Electrolytic Cells Electrolysis Reactions NonSpontaneous Redox Reaction Outside







- Slides: 24

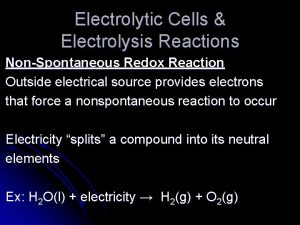
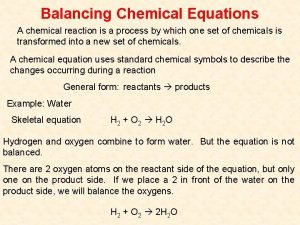
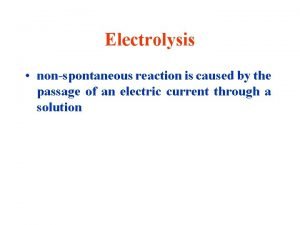
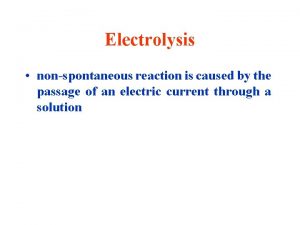
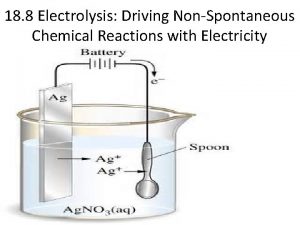
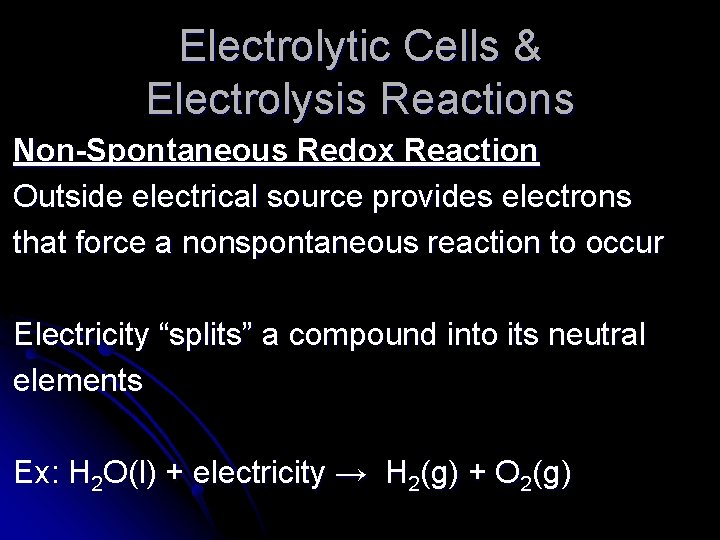
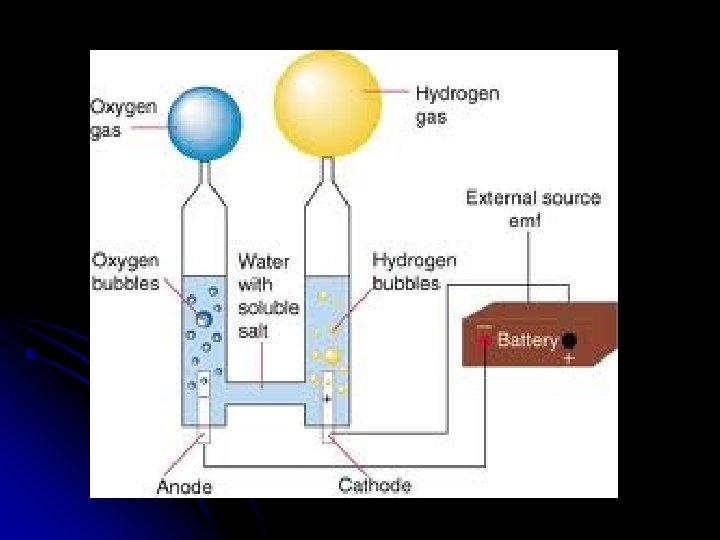
Electrolytic Cells & Electrolysis Reactions Non-Spontaneous Redox Reaction Outside electrical source provides electrons that force a nonspontaneous reaction to occur Electricity “splits” a compound into its neutral elements Ex: H 2 O(l) + electricity → H 2(g) + O 2(g)

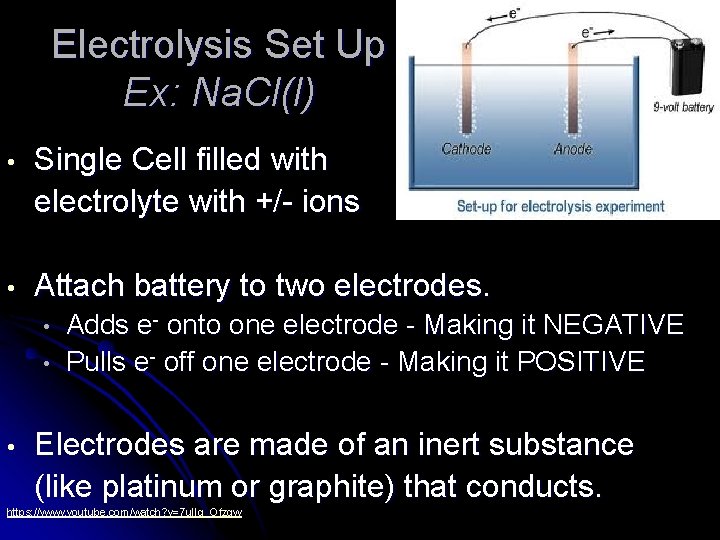
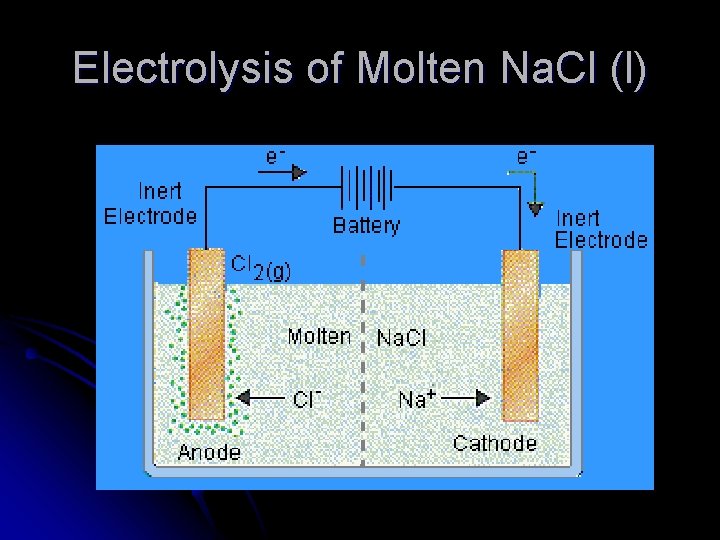
Electrolysis Set Up Ex: Na. Cl(l) • Single Cell filled with electrolyte with +/- ions • Attach battery to two electrodes. • • • Adds e- onto one electrode - Making it NEGATIVE Pulls e- off one electrode - Making it POSITIVE Electrodes are made of an inert substance (like platinum or graphite) that conducts. https: //www. youtube. com/watch? v=7 u. IIq_Ofzgw

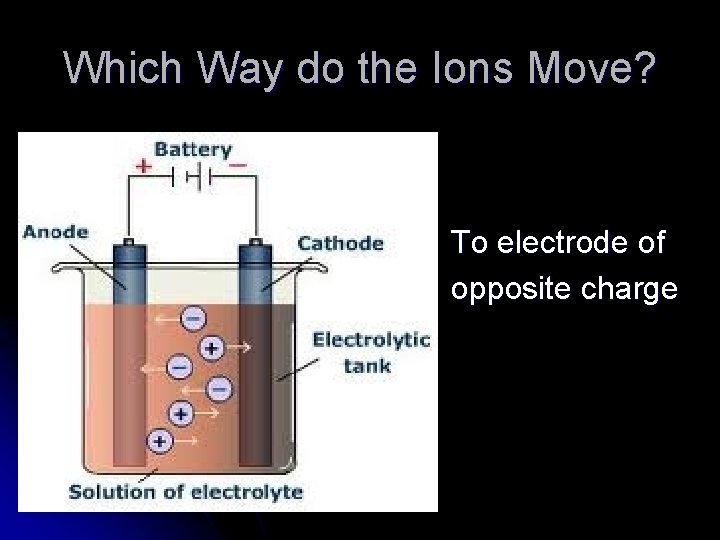
Which Way do the Ions Move? To electrode of opposite charge

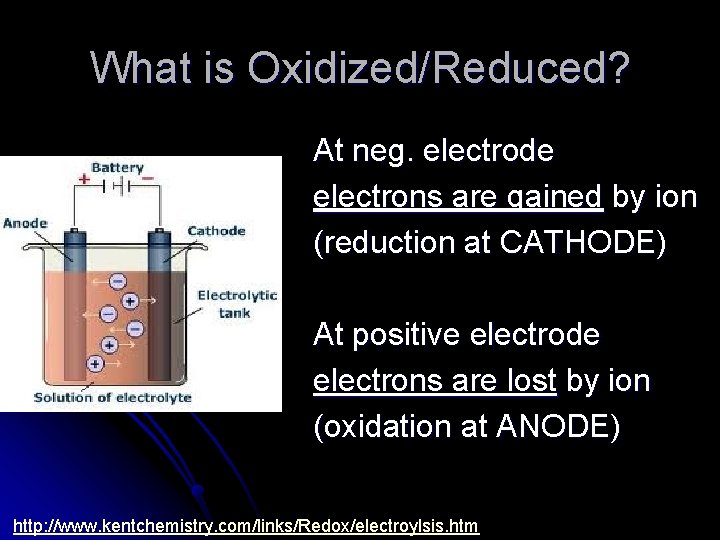
What is Oxidized/Reduced? At neg. electrode electrons are gained by ion (reduction at CATHODE) At positive electrode electrons are lost by ion (oxidation at ANODE) http: //www. kentchemistry. com/links/Redox/electroylsis. htm

Remember AN OX RED CAT Anode is where oxidation happens Cathode is where reduction happens

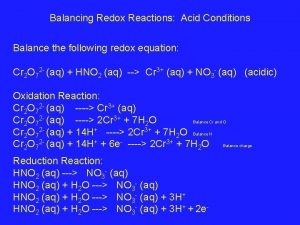
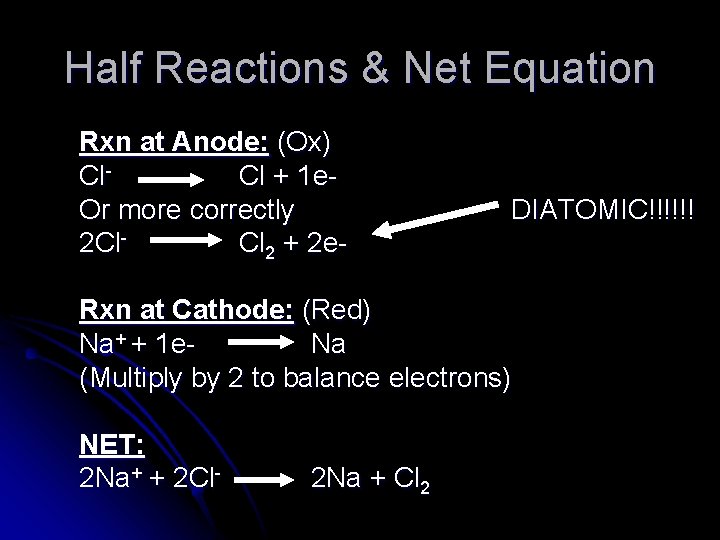
Half Reactions & Net Equation Rxn at Anode: (Ox) Cl Cl + 1 e. Or more correctly 2 Cl Cl 2 + 2 e- DIATOMIC!!!!!! Rxn at Cathode: (Red) Na+ + 1 e Na (Multiply by 2 to balance electrons) NET: 2 Na+ + 2 Cl- 2 Na + Cl 2

Electrolysis of Molten Na. Cl (l)

Determining Voltage Needed (Honors) Use the Voltage Table to determine the total voltage “needed” to run the Electrolytic cell. Total voltage should be a NEGATIVE number

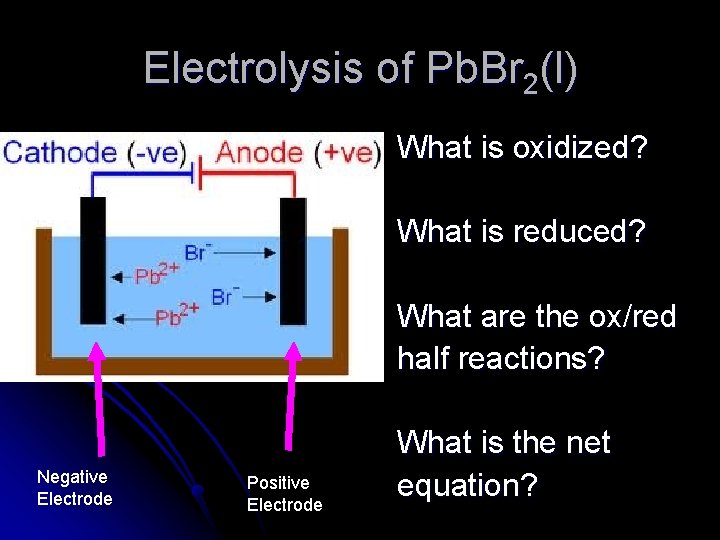
Electrolysis of Pb. Br 2(l) What is oxidized? What is reduced? What are the ox/red half reactions? Negative Electrode Positive Electrode What is the net equation?

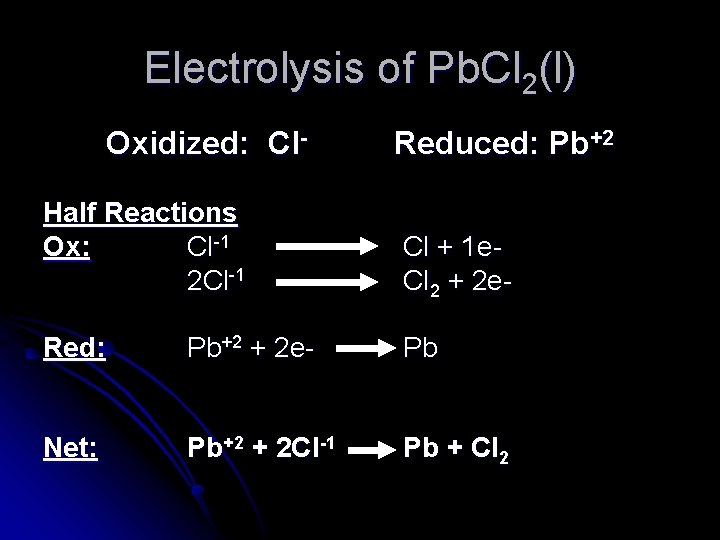
Electrolysis of Pb. Cl 2(l) Oxidized: Cl- Reduced: Pb+2 Half Reactions Ox: Cl-1 2 Cl-1 Cl + 1 e. Cl 2 + 2 e- Red: Pb+2 + 2 e- Pb Net: Pb+2 + 2 Cl-1 Pb + Cl 2

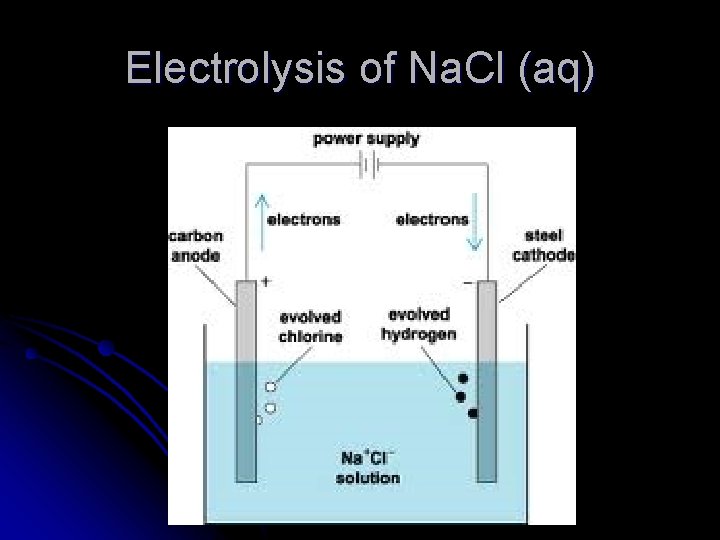
Electrolysis of Na. Cl (aq)

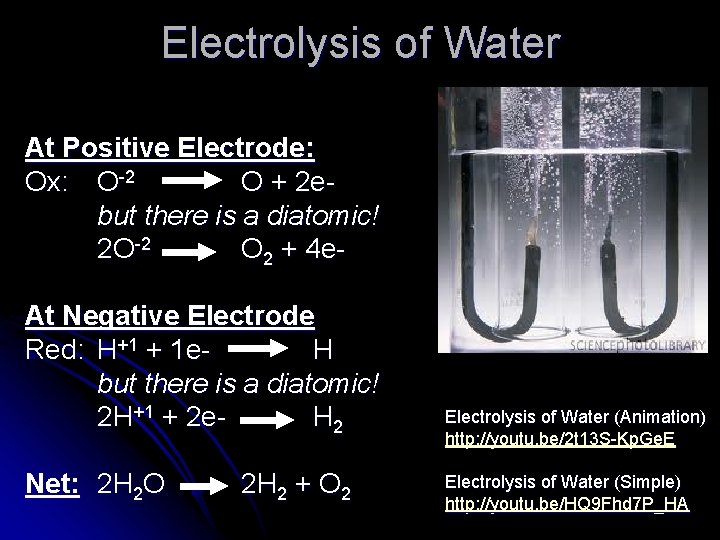
Electrolysis of Water At Positive Electrode: Ox: O-2 O + 2 e- but there is a diatomic! 2 O-2 O 2 + 4 e. At Negative Electrode Red: H+1 + 1 e. H but there is a diatomic! 2 H+1 + 2 e. H 2 Net: 2 H 2 O 2 H 2 + O 2 Electrolysis of Water (Animation) http: //youtu. be/2 t 13 S-Kp. Ge. E Electrolysis of Water (Simple) http: //youtu. be/HQ 9 Fhd 7 P_HA


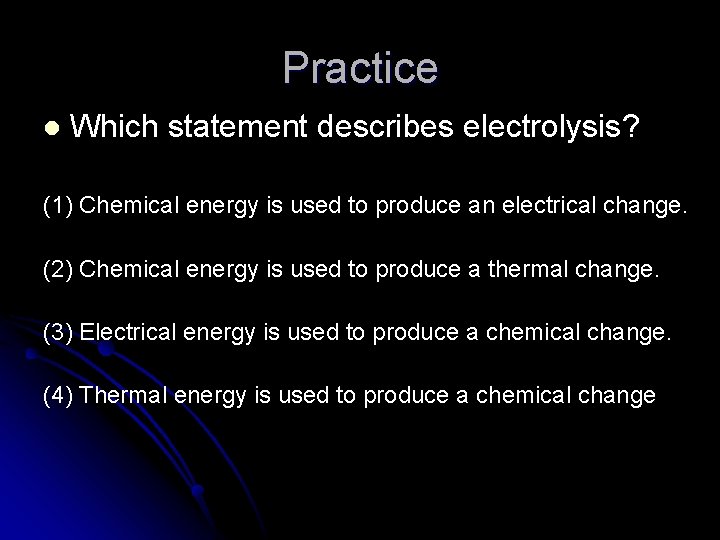
Practice l Which statement describes electrolysis? (1) Chemical energy is used to produce an electrical change. (2) Chemical energy is used to produce a thermal change. (3) Electrical energy is used to produce a chemical change. (4) Thermal energy is used to produce a chemical change

l Given the balanced equation representing a reaction occurring in an electrolytic cell: 2 Na. Cl(l) 2 Na(l) + Cl 2(g) Where is Na(l) produced in the cell? (1) at the anode, where oxidation occurs (2) at the anode, where reduction occurs (3) at the cathode, where oxidation occurs (4) at the cathode, where reduction occurs

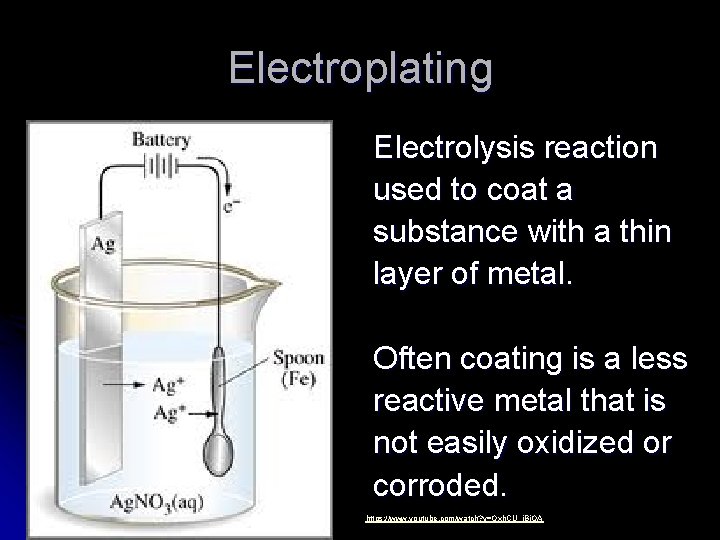
Electroplating Electrolysis reaction used to coat a substance with a thin layer of metal. Often coating is a less reactive metal that is not easily oxidized or corroded. https: //www. youtube. com/watch? v=Oxh. CU_j. Bi. OA

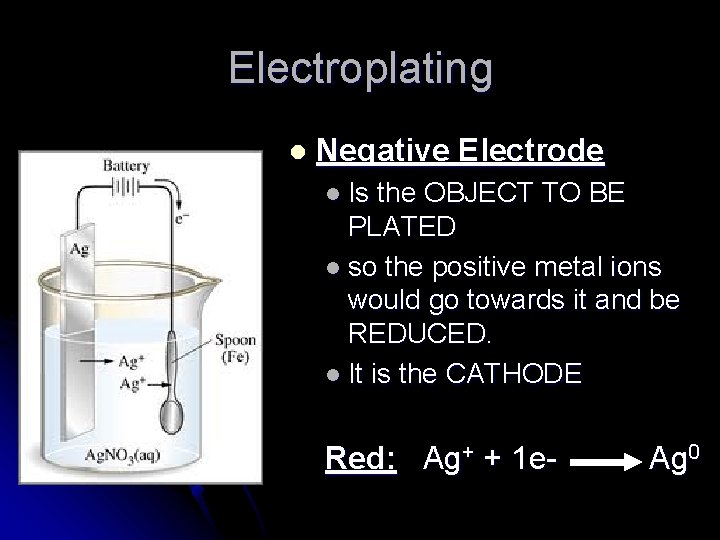
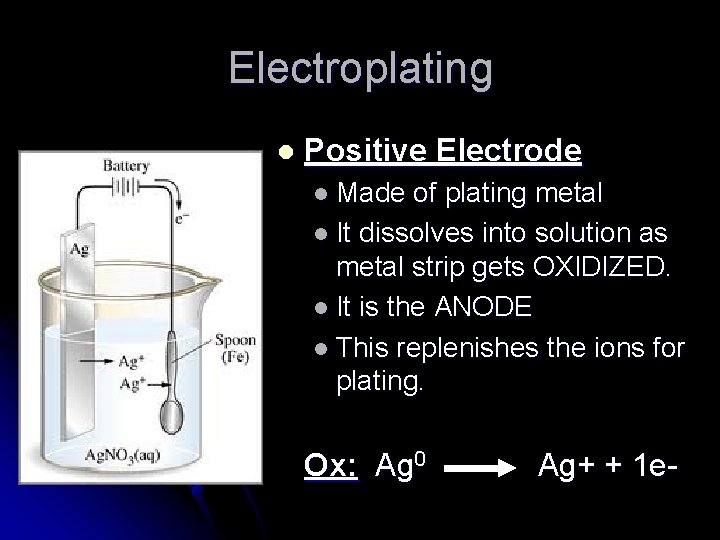
Electroplating l Negative Electrode l Is the OBJECT TO BE PLATED l so the positive metal ions would go towards it and be REDUCED. l It is the CATHODE Red: Ag+ + 1 e- Ag 0

Electroplating l Positive Electrode l Made of plating metal l It dissolves into solution as metal strip gets OXIDIZED. l It is the ANODE l This replenishes the ions for plating. Ox: Ag 0 Ag+ + 1 e-

Electroplating Problems (Honors) Coulomb = measure of electrical charge, related to certain amount of electrons passing through system Amp = how many coulombs per second REMEMBER: 1 mole e- = 96, 500 coulombs # coulombs = # amps x seconds

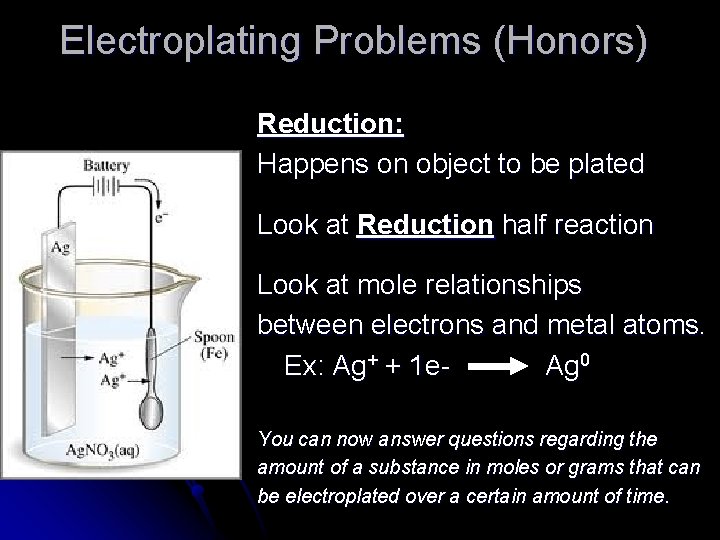
Electroplating Problems (Honors) Reduction: Happens on object to be plated Look at Reduction half reaction Look at mole relationships between electrons and metal atoms. Ex: Ag+ + 1 e. Ag 0 You can now answer questions regarding the amount of a substance in moles or grams that can be electroplated over a certain amount of time.

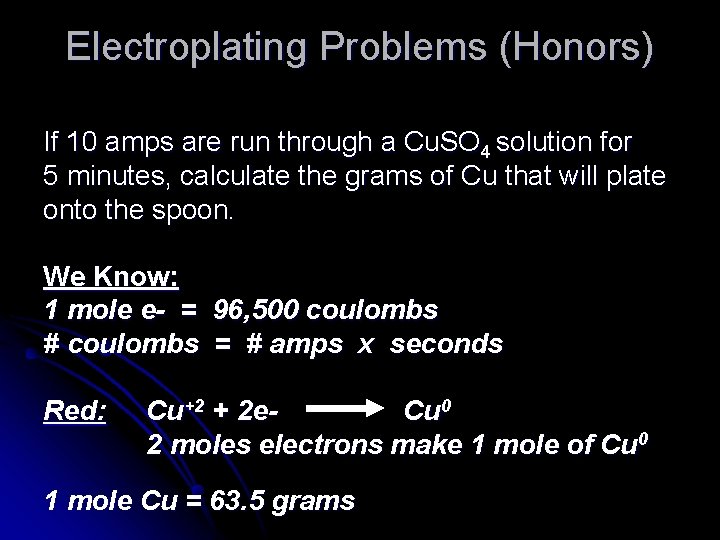
Electroplating Problems (Honors) If 10 amps are run through a Cu. SO 4 solution for 5 minutes, calculate the grams of Cu that will plate onto the spoon. We Know: 1 mole e- = 96, 500 coulombs # coulombs = # amps x seconds Red: Cu+2 + 2 e. Cu 0 2 moles electrons make 1 mole of Cu 0 1 mole Cu = 63. 5 grams

So…. Let’s start here # coulombs = 10 amps x 300 seconds = 3000 coulombs 3000 coul. x 1 mole e- x 1 mole Cu x 63. 5 g Cu =. 987 grams 96, 500 coul 2 mole e- 1 mole Cu Mole ratio from Reduction half reaction Cu+2 + 2 e. Cu 0

You Try One How long will it take to deposit 20 grams of silver from a solution of Ag. Cl onto a copper tray if a current of 5 amps is used? Answer = 3, 574 sec or 59. 5 minutes or about 1 hour

You Try One How many amps are needed to deposit. 504 g. of Iron in 40 minutes by passing a current through a solution of Iron II Sulfate? Answer: . 72 amps
 Nuclear reaction equation
Nuclear reaction equation Example of redox reaction
Example of redox reaction Electrolysis vs voltaic cell
Electrolysis vs voltaic cell Galvanic cell khan academy
Galvanic cell khan academy Application of electrolytic cell
Application of electrolytic cell Example of electrolytic decomposition reaction
Example of electrolytic decomposition reaction Are nonspontaneous processes impossible
Are nonspontaneous processes impossible Classify each process as spontaneous or nonspontaneous.
Classify each process as spontaneous or nonspontaneous. How redox reactions work
How redox reactions work Balance redox reactions
Balance redox reactions Building redox tables
Building redox tables Reactions that produce gas
Reactions that produce gas Is photosynthesis a redox reaction
Is photosynthesis a redox reaction Leo goes ger
Leo goes ger Chapter 19 review oxidation-reduction reactions answers
Chapter 19 review oxidation-reduction reactions answers Concept map redox reactions
Concept map redox reactions Rules for balancing redox reactions
Rules for balancing redox reactions Oxidation half reaction
Oxidation half reaction Redox reactions ncea level 2
Redox reactions ncea level 2 Balancing complex redox reactions
Balancing complex redox reactions Balancing redox reactions
Balancing redox reactions Types of chemical reactions redox
Types of chemical reactions redox How to balance an equation step by step
How to balance an equation step by step S
S Oxidation states chemsheets answers
Oxidation states chemsheets answers