Mullis 1 Steps for Net Ionic Equations Write

- Slides: 6

Mullis 1

Steps for Net Ionic Equations Write the chemical formula equation. 2. Decide which are strong electrolytes and put a line between the anion and the cation. Also, show the charge of the ion. 3. If the species is not a strong electrolyte, draw a box around it so you will remember to leave it as a compound. 4. Cancel the spectator ions. 1. Mullis 2

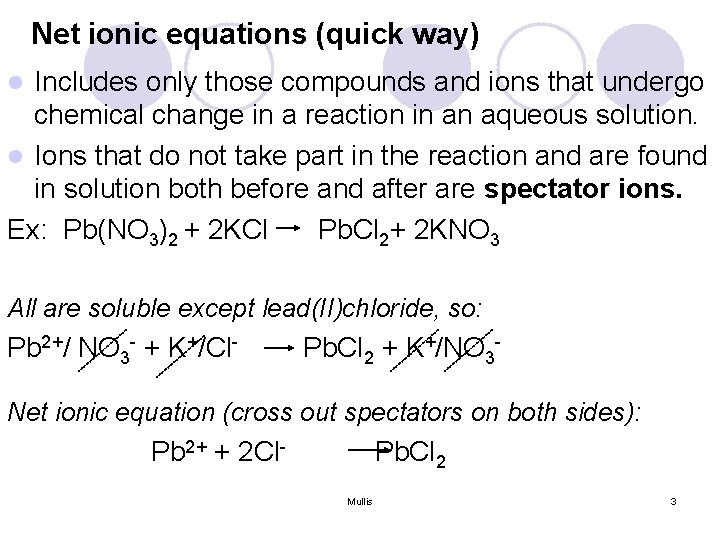
Net ionic equations (quick way) Includes only those compounds and ions that undergo chemical change in a reaction in an aqueous solution. l Ions that do not take part in the reaction and are found in solution both before and after are spectator ions. Ex: Pb(NO 3)2 + 2 KCl Pb. Cl 2+ 2 KNO 3 l All are soluble except lead(II)chloride, so: Pb 2+/ NO 3 - + K+/Cl- Pb. Cl 2 + K+/NO 3 - Net ionic equation (cross out spectators on both sides): Pb 2+ + 2 Cl- Pb. Cl 2 Mullis 3

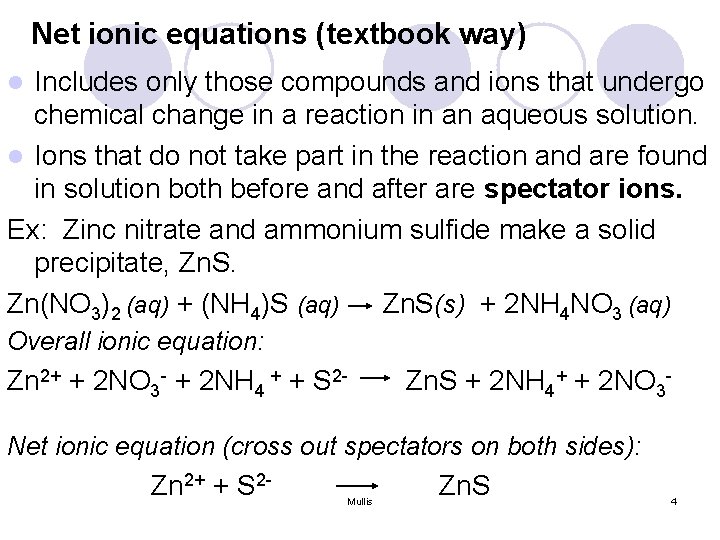
Net ionic equations (textbook way) Includes only those compounds and ions that undergo chemical change in a reaction in an aqueous solution. l Ions that do not take part in the reaction and are found in solution both before and after are spectator ions. Ex: Zinc nitrate and ammonium sulfide make a solid precipitate, Zn. S. Zn(NO 3)2 (aq) + (NH 4)S (aq) Zn. S(s) + 2 NH 4 NO 3 (aq) l Overall ionic equation: Zn 2+ + 2 NO 3 - + 2 NH 4 + + S 2 - Zn. S + 2 NH 4+ + 2 NO 3 - Net ionic equation (cross out spectators on both sides): Zn 2+ + S 2 - Mullis Zn. S 4

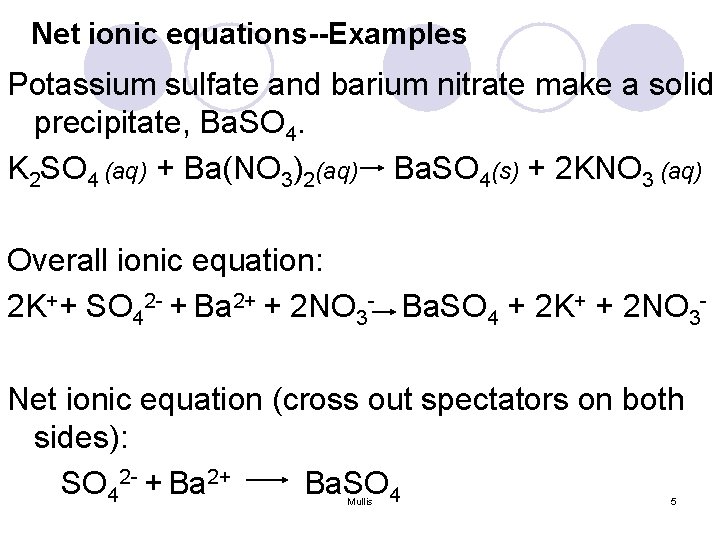
Net ionic equations--Examples Potassium sulfate and barium nitrate make a solid precipitate, Ba. SO 4. K 2 SO 4 (aq) + Ba(NO 3)2(aq) Ba. SO 4(s) + 2 KNO 3 (aq) Overall ionic equation: 2 K++ SO 42 - + Ba 2+ + 2 NO 3 - Ba. SO 4 + 2 K+ + 2 NO 3 Net ionic equation (cross out spectators on both sides): SO 42 - + Ba 2+ Ba. SO 4 Mullis 5
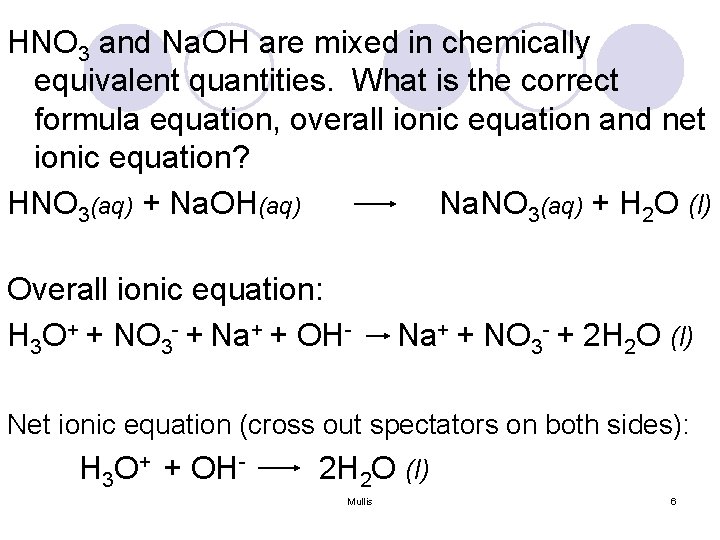
HNO 3 and Na. OH are mixed in chemically equivalent quantities. What is the correct formula equation, overall ionic equation and net ionic equation? HNO 3(aq) + Na. OH(aq) Na. NO 3(aq) + H 2 O (l) Overall ionic equation: H 3 O+ + NO 3 - + Na+ + OH- Na+ + NO 3 - + 2 H 2 O (l) Net ionic equation (cross out spectators on both sides): H 3 O+ + OH- 2 H 2 O (l) Mullis 6