Complete Ionic and Net Ionic Equations Writing Complete

















- Slides: 32

Complete Ionic and Net Ionic Equations

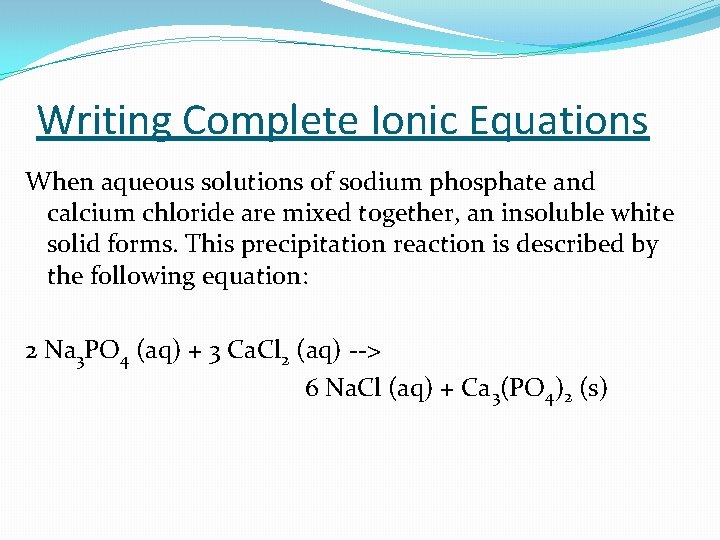
Writing Complete Ionic Equations When aqueous solutions of sodium phosphate and calcium chloride are mixed together, an insoluble white solid forms. This precipitation reaction is described by the following equation: 2 Na 3 PO 4 (aq) + 3 Ca. Cl 2 (aq) --> 6 Na. Cl (aq) + Ca 3(PO 4)2 (s)

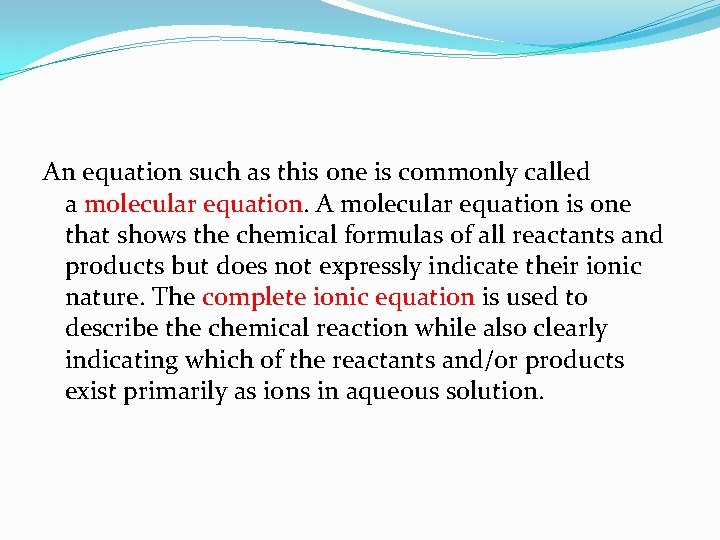
An equation such as this one is commonly called a molecular equation. A molecular equation is one that shows the chemical formulas of all reactants and products but does not expressly indicate their ionic nature. The complete ionic equation is used to describe the chemical reaction while also clearly indicating which of the reactants and/or products exist primarily as ions in aqueous solution.

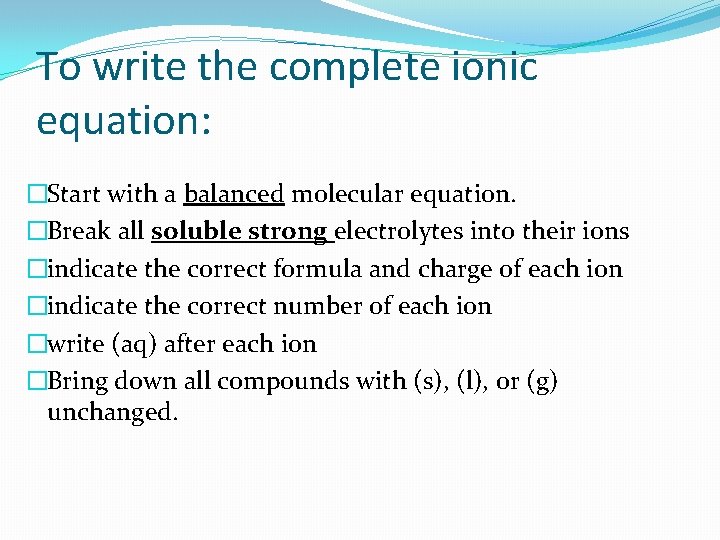
To write the complete ionic equation: �Start with a balanced molecular equation. �Break all soluble strong electrolytes into their ions �indicate the correct formula and charge of each ion �indicate the correct number of each ion �write (aq) after each ion �Bring down all compounds with (s), (l), or (g) unchanged.

Remember: �Strong electrolytes are: �Strong acids: HCl, HBr, HI, HCl. O 3, HCl. O 4, H 2 SO 4, HNO 3 �Strong bases: Group Hydroxides (OH), Ba, Sr, and Ca Hydroxides �Salts: Ionic Compounds that are not bases �Weak electrolytes are: All other acids and bases (NH 3 also) �Nonelectrolytes are: �Organic Substances: Alcohols, Sugars, Hydrocarbons �Many other covalent substances

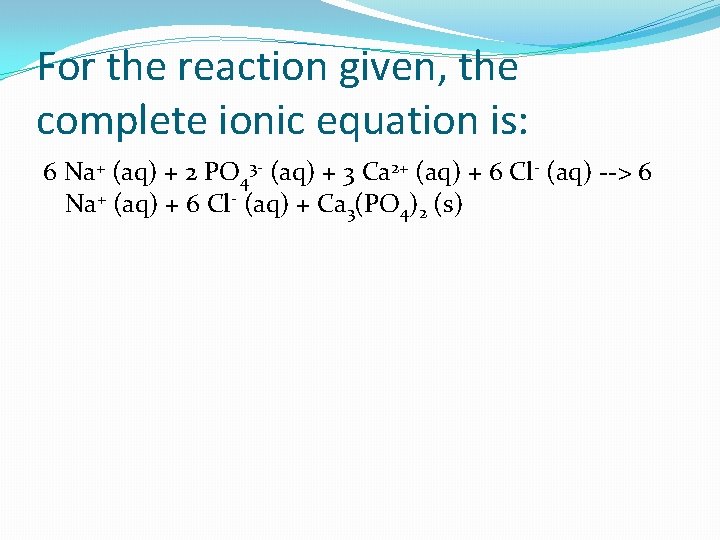
For the reaction given, the complete ionic equation is: 6 Na+ (aq) + 2 PO 43 - (aq) + 3 Ca 2+ (aq) + 6 Cl- (aq) --> 6 Na+ (aq) + 6 Cl- (aq) + Ca 3(PO 4)2 (s)

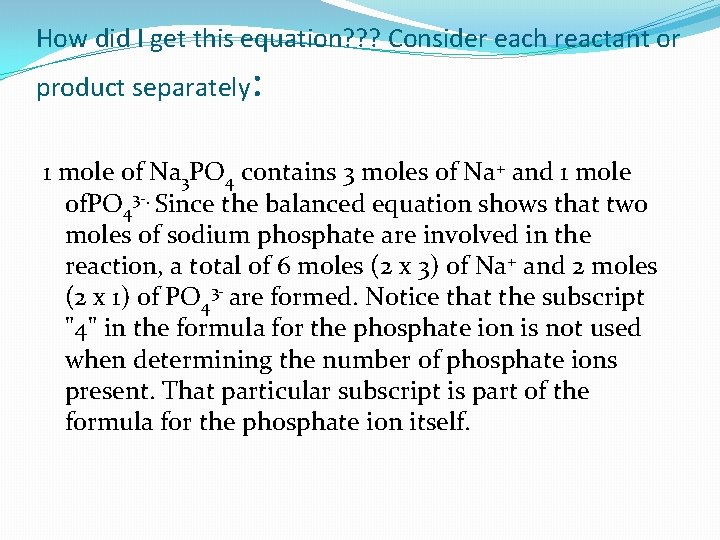
How did I get this equation? ? ? Consider each reactant or product separately : 1 mole of Na 3 PO 4 contains 3 moles of Na+ and 1 mole of. PO 43 -. Since the balanced equation shows that two moles of sodium phosphate are involved in the reaction, a total of 6 moles (2 x 3) of Na+ and 2 moles (2 x 1) of PO 43 - are formed. Notice that the subscript "4" in the formula for the phosphate ion is not used when determining the number of phosphate ions present. That particular subscript is part of the formula for the phosphate ion itself.

2 Na 3 PO 4 (aq) --> 6 Na+ (aq) + 2 PO 43 - (aq)

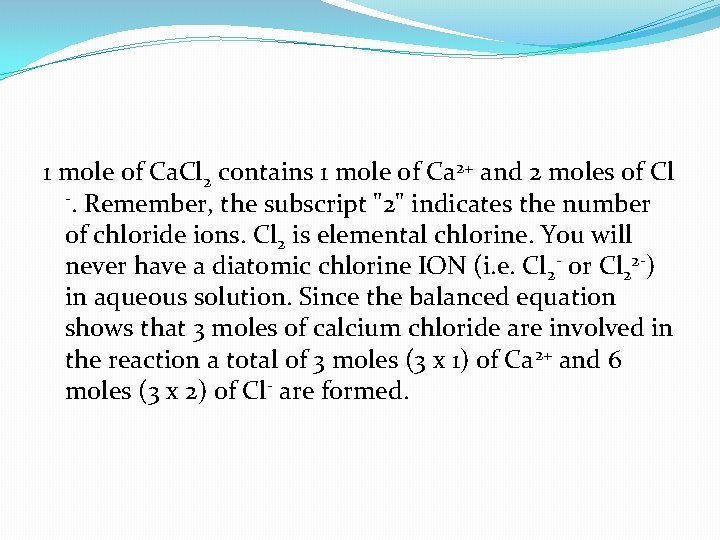
1 mole of Ca. Cl 2 contains 1 mole of Ca 2+ and 2 moles of Cl -. Remember, the subscript "2" indicates the number of chloride ions. Cl 2 is elemental chlorine. You will never have a diatomic chlorine ION (i. e. Cl 2 - or Cl 22 -) in aqueous solution. Since the balanced equation shows that 3 moles of calcium chloride are involved in the reaction a total of 3 moles (3 x 1) of Ca 2+ and 6 moles (3 x 2) of Cl- are formed.

3 Ca. Cl 2 (aq) --> 3 Ca 2+ (aq) + 6 Cl- (aq)

1 mole of Na. Cl contains 1 mole of Na+ and 1 mole of Cl-. Since the balanced equation shows that 6 moles of Na. Cl are produced by the reaction, 6 moles (6 x 1) of Na+ and 6 moles (6 x 1)of Cl- will be formed.

6 Na. Cl (aq) --> 6 Na+ (aq) + 6 Cl- (aq)

Since calcium phosphate is an insoluble solid (indicated by the (s) beside its formula), it will not form appreciable amounts of ions in water. It is brought down unchanged into the complete ionic equation.

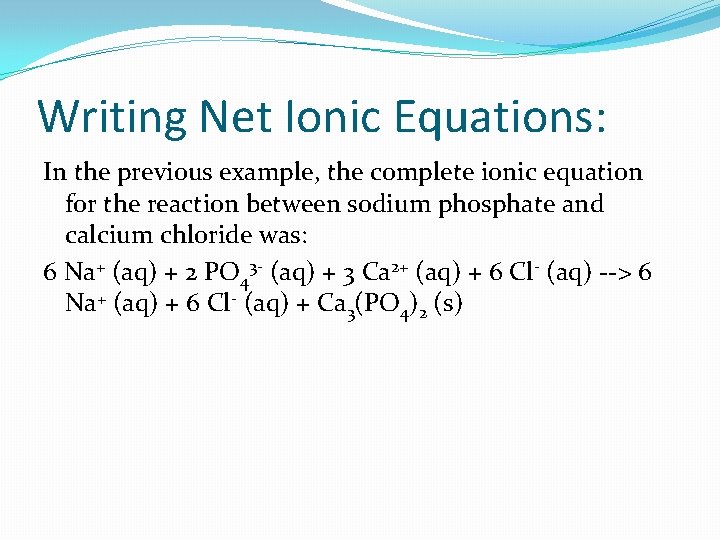
Writing Net Ionic Equations: In the previous example, the complete ionic equation for the reaction between sodium phosphate and calcium chloride was: 6 Na+ (aq) + 2 PO 43 - (aq) + 3 Ca 2+ (aq) + 6 Cl- (aq) --> 6 Na+ (aq) + 6 Cl- (aq) + Ca 3(PO 4)2 (s)

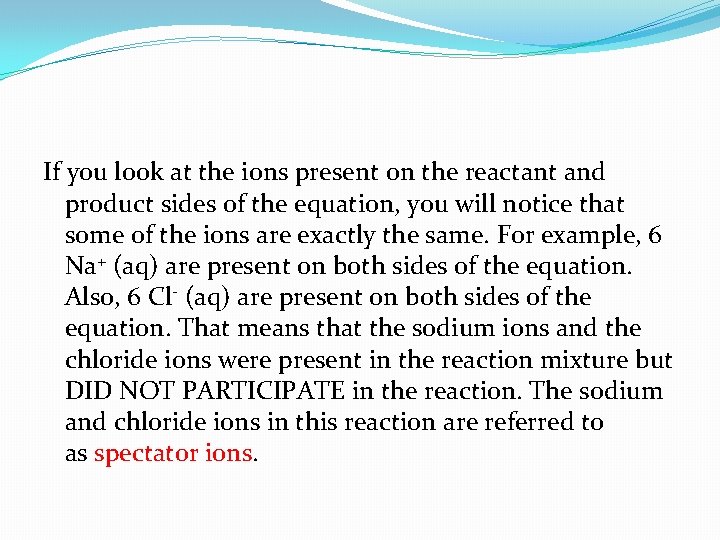
If you look at the ions present on the reactant and product sides of the equation, you will notice that some of the ions are exactly the same. For example, 6 Na+ (aq) are present on both sides of the equation. Also, 6 Cl- (aq) are present on both sides of the equation. That means that the sodium ions and the chloride ions were present in the reaction mixture but DID NOT PARTICIPATE in the reaction. The sodium and chloride ions in this reaction are referred to as spectator ions.

Spectator ions are ions that are present in the reaction mixture but do not participate in it. They "sit around and watch the reaction take place" just like a spectator at a basketball game watches the players in the game but doesn't play the game himself. You can recognize spectator ions by looking for ions that are present on both sides of the equation. They will always have the same exact formula, charge, and physical state. They will also be present in exactly the same number on both sides of the equation.

To write a net ionic equation: 1. 2. 3. 4. Write the balanced molecular equation. Write the balanced complete ionic equation. Cross out the spectator ions that are present. Write the "leftovers" as the net ionic equation.

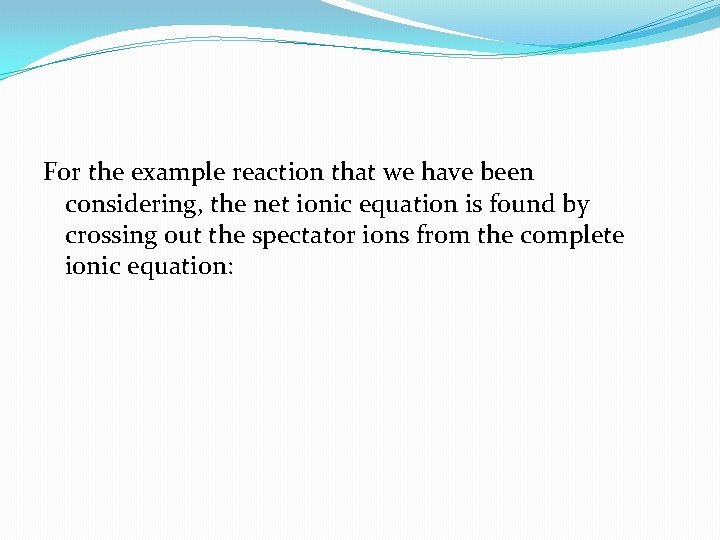
For the example reaction that we have been considering, the net ionic equation is found by crossing out the spectator ions from the complete ionic equation:

6 Na+ (aq) + 2 PO 43 - (aq) + 3 Ca 2+ (aq) + 6 Cl- (aq) --> 6 Na+ (aq)+ 6 Cl- (aq) + Ca 3(PO 4)2 (s)

and then re-writing the "leftovers: " 2 PO 43 - (aq) + 3 Ca 2+ (aq) --> Ca 3(PO 4)2 (s)

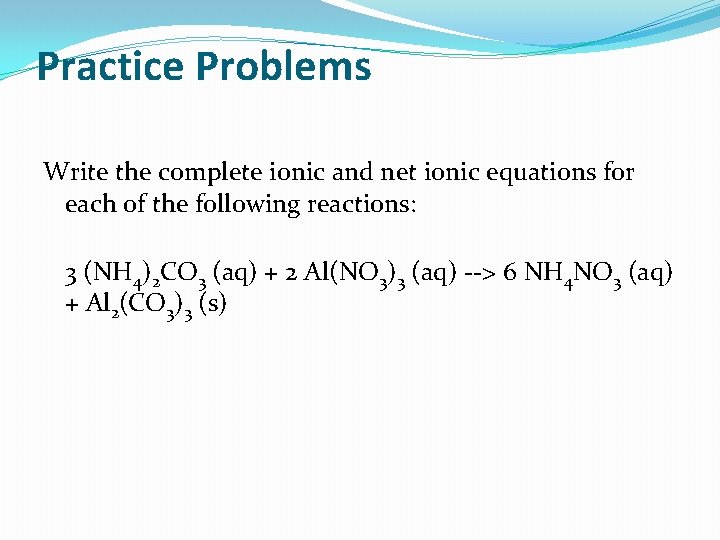
Practice Problems Write the complete ionic and net ionic equations for each of the following reactions: 3 (NH 4)2 CO 3 (aq) + 2 Al(NO 3)3 (aq) --> 6 NH 4 NO 3 (aq) + Al 2(CO 3)3 (s)

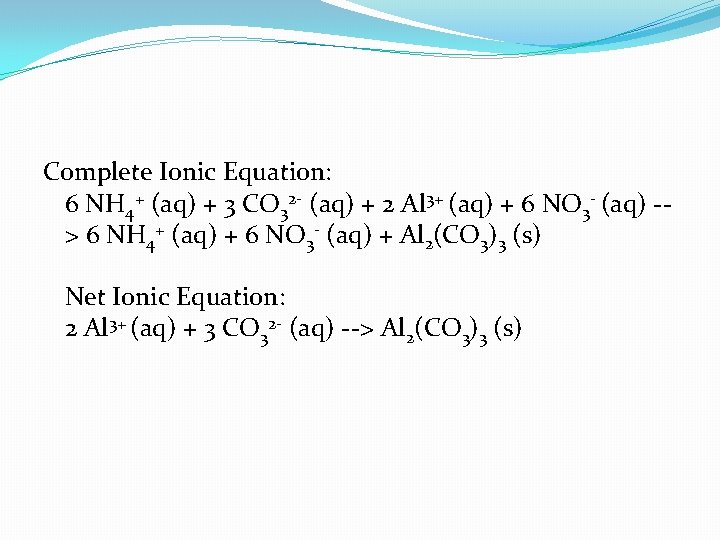
Complete Ionic Equation: 6 NH 4+ (aq) + 3 CO 32 - (aq) + 2 Al 3+ (aq) + 6 NO 3 - (aq) -> 6 NH 4+ (aq) + 6 NO 3 - (aq) + Al 2(CO 3)3 (s) Net Ionic Equation: 2 Al 3+ (aq) + 3 CO 32 - (aq) --> Al 2(CO 3)3 (s)

2 Na. OH (aq) + H 2 SO 4 (aq) --> Na 2 SO 4 (aq) + 2 H 2 O (l)

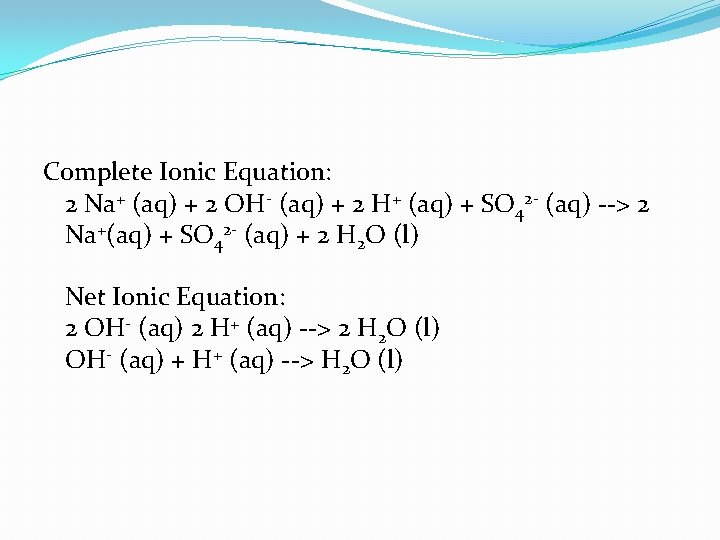
Complete Ionic Equation: 2 Na+ (aq) + 2 OH- (aq) + 2 H+ (aq) + SO 42 - (aq) --> 2 Na+(aq) + SO 42 - (aq) + 2 H 2 O (l) Net Ionic Equation: 2 OH- (aq) 2 H+ (aq) --> 2 H 2 O (l) OH- (aq) + H+ (aq) --> H 2 O (l)

Notice that you should always use the lowest whole number ratio of the reactants and products. In this case, all of the coefficients were divided by two to get the final net ionic equation.

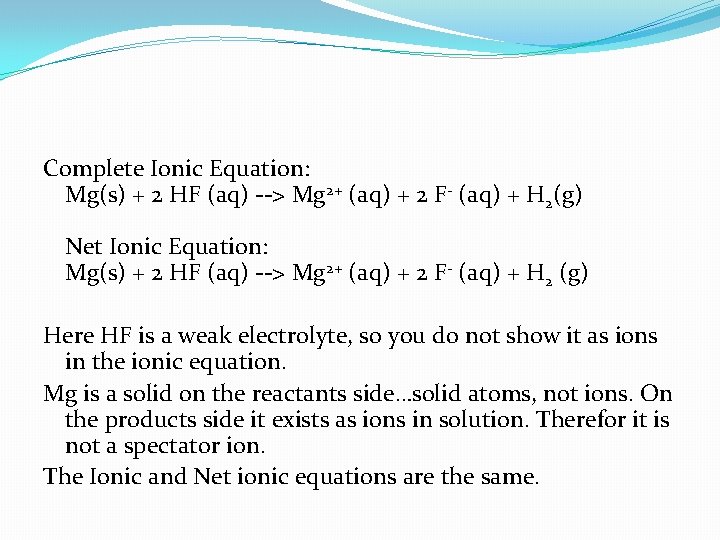
Mg (s) + 2 HF (aq) --> Mg. Cl 2 (aq) + H 2 (g)

Complete Ionic Equation: Mg(s) + 2 HF (aq) --> Mg 2+ (aq) + 2 F- (aq) + H 2(g) Net Ionic Equation: Mg(s) + 2 HF (aq) --> Mg 2+ (aq) + 2 F- (aq) + H 2 (g) Here HF is a weak electrolyte, so you do not show it as ions in the ionic equation. Mg is a solid on the reactants side…solid atoms, not ions. On the products side it exists as ions in solution. Therefor it is not a spectator ion. The Ionic and Net ionic equations are the same.

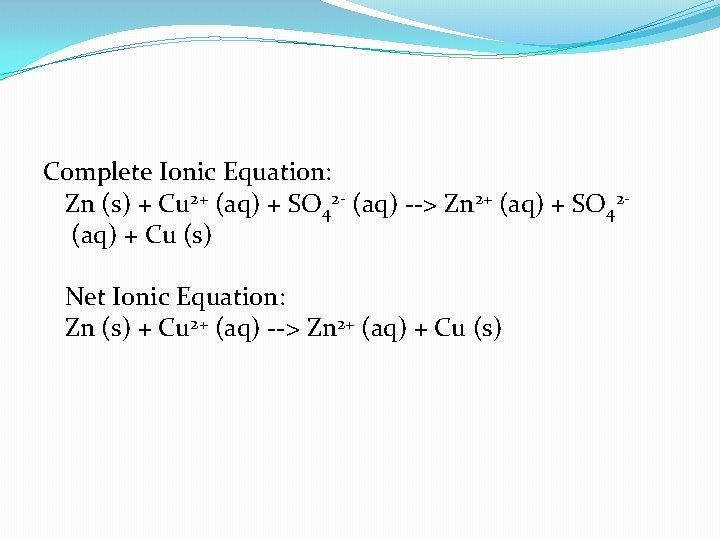
Zn (s) + Cu. SO 4 (aq) --> Zn. SO 4 (aq) + Cu (s)

Complete Ionic Equation: Zn (s) + Cu 2+ (aq) + SO 42 - (aq) --> Zn 2+ (aq) + SO 42(aq) + Cu (s) Net Ionic Equation: Zn (s) + Cu 2+ (aq) --> Zn 2+ (aq) + Cu (s)

Zn(s) and Zn 2+ (aq) are not the same. One is elemental while the other is a monoatomic ion. The same applies to Cu(s) and Cu 2+ (aq).

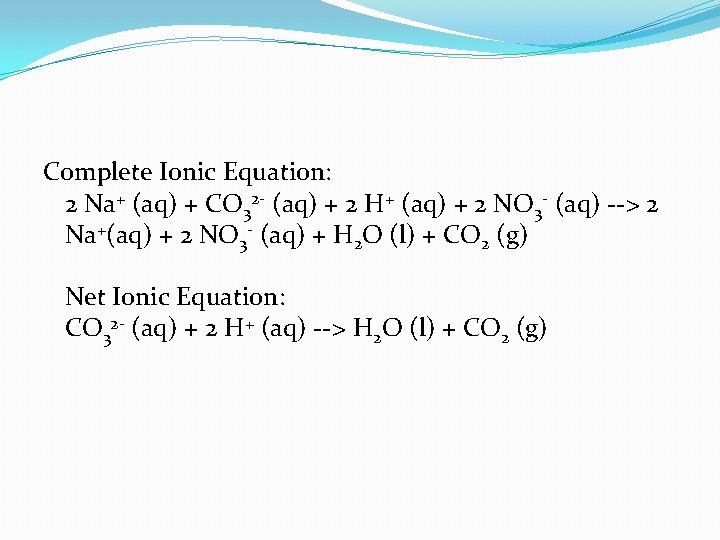
Na 2 CO 3 (aq) + 2 HNO 3 (aq) --> 2 Na. NO 3 (aq) + H 2 O (l) + CO 2 (g)

Complete Ionic Equation: 2 Na+ (aq) + CO 32 - (aq) + 2 H+ (aq) + 2 NO 3 - (aq) --> 2 Na+(aq) + 2 NO 3 - (aq) + H 2 O (l) + CO 2 (g) Net Ionic Equation: CO 32 - (aq) + 2 H+ (aq) --> H 2 O (l) + CO 2 (g)