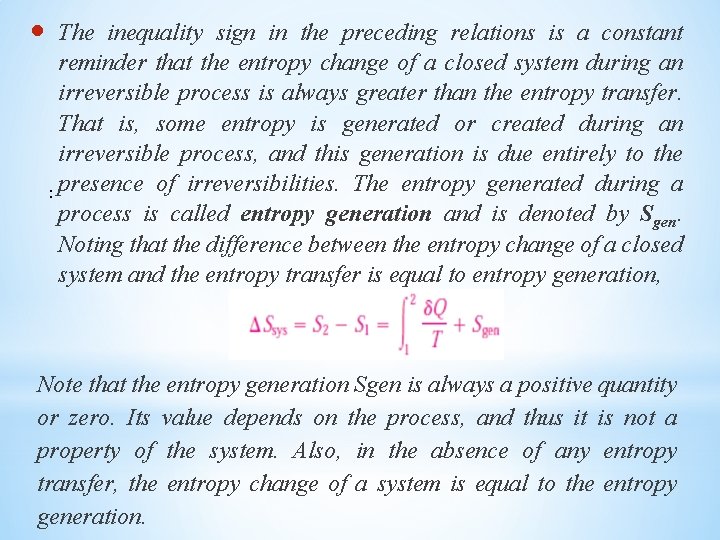
The inequality sign in the preceding relations is a constant reminder that the entropy change of a closed system during an irreversible process is always greater than the entropy transfer. That is, some entropy is generated or created during an irreversible process, and this generation is due entirely to the : presence of irreversibilities. The entropy generated during a process is called entropy generation and is denoted by Sgen. Noting that the difference between the entropy change of a closed system and the entropy transfer is equal to entropy generation, Note that the entropy generation Sgen is always a positive quantity or zero. Its value depends on the process, and thus it is not a property of the system. Also, in the absence of any entropy transfer, the entropy change of a system is equal to the entropy generation.

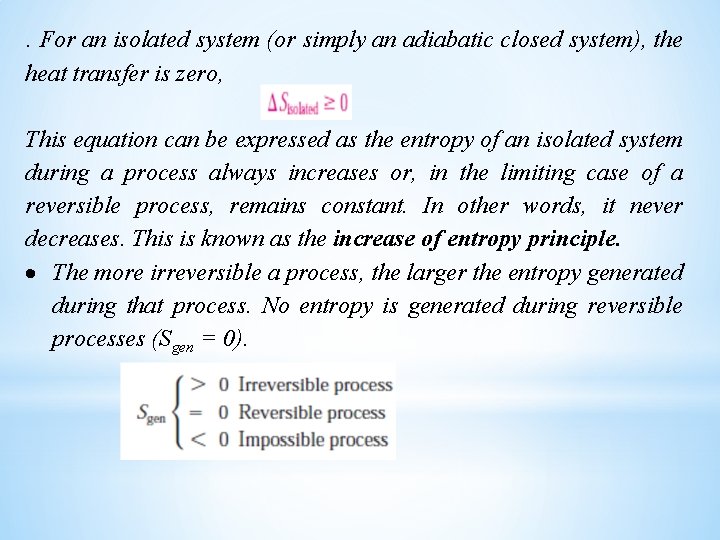
. For an isolated system (or simply an adiabatic closed system), the heat transfer is zero, This equation can be expressed as the entropy of an isolated system during a process always increases or, in the limiting case of a reversible process, remains constant. In other words, it never decreases. This is known as the increase of entropy principle. The more irreversible a process, the larger the entropy generated during that process. No entropy is generated during reversible processes (Sgen = 0).

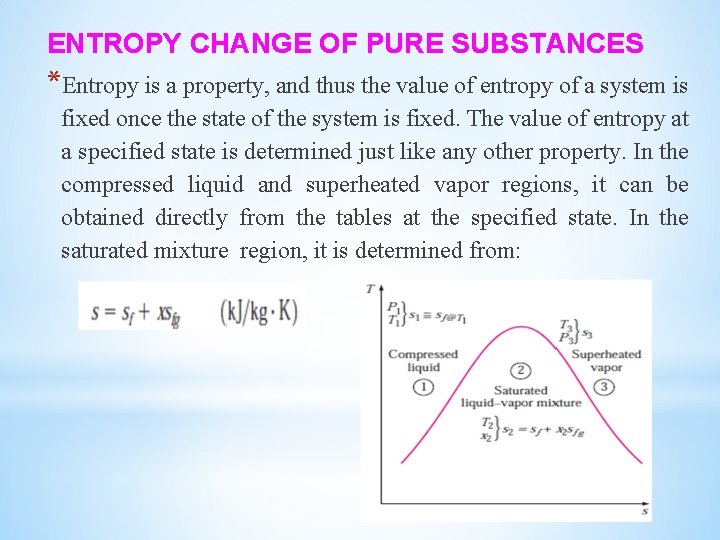
ENTROPY CHANGE OF PURE SUBSTANCES *Entropy is a property, and thus the value of entropy of a system is fixed once the state of the system is fixed. The value of entropy at a specified state is determined just like any other property. In the compressed liquid and superheated vapor regions, it can be obtained directly from the tables at the specified state. In the saturated mixture region, it is determined from:

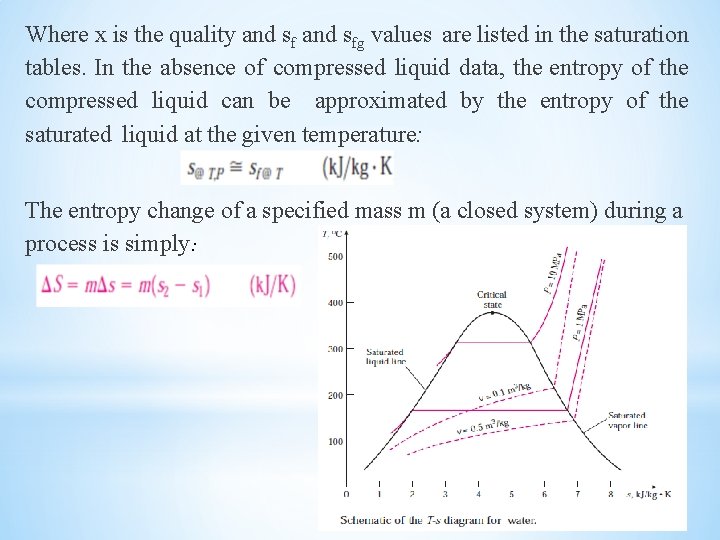
Where x is the quality and sfg values are listed in the saturation tables. In the absence of compressed liquid data, the entropy of the compressed liquid can be approximated by the entropy of the saturated liquid at the given temperature: The entropy change of a specified mass m (a closed system) during a process is simply:

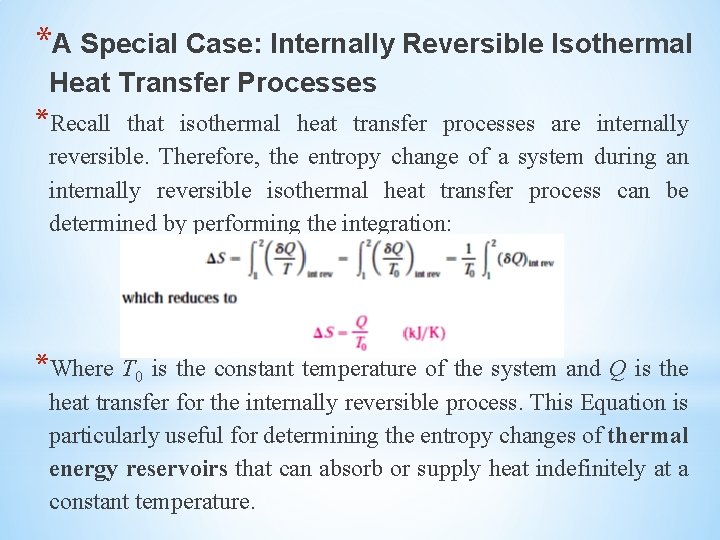
*A Special Case: Internally Reversible Isothermal Heat Transfer Processes *Recall that isothermal heat transfer processes are internally reversible. Therefore, the entropy change of a system during an internally reversible isothermal heat transfer process can be determined by performing the integration: *Where T 0 is the constant temperature of the system and Q is the heat transfer for the internally reversible process. This Equation is particularly useful for determining the entropy changes of thermal energy reservoirs that can absorb or supply heat indefinitely at a constant temperature.