CHAPTER 6 ENTROPY ENTROPY l The first law














- Slides: 40

CHAPTER 6 ENTROPY

ENTROPY l The first law of thermodynamics deals with the property energy and the conservation of it. l The second law leads to the definition of a new property called entropy. l Unlike energy, entropy is a nonconserved property, and there is no such thing as conservation of entropy principle.



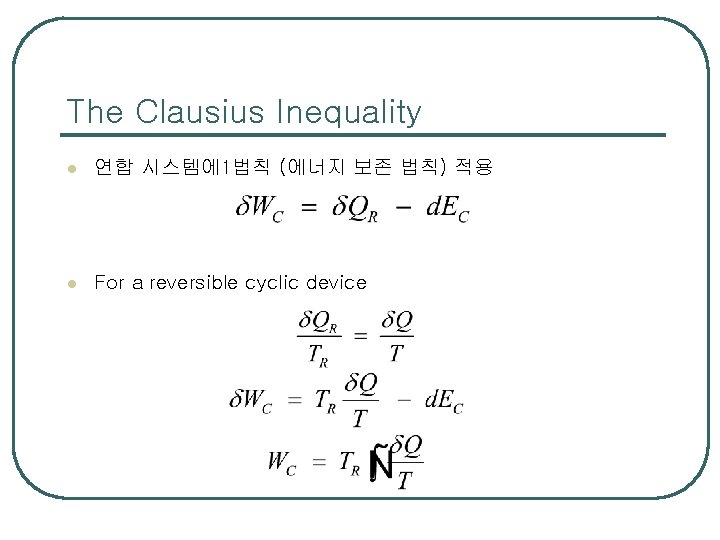
The Clausius Inequality l 연합 시스템에 1법칙 (에너지 보존 법칙) 적용 l For a reversible cyclic device

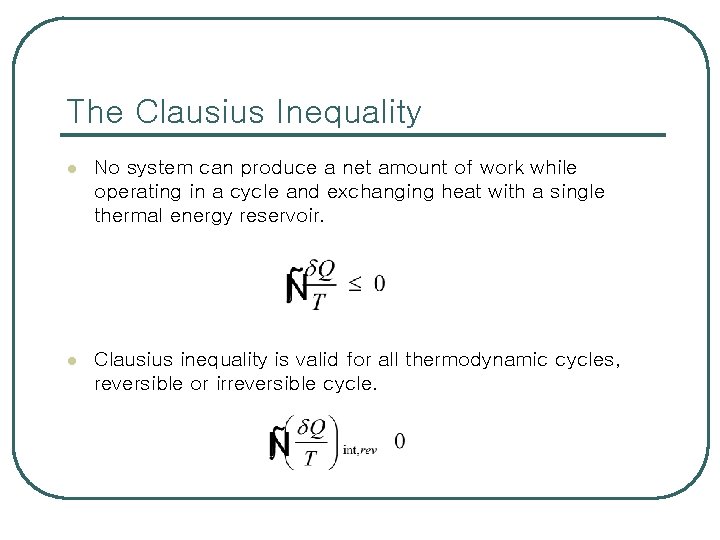
The Clausius Inequality l No system can produce a net amount of work while operating in a cycle and exchanging heat with a single thermal energy reservoir. l Clausius inequality is valid for all thermodynamic cycles, reversible or irreversible cycle.

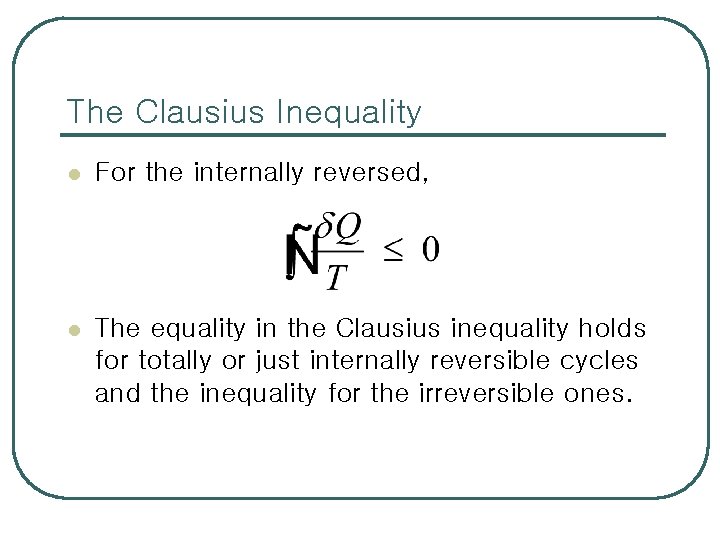
The Clausius Inequality l For the internally reversed, l The equality in the Clausius inequality holds for totally or just internally reversible cycles and the inequality for the irreversible ones.



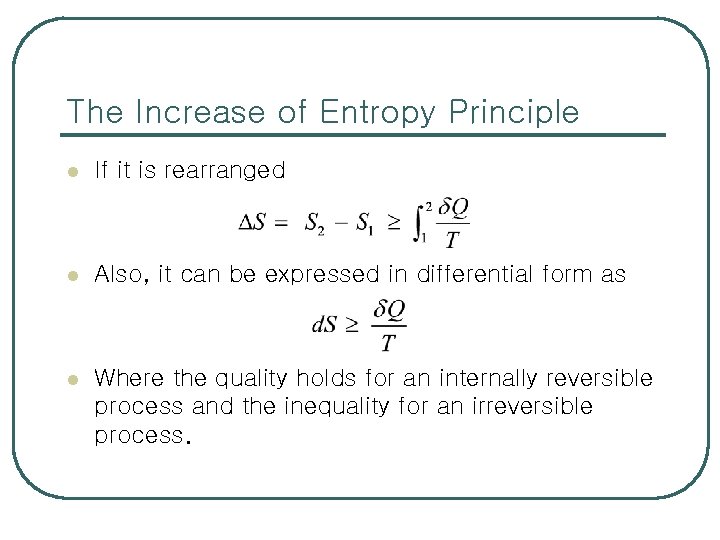
6 -3 엔트로피 증가 법칙 (The Increase of Entropy Principle) l Consider a cycle that is made up of 2 processes: Process 1 -2 and process 2 -1 or The 2 nd integral in the above relation is readily recognized as the entropy change S 1 – S 2.

The Increase of Entropy Principle l If it is rearranged l Also, it can be expressed in differential form as l Where the quality holds for an internally reversible process and the inequality for an irreversible process.


The Increase of Entropy Principle l The increase of entropy principle can be summarized as follows; l The things in nature have a tendency to change until they attain a state of equilibrium. The increase of entropy principle dictates that the entropy of an isolated system will increase until the entropy of the system reaches a maximum value.

Some Remarks about Entropy l l l Process can occur in a certain direction only, not in any direction. A process must proceed in the direction that complies with the increase of entropy principle, that is, Sgen≥ 0. A process that violates this principle is impossible. Entropy is a nonconserved property, and there is no such thing as the conservation of entropy principle. Entropy is conserved during the idealized reversible processes only and increases during all actual process. Therefore, the entropy of the universe is continuously increasing. The performance of engineering systems is degraded by the presence of irreversibilities, and the entropy generation is a measure of the magnitudes of the irreversibilities present during that process. The greater the extent of irreversibilities, the greater the entropy generation. Therefore, entropy can be used as a quantitative measure of irreversibilities associated with a process.






What is Entropy l l Entropy can be viewed as a measure of molecular disorder, or molecular randomness. The entropy of a substance is lowest in the solid phase and highest in the gas phase.

6 -6 T-S 선도 (The T-s Diagram) l 열역학 2법칙에서는, the temperature-entropy and the enthalpy-entropy diagrams are very helpful to analysis. l The defining equation of entropy can be rearranged as Qint rev = T d. S (k. J)

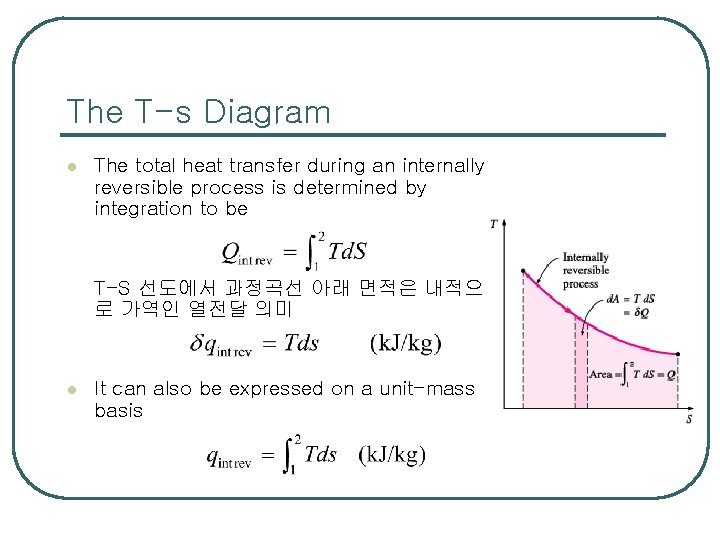
The T-s Diagram l The total heat transfer during an internally reversible process is determined by integration to be T-S 선도에서 과정곡선 아래 면적은 내적으 로 가역인 열전달 의미 l It can also be expressed on a unit-mass basis


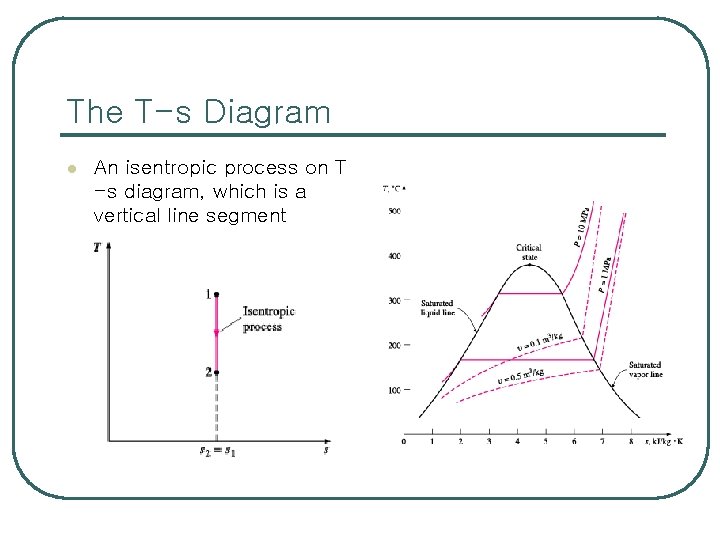
The T-s Diagram l An isentropic process on T -s diagram, which is a vertical line segment


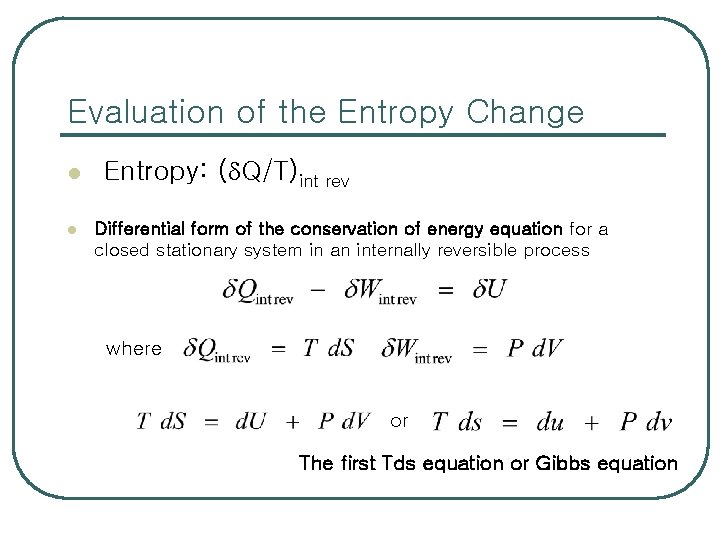
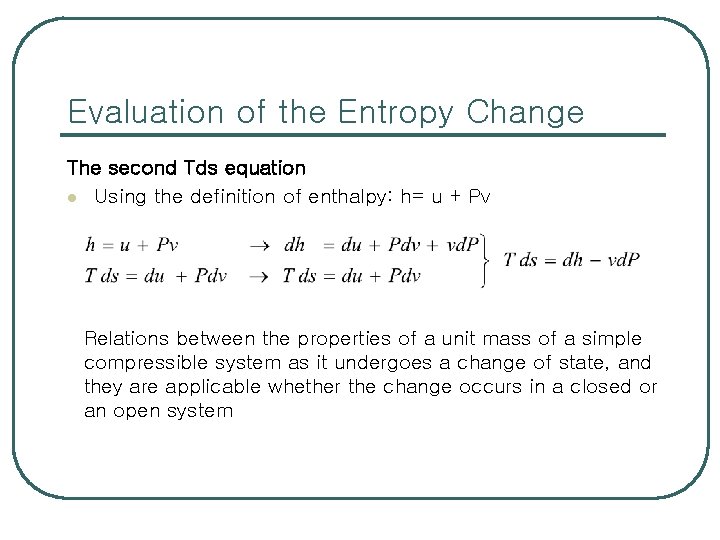
Evaluation of the Entropy Change l l Entropy: ( Q/T)int rev Differential form of the conservation of energy equation for a closed stationary system in an internally reversible process where or The first Tds equation or Gibbs equation

Evaluation of the Entropy Change The second Tds equation l Using the definition of enthalpy: h= u + Pv Relations between the properties of a unit mass of a simple compressible system as it undergoes a change of state, and they are applicable whether the change occurs in a closed or an open system

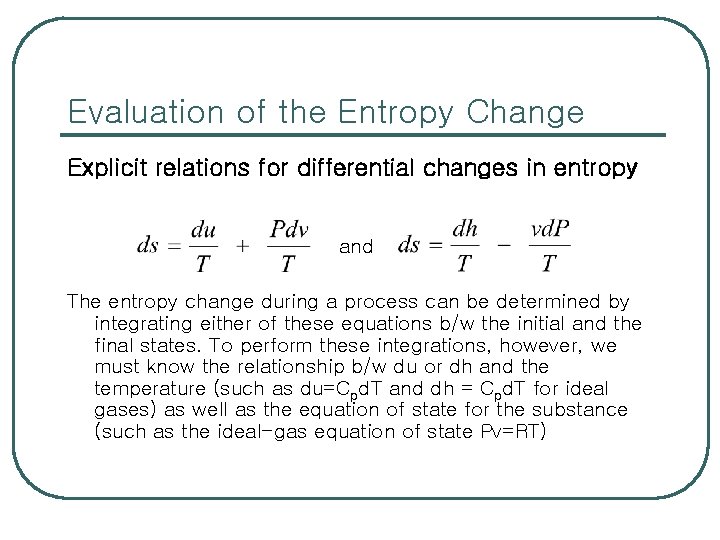
Evaluation of the Entropy Change Explicit relations for differential changes in entropy and The entropy change during a process can be determined by integrating either of these equations b/w the initial and the final states. To perform these integrations, however, we must know the relationship b/w du or dh and the temperature (such as du=Cpd. T and dh = Cpd. T for ideal gases) as well as the equation of state for the substance (such as the ideal-gas equation of state Pv=RT)

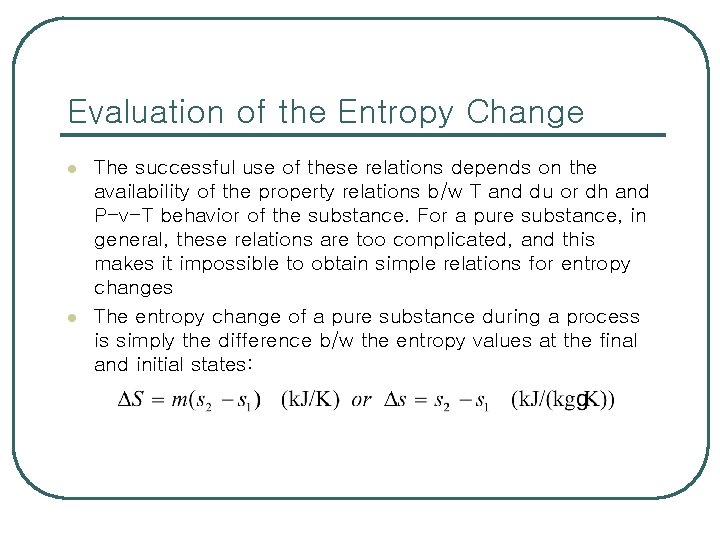
Evaluation of the Entropy Change l l The successful use of these relations depends on the availability of the property relations b/w T and du or dh and P-v-T behavior of the substance. For a pure substance, in general, these relations are too complicated, and this makes it impossible to obtain simple relations for entropy changes The entropy change of a pure substance during a process is simply the difference b/w the entropy values at the final and initial states:

Evaluation of the Entropy Change Isentropic Processes l l The entropy of a fixed mass will not change during an internally reversible, adiabatic process, which is called an isentropic (constant entropy) process. An isentropic process appears as a vertical line on a T-s diagram. Most engineering systems perform best when the irreversibilities are minimized. Therefore, an isentropic process can serve as an appropriate model for actual processes. Also isentropic processes enables us to these define efficiencies for processes to compare the actual performance of these devices to the performance under idealized conditions.

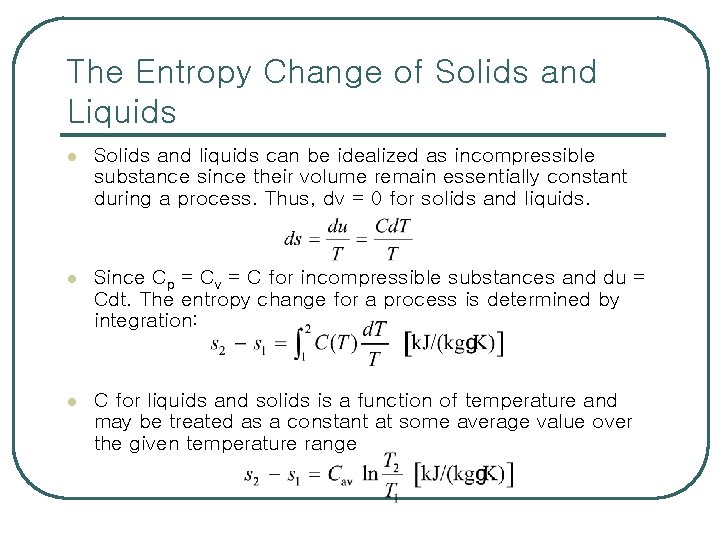
The Entropy Change of Solids and Liquids l Solids and liquids can be idealized as incompressible substance since their volume remain essentially constant during a process. Thus, dv = 0 for solids and liquids. l Since Cp = Cv = C for incompressible substances and du = Cdt. The entropy change for a process is determined by integration: l C for liquids and solids is a function of temperature and may be treated as a constant at some average value over the given temperature range

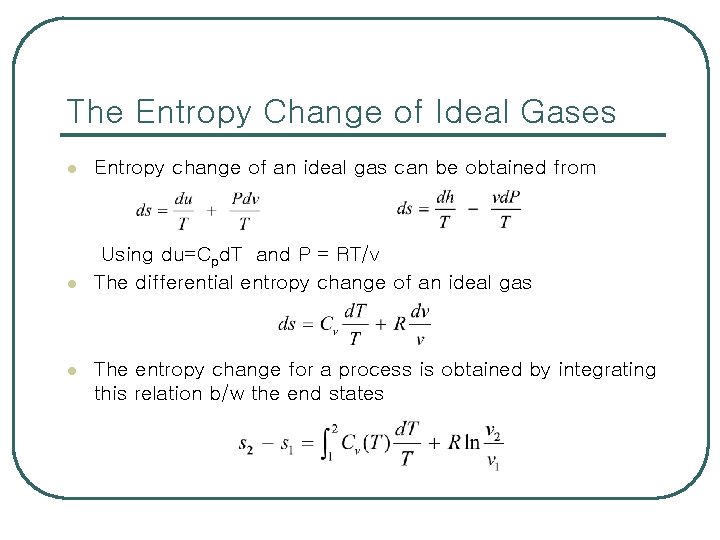
The Entropy Change of Ideal Gases l Entropy change of an ideal gas can be obtained from l Using du=Cpd. T and P = RT/v The differential entropy change of an ideal gas l The entropy change for a process is obtained by integrating this relation b/w the end states

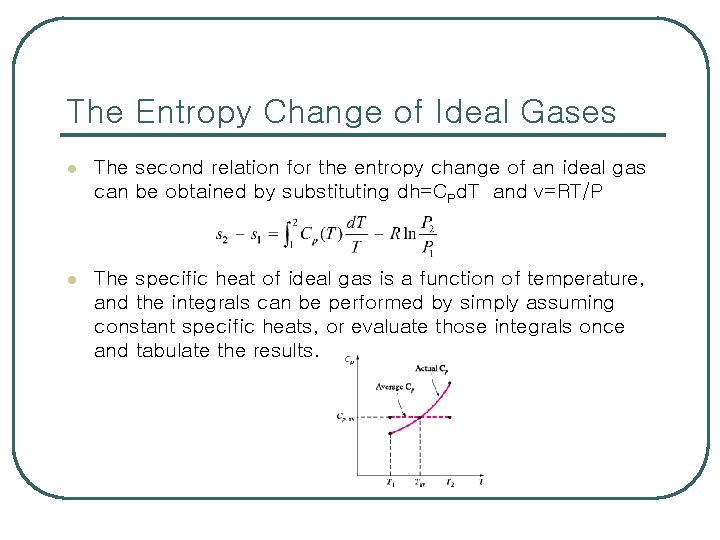
The Entropy Change of Ideal Gases l The second relation for the entropy change of an ideal gas can be obtained by substituting dh=CPd. T and v=RT/P l The specific heat of ideal gas is a function of temperature, and the integrals can be performed by simply assuming constant specific heats, or evaluate those integrals once and tabulate the results.

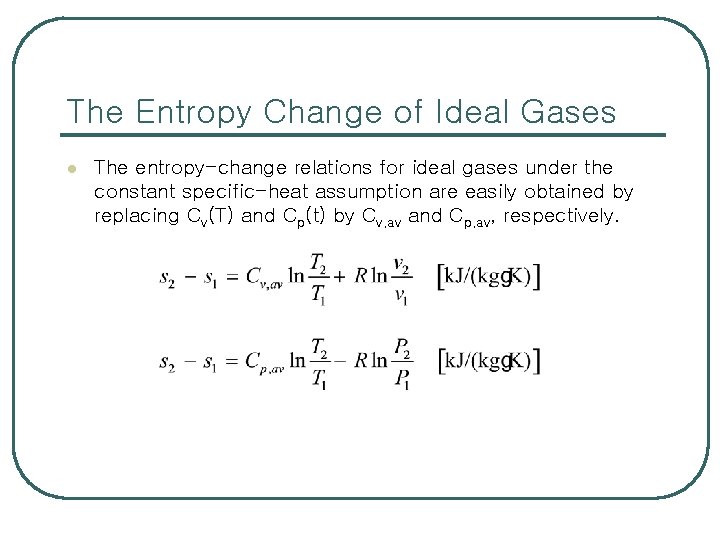
The Entropy Change of Ideal Gases l The entropy-change relations for ideal gases under the constant specific-heat assumption are easily obtained by replacing Cv(T) and Cp(t) by Cv, av and Cp, av, respectively.

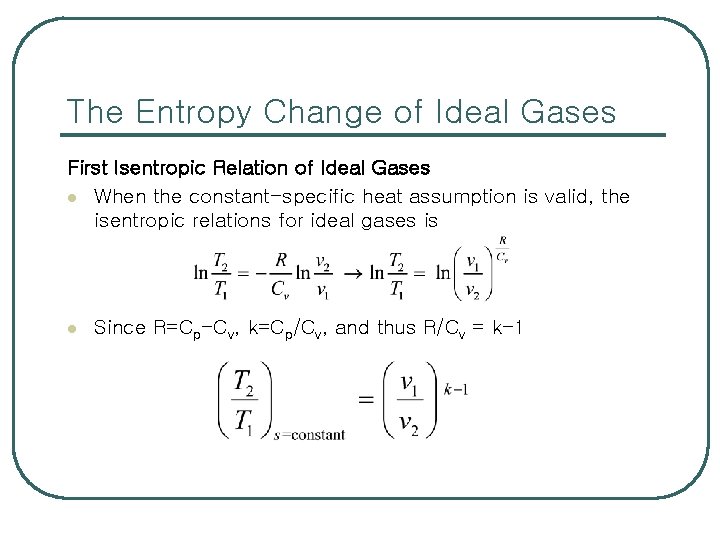
The Entropy Change of Ideal Gases First Isentropic Relation of Ideal Gases l When the constant-specific heat assumption is valid, the isentropic relations for ideal gases is l Since R=Cp-Cv, k=Cp/Cv, and thus R/Cv = k-1

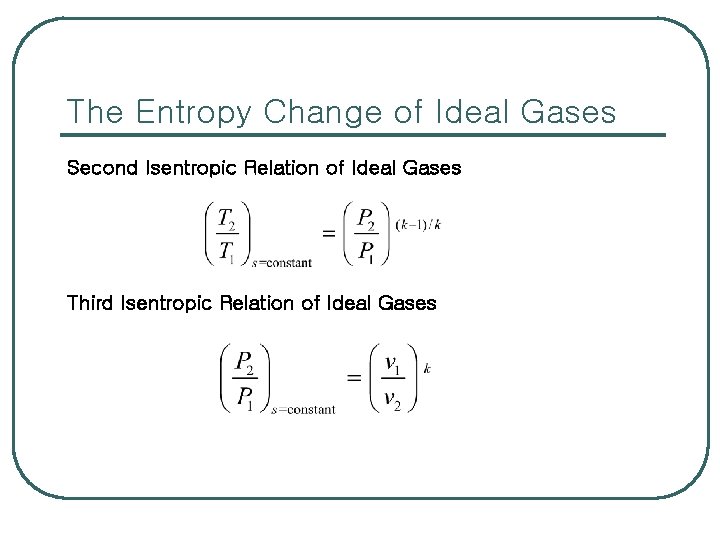
The Entropy Change of Ideal Gases Second Isentropic Relation of Ideal Gases Third Isentropic Relation of Ideal Gases

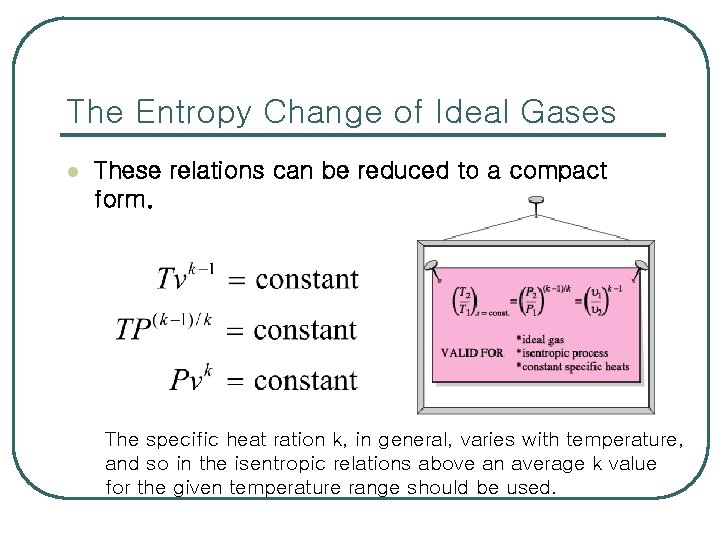
The Entropy Change of Ideal Gases l These relations can be reduced to a compact form. The specific heat ration k, in general, varies with temperature, and so in the isentropic relations above an average k value for the given temperature range should be used.

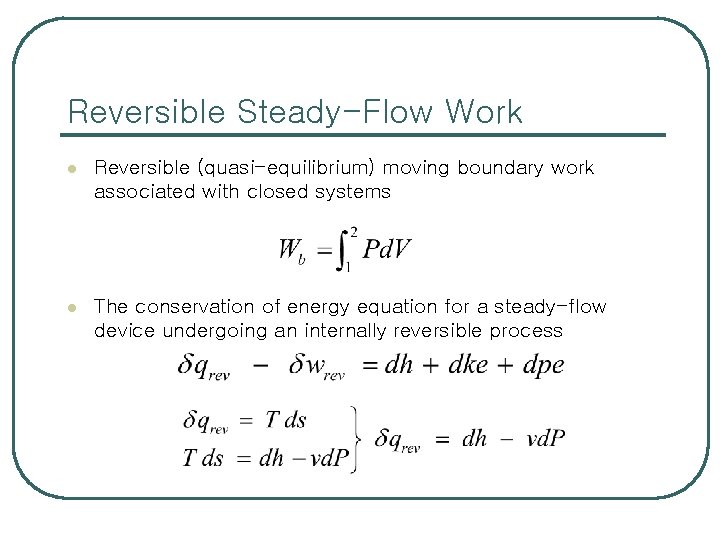
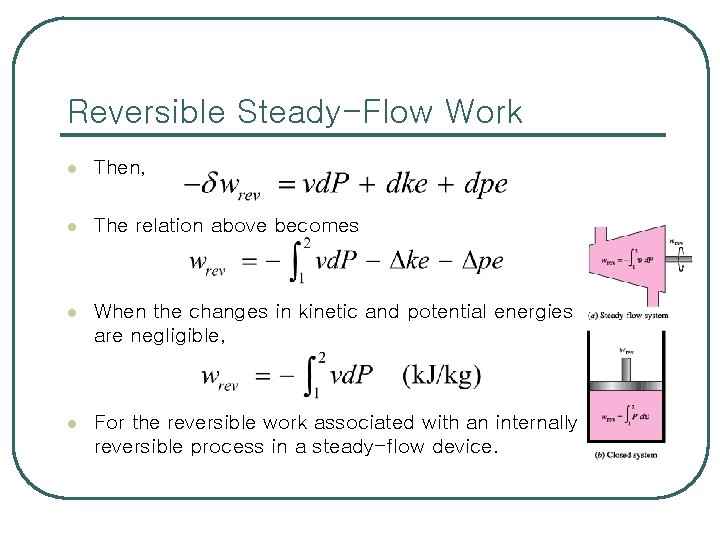
Reversible Steady-Flow Work l Reversible (quasi-equilibrium) moving boundary work associated with closed systems l The conservation of energy equation for a steady-flow device undergoing an internally reversible process

Reversible Steady-Flow Work l Then, l The relation above becomes l When the changes in kinetic and potential energies are negligible, l For the reversible work associated with an internally reversible process in a steady-flow device.

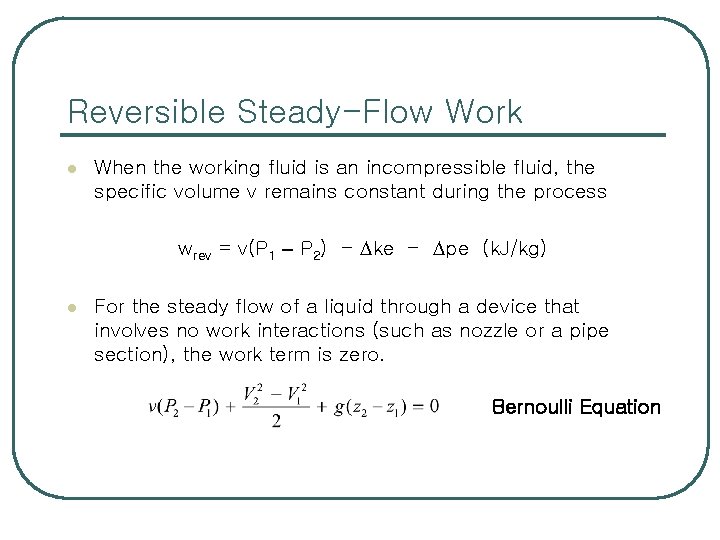
Reversible Steady-Flow Work l When the working fluid is an incompressible fluid, the specific volume v remains constant during the process wrev = v(P 1 – P 2) - ke - pe (k. J/kg) l For the steady flow of a liquid through a device that involves no work interactions (such as nozzle or a pipe section), the work term is zero. Bernoulli Equation