Net Ionic Equations NET IONIC EQUATIONS I CAN





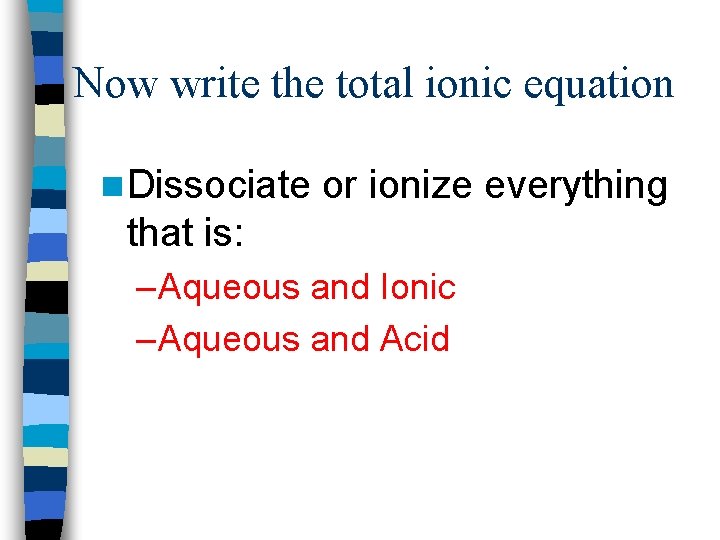



- Slides: 15

Net Ionic Equations

NET IONIC EQUATIONS I CAN describe a chemical reaction in solution by writing is total and net ionic equations.

SOLUBLE vs INSOLUBLE n Not all substances are soluble in water. n Generally speaking, IONIC COMPOUND formula units separate into their positive cations and negative anions in water. n Covalent compounds simply spread out…their molecules do not separate. n Since chemical equations must represent what is happening in a reaction, we often write a NET IONIC equation to show what actually happens in the reaction.

There are three steps involved in getting to the net ionic equation: n Write the non-ionic equation (aka a balanced chemical equation) n Write the total ionic equation n Write net ionic equation

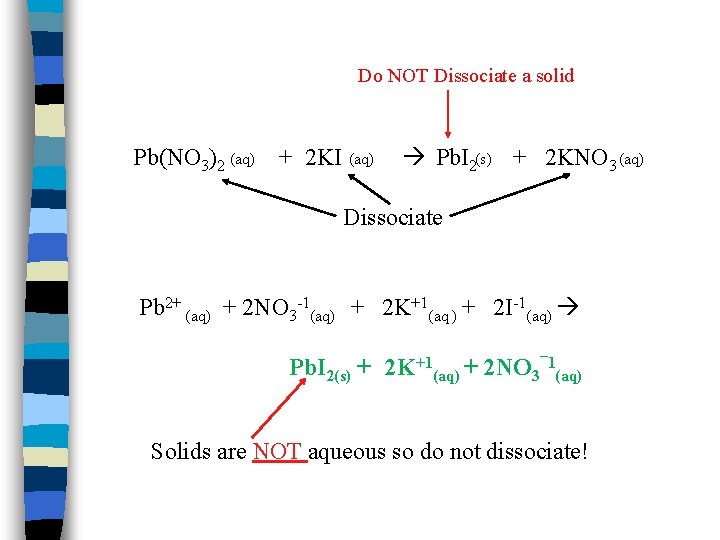
EXAMPLE n Solutions* of lead (II) nitrate and potassium iodide are mixed according to the following equation: Pb(NO 3)2 (aq) + 2 KI (aq) Pb. I 2 (s) + 2 KNO 3 (aq) n *Remember that solution means it is soluble in water! n When you see (aq) following a compound, it means it is soluble in water. An (s) means it is an insoluble solid. A (g) represents a gas and is also INSOLUBLE in water.
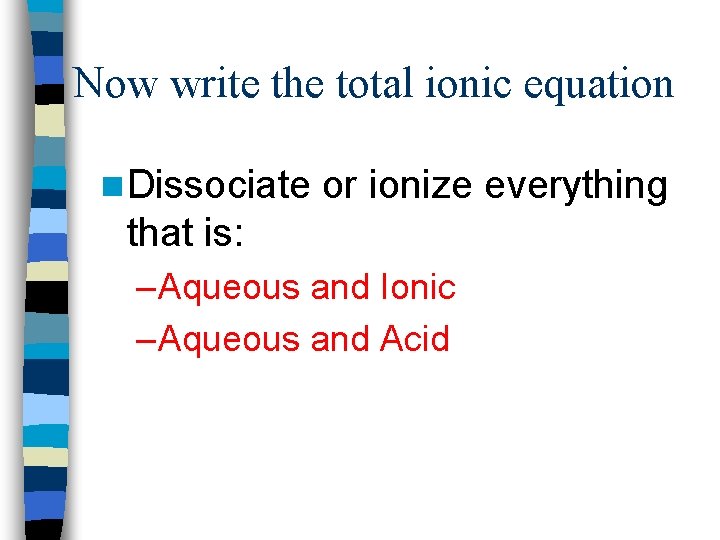
Now write the total ionic equation n Dissociate or ionize everything that is: – Aqueous and Ionic – Aqueous and Acid

Do NOT Dissociate a solid Pb(NO 3)2 (aq) + 2 KI (aq) Pb. I 2(s) + 2 KNO 3 (aq) Dissociate Pb 2+ (aq) + 2 NO 3 -1(aq) + 2 K+1(aq ) + 2 I-1(aq) Pb. I 2(s) + 2 K+1(aq) + 2 NO 3¯ 1(aq) Solids are NOT aqueous so do not dissociate!

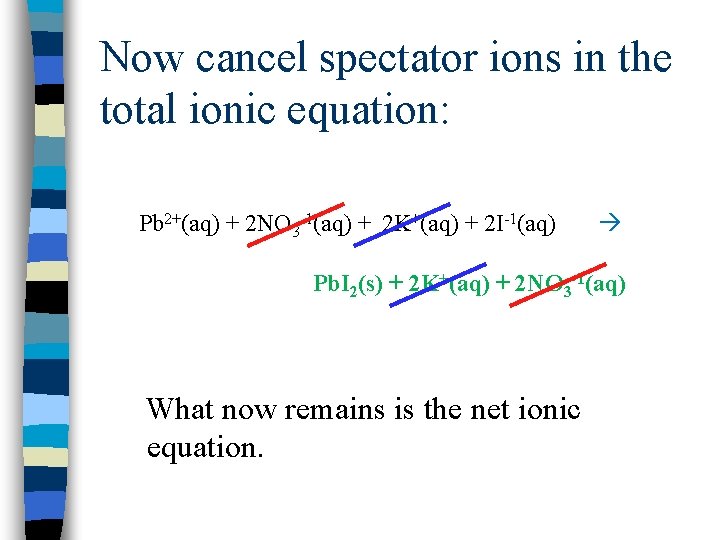
SPECTATOR IONS n. A SPECTATOR ION is one that does not change in the reaction…it is not involved in the reaction and stays the same on BOTH SIDES of the equation.

Now cancel spectator ions in the total ionic equation: Pb 2+(aq) + 2 NO 3 -1(aq) + 2 K+(aq) + 2 I-1(aq) Pb. I 2(s) + 2 K+(aq) + 2 NO 3 -1(aq) What now remains is the net ionic equation.

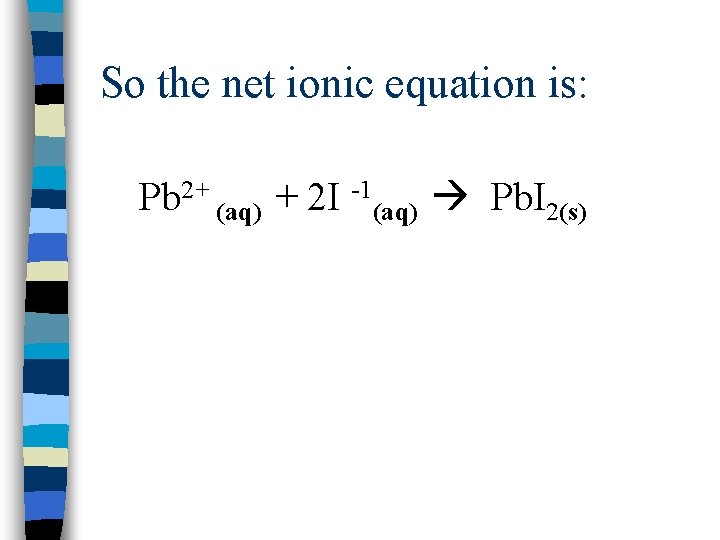
So the net ionic equation is: Pb 2+ (aq) + 2 I -1(aq) Pb. I 2(s)

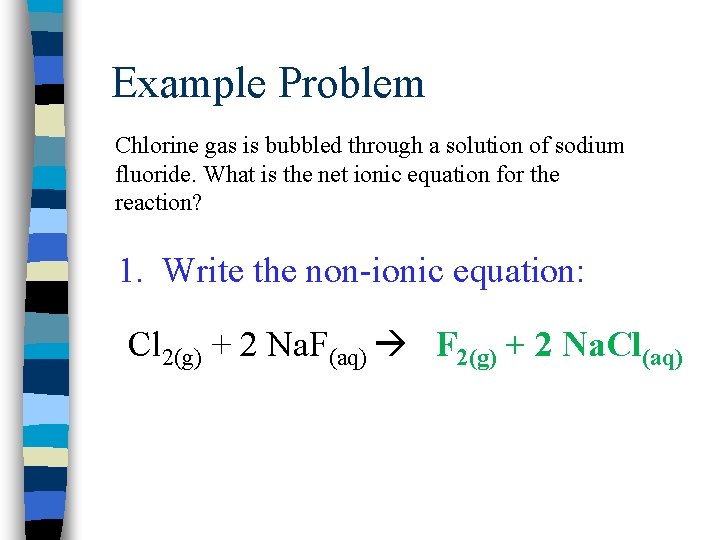
Example Problem Chlorine gas is bubbled through a solution of sodium fluoride. What is the net ionic equation for the reaction? 1. Write the non-ionic equation: Cl 2(g) + 2 Na. F(aq) F 2(g) + 2 Na. Cl(aq)

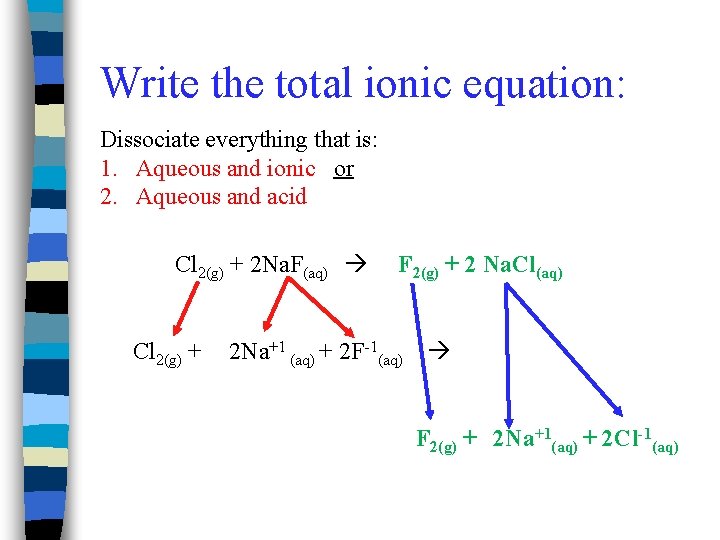
Write the total ionic equation: Dissociate everything that is: 1. Aqueous and ionic or 2. Aqueous and acid Cl 2(g) + 2 Na. F(aq) Cl 2(g) + F 2(g) + 2 Na. Cl(aq) 2 Na+1 (aq) + 2 F-1(aq) F 2(g) + 2 Na+1(aq) + 2 Cl-1(aq)

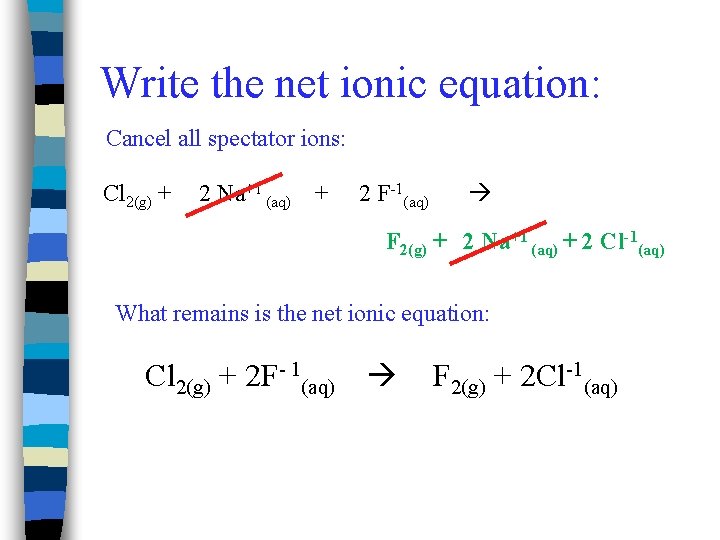
Write the net ionic equation: Cancel all spectator ions: Cl 2(g) + 2 Na+1 (aq) + 2 F-1(aq) F 2(g) + 2 Na+1 (aq) + 2 Cl-1(aq) What remains is the net ionic equation: Cl 2(g) + 2 F- 1(aq) F 2(g) + 2 Cl-1(aq)

n Occasionally there are NO SPECTATOR IONS. n In this situation, the TOTAL and the NET IONIC EQUATIONS are the same.

Practice Problems n Complete the practice problems at the end of the notesheet.