CHEM 3310 Thermodynamics Work There are two ways






- Slides: 32

CHEM 3310 Thermodynamics Work

There are two ways to change the internal energy of a system: 1. By flow of heat, q Heat is the transfer of thermal energy between the system and the surroundings 2. By doing work, w Work can be converted into heat and vice versa. q and w are process dependent, and are not state functions. CHEM 3310 2

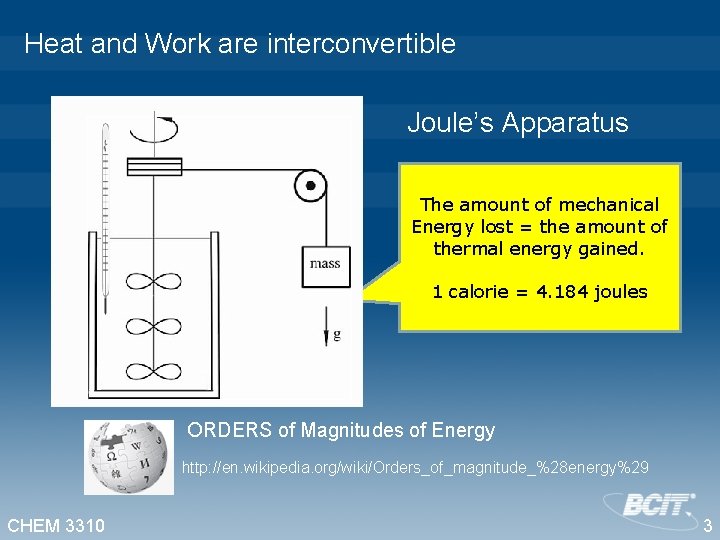
Heat and Work are interconvertible Joule’s Apparatus The amount of mechanical Energy lost = the amount of thermal energy gained. 1 calorie = 4. 184 joules ORDERS of Magnitudes of Energy http: //en. wikipedia. org/wiki/Orders_of_magnitude_%28 energy%29 CHEM 3310 3

Change in internal energy can be a result of a chemical reaction System: The chemical reaction Surroundings: Everything that interacts with the system All matter has chemical energy. • Chemical bonds are a source of energy (PE). • The movement of molecules in space is a source of energy (KE). • The vibrations and rotations of molecules is another source of chemical energy (KE). All of these forms of chemical energy contribute in one way or another to chemical reactions. CHEM 3310 4

Change in internal energy can be a result of a chemical reaction System: The chemical reaction Surroundings: Everything that interacts with the system In a chemical reaction, when bonds are broken and new bonds are formed, the internal energy of the system changes. Exothermic reaction – heat flows out of the system Endothermic reaction – heat flows into the system CHEM 3310 5

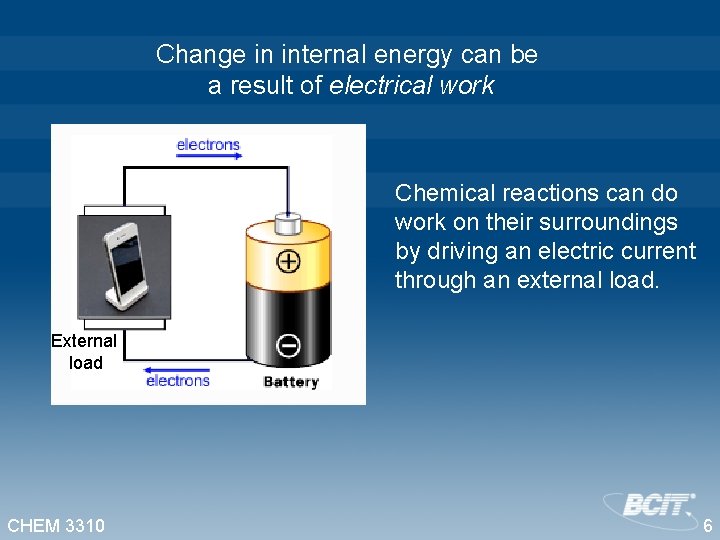
Change in internal energy can be a result of electrical work Chemical reactions can do work on their surroundings by driving an electric current through an external load. External load CHEM 3310 6

Change in internal energy can be a result of work of expansion Chemical reactions can do work on their surroundings when the volume of the system expands during the course of the reaction. Internal combustion engine burns a fuel with air and uses the hot gases for generating power. Image credit: http: //4 mechanical. com/wp-content/uploads/2011/06/engine 1. gif CHEM 3310 7

Work, w Mechanical work is defined as the force exerted on an object multiplied by the distance which the object moves resulting from the applied force. w=f x If there is no movement (x=0), then w = 0. For infinitesimal change in position, dx, dw = f dx CHEM 3310 8

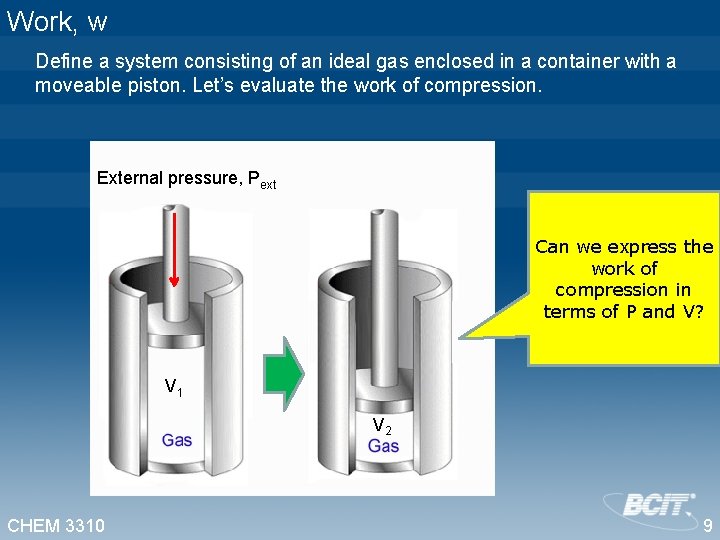
Work, w Define a system consisting of an ideal gas enclosed in a container with a moveable piston. Let’s evaluate the work of compression. External pressure, Pext Can we express the work of compression in terms of P and V? V 1 V 2 CHEM 3310 9

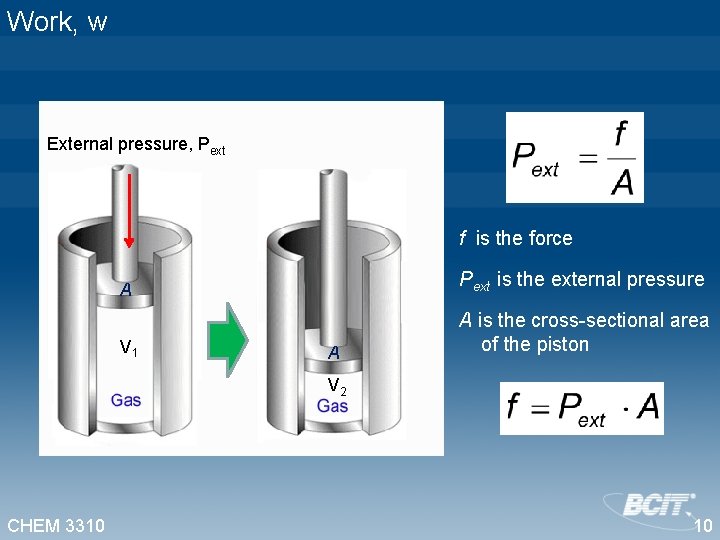
Work, w External pressure, Pext f is the force A Pext is the external pressure V 1 A is the cross-sectional area of the piston A V 2 CHEM 3310 10

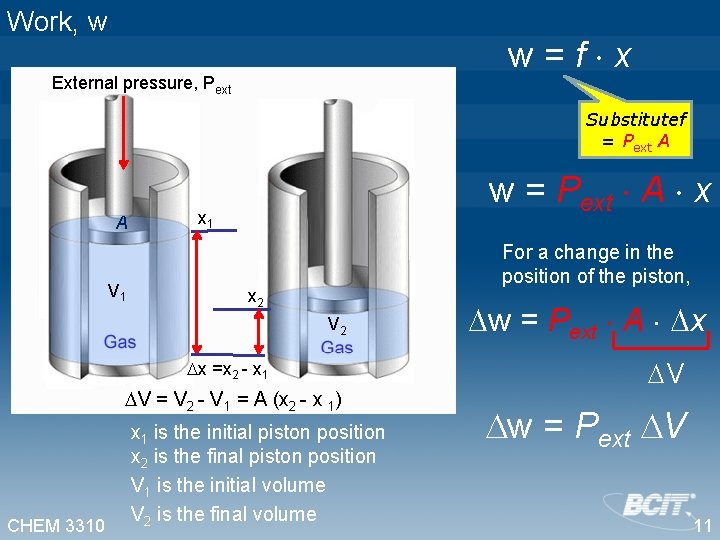
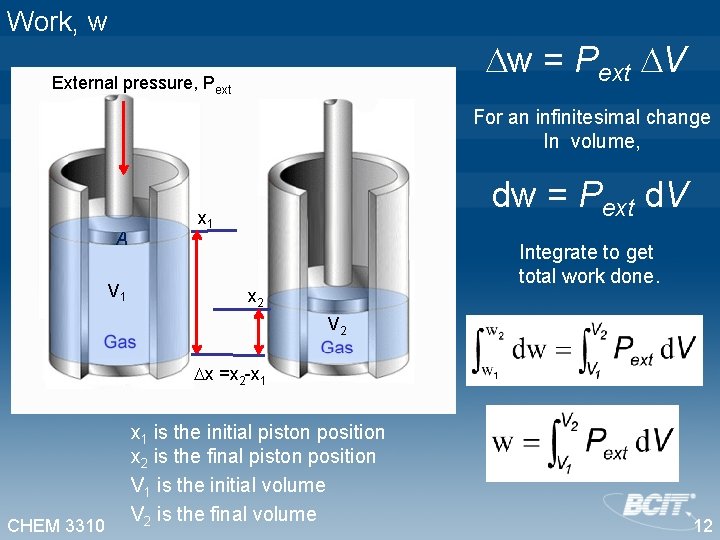
Work, w w=f x External pressure, Pext Substitutef = Pext A A V 1 w = Pext A x x 1 For a change in the position of the piston, x 2 V 2 x =x 2 - x 1 V = V 2 - V 1 = A (x 2 - x 1) CHEM 3310 x 1 is the initial piston position x 2 is the final piston position V 1 is the initial volume V 2 is the final volume w = Pext A x V w = Pext V 11

Work, w w = Pext V External pressure, Pext For an infinitesimal change In volume, A V 1 dw = Pext d. V x 1 Integrate to get total work done. x 2 V 2 x =x 2 -x 1 CHEM 3310 x 1 is the initial piston position x 2 is the final piston position V 1 is the initial volume V 2 is the final volume 12

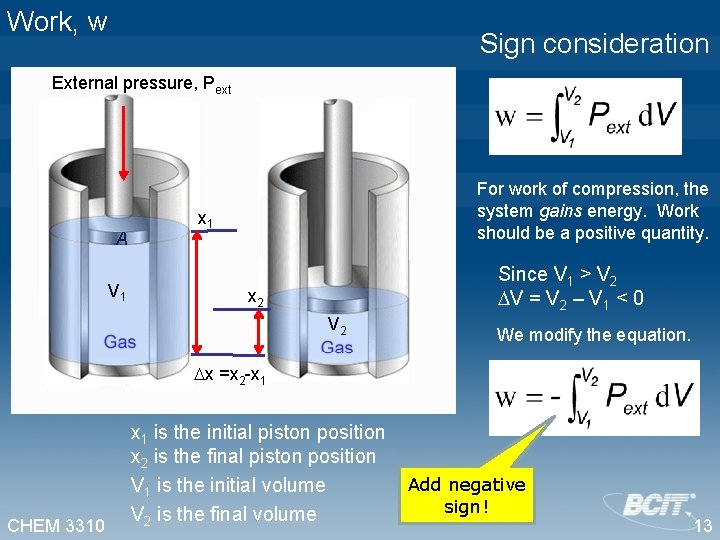
Work, w Sign consideration External pressure, Pext A V 1 For work of compression, the system gains energy. Work should be a positive quantity. x 1 Since V 1 > V 2 V = V 2 – V 1 < 0 x 2 V 2 We modify the equation. x =x 2 -x 1 CHEM 3310 x 1 is the initial piston position x 2 is the final piston position V 1 is the initial volume V 2 is the final volume Add negative sign! 13

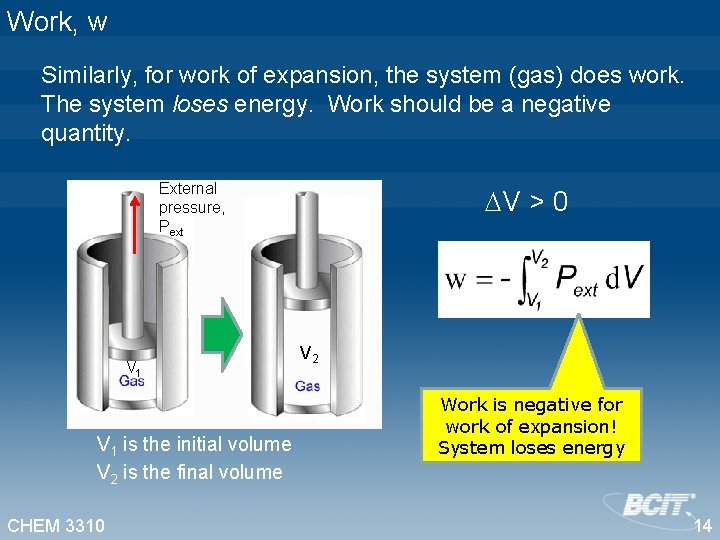
Work, w Similarly, for work of expansion, the system (gas) does work. The system loses energy. Work should be a negative quantity. External pressure, Pext V 1 is the initial volume V 2 is the final volume CHEM 3310 V > 0 V 2 Work is negative for work of expansion! System loses energy 14

Work, w w=f x Mechanical work CHEM 3310 PV work done by or on a gas confined in a piston and cylinder configuration. 15

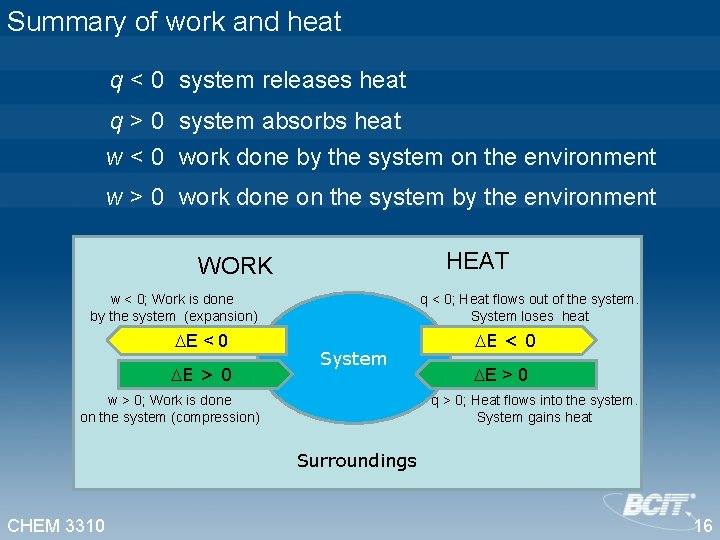
Summary of work and heat q < 0 system releases heat q > 0 system absorbs heat w < 0 work done by the system on the environment w > 0 work done on the system by the environment HEAT WORK w < 0; Work is done by the system (expansion) E < 0 E > 0 q < 0; Heat flows out of the system. System loses heat System w > 0; Work is done on the system (compression) E < 0 E > 0 q > 0; Heat flows into the system. System gains heat Surroundings CHEM 3310 16

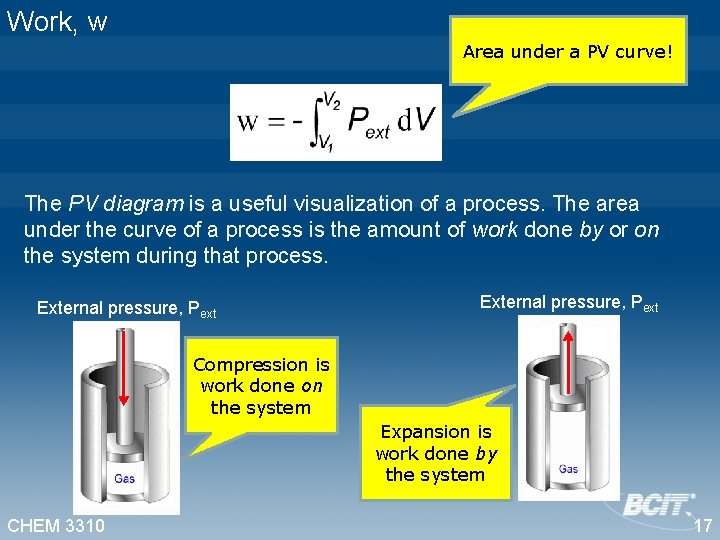
Work, w Area under a PV curve! The PV diagram is a useful visualization of a process. The area under the curve of a process is the amount of work done by or on the system during that process. External pressure, Pext Compression is work done on the system Expansion is work done by the system CHEM 3310 17

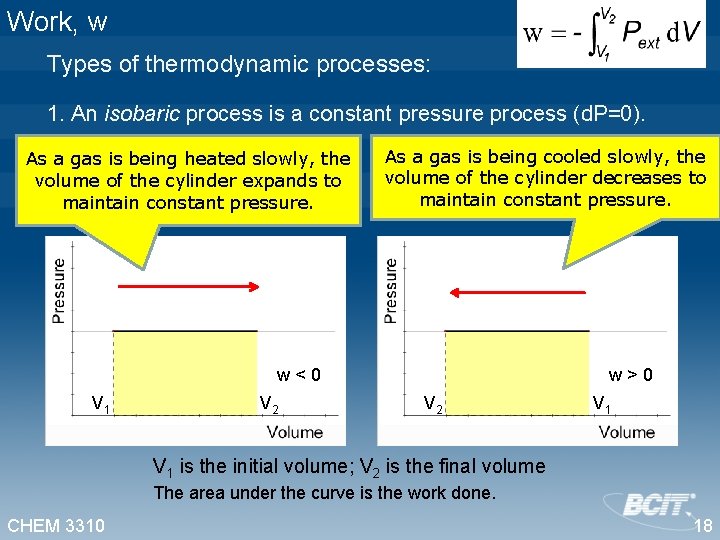
Work, w Types of thermodynamic processes: 1. An isobaric process is a constant pressure process (d. P=0). As a gas is being heated slowly, the volume of the cylinder expands to maintain constant pressure. As a gas is being cooled slowly, the volume of the cylinder decreases to maintain constant pressure. w<0 V 1 V 2 w>0 V 2 V 1 is the initial volume; V 2 is the final volume The area under the curve is the work done. CHEM 3310 18

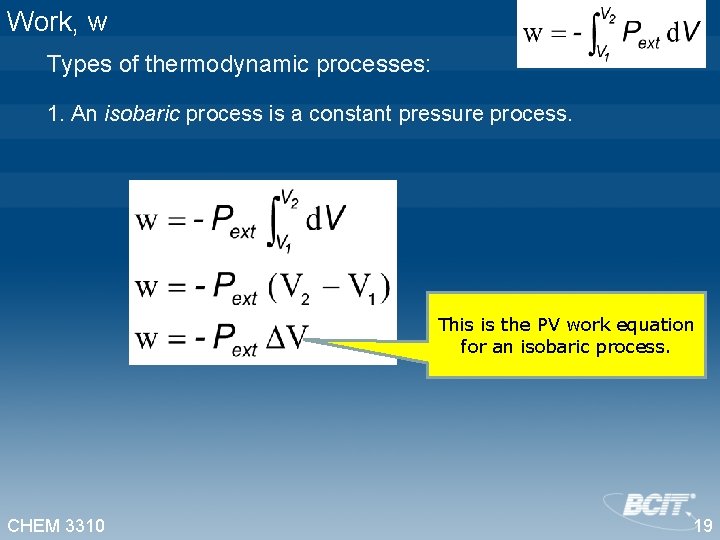
Work, w Types of thermodynamic processes: 1. An isobaric process is a constant pressure process. This is the PV work equation for an isobaric process. CHEM 3310 19

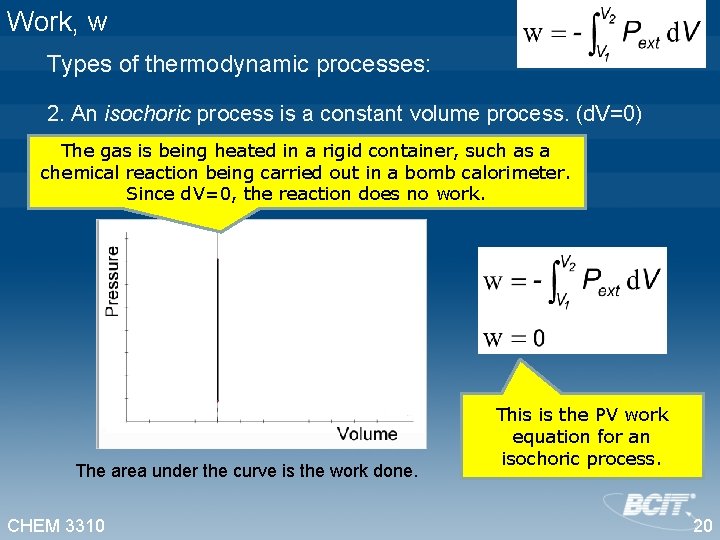
Work, w Types of thermodynamic processes: 2. An isochoric process is a constant volume process. (d. V=0) The gas is being heated in a rigid container, such as a chemical reaction being carried out in a bomb calorimeter. Since d. V=0, the reaction does no work. The area under the curve is the work done. CHEM 3310 This is the PV work equation for an isochoric process. 20

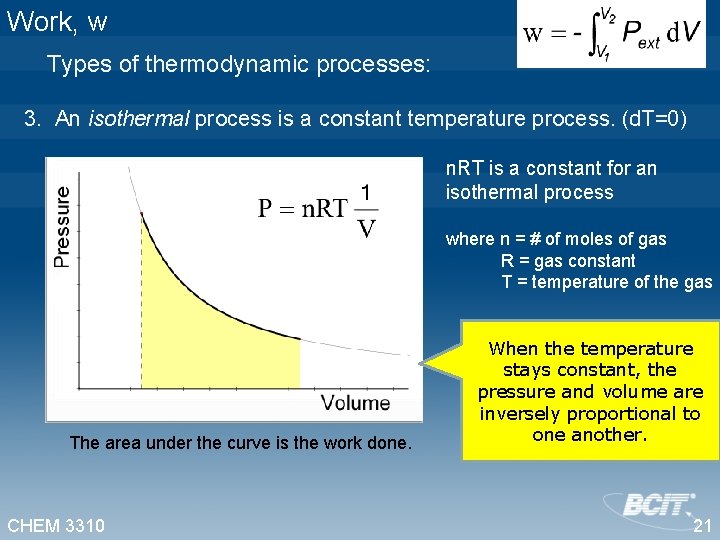
Work, w Types of thermodynamic processes: 3. An isothermal process is a constant temperature process. (d. T=0) n. RT is a constant for an isothermal process where n = # of moles of gas R = gas constant T = temperature of the gas The area under the curve is the work done. CHEM 3310 When the temperature stays constant, the pressure and volume are inversely proportional to one another. 21

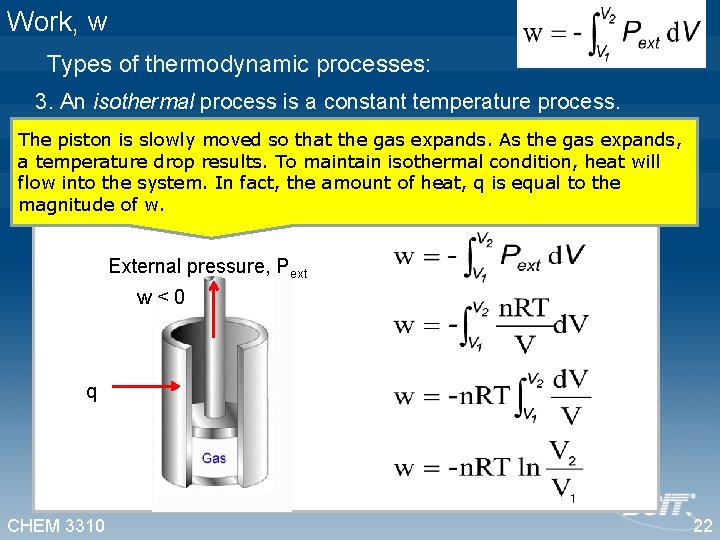
Work, w Types of thermodynamic processes: 3. An isothermal process is a constant temperature process. The piston is slowly moved so that the gas expands. As the gas expands, a temperature drop results. To maintain isothermal condition, heat will flow into the system. In fact, the amount of heat, q is equal to the magnitude of w. External pressure, Pext w<0 q q CHEM 3310 22

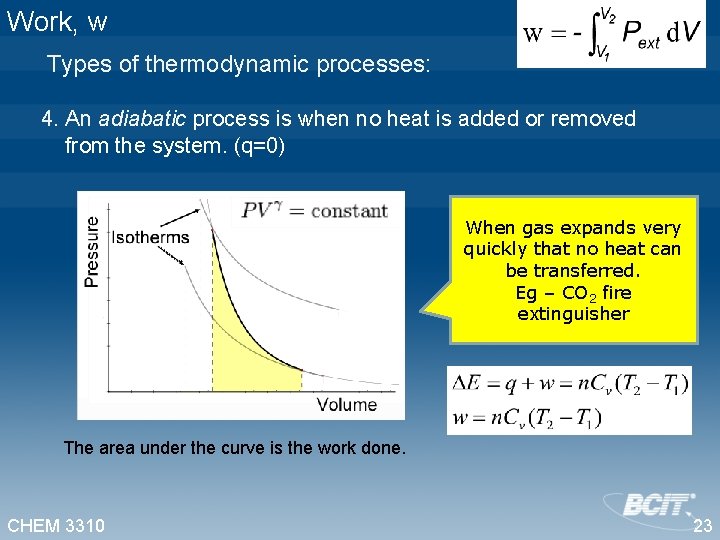
Work, w Types of thermodynamic processes: 4. An adiabatic process is when no heat is added or removed from the system. (q=0) When gas expands very quickly that no heat can be transferred. Eg – CO 2 fire extinguisher The area under the curve is the work done. CHEM 3310 23

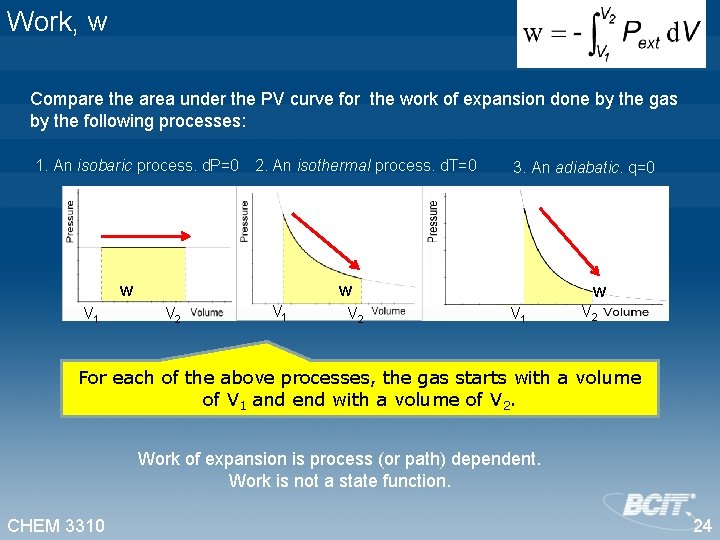
Work, w Compare the area under the PV curve for the work of expansion done by the gas by the following processes: 1. An isobaric process. d. P=0 2. An isothermal process. d. T=0 w V 1 3. An adiabatic. q=0 w V 2 V 1 V 2 w V 1 V 2 For each of the above processes, the gas starts with a volume of V 1 and end with a volume of V 2. Work of expansion is process (or path) dependent. Work is not a state function. CHEM 3310 24

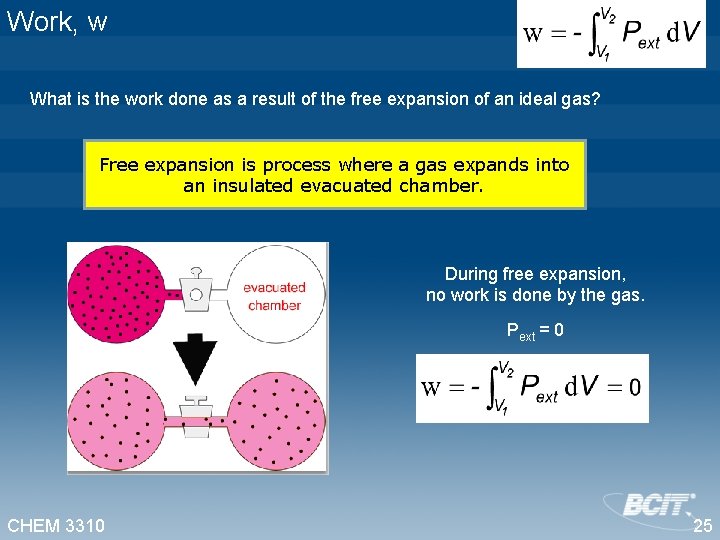
Work, w What is the work done as a result of the free expansion of an ideal gas? Free expansion is process where a gas expands into an insulated evacuated chamber. During free expansion, no work is done by the gas. Pext = 0 CHEM 3310 25

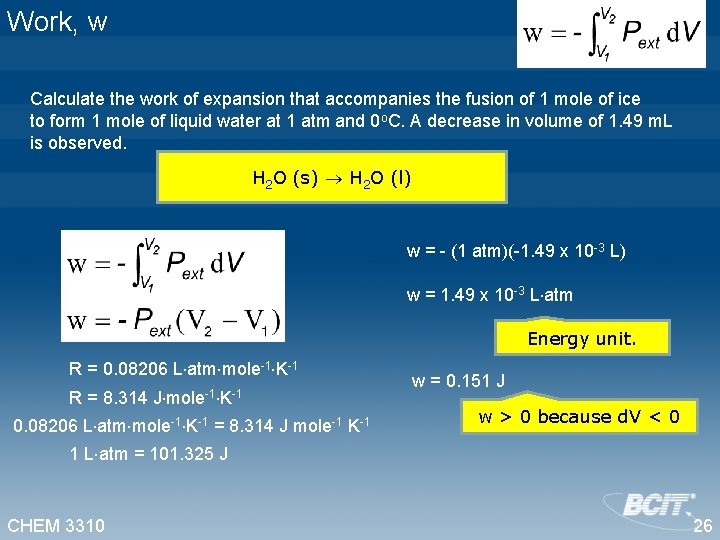
Work, w Calculate the work of expansion that accompanies the fusion of 1 mole of ice to form 1 mole of liquid water at 1 atm and 0 o. C. A decrease in volume of 1. 49 m. L is observed. H 2 O (s) H 2 O (l) w = - (1 atm)(-1. 49 x 10 -3 L) w = 1. 49 x 10 -3 L atm Energy unit. R = 0. 08206 L atm mole-1 K-1 R = 8. 314 J mole-1 K-1 0. 08206 L atm mole-1 K-1 = 8. 314 J mole-1 K-1 w = 0. 151 J w > 0 because d. V < 0 1 L atm = 101. 325 J CHEM 3310 26

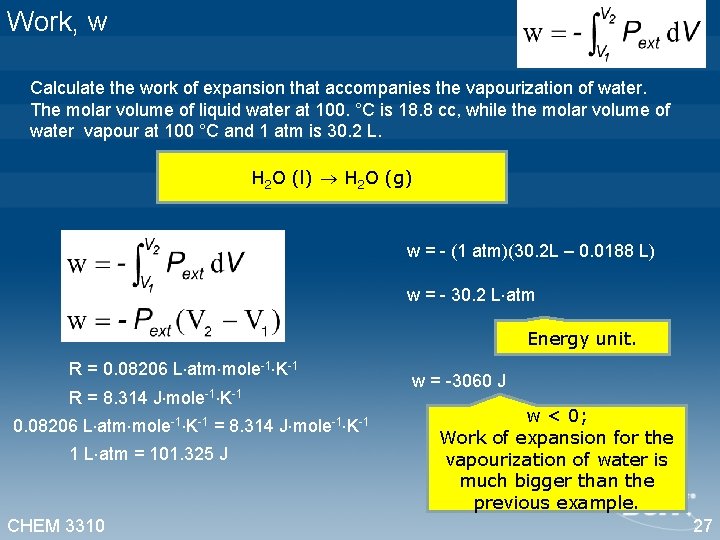
Work, w Calculate the work of expansion that accompanies the vapourization of water. The molar volume of liquid water at 100. °C is 18. 8 cc, while the molar volume of water vapour at 100 °C and 1 atm is 30. 2 L. H 2 O (l) H 2 O (g) w = - (1 atm)(30. 2 L – 0. 0188 L) w = - 30. 2 L atm Energy unit. R = 0. 08206 L atm mole-1 K-1 R = 8. 314 J mole-1 K-1 0. 08206 L atm mole-1 K-1 = 8. 314 J mole-1 K-1 1 L atm = 101. 325 J CHEM 3310 w = -3060 J w < 0; Work of expansion for the vapourization of water is much bigger than the previous example. 27

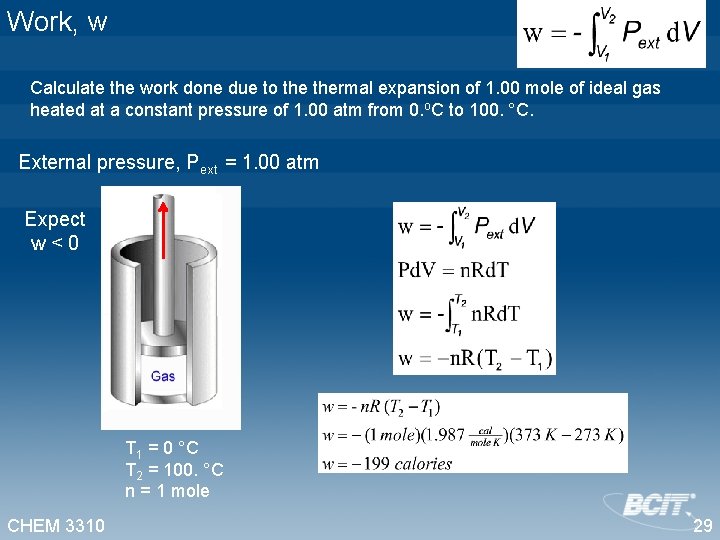
Work, w Calculate the work done due to thermal expansion of 1. 00 mole of ideal gas heated at a constant pressure of 1. 00 atm from 0. 00 °C to 100. °C. External pressure, Pext = 1. 00 atm Expect w<0 V 2 =30. 623 L V 1 =22. 413 L T 1 = 0. 00 °C T 2 = 100. °C n = 1. 00 mole CHEM 3310 w = -199 calories 28

Work, w Calculate the work done due to thermal expansion of 1. 00 mole of ideal gas heated at a constant pressure of 1. 00 atm from 0. o. C to 100. °C. External pressure, Pext = 1. 00 atm Expect w<0 T 1 = 0 °C T 2 = 100. °C n = 1 mole CHEM 3310 29

Work, w Calculate the work compression of 2. 00 moles of an ideal gas from 1. 00 bar to 100. 0 bar at 25. 0 °C. (1 bar = 0. 987 atm) External pressure, Pext Expect w>0 P 1 = 1. 00 bar P 2 = 100. bar CHEM 3310 T = 298 K n = 2. 00 moles P 1 = 0. 987 atm P 2 = 98. 7 atm w = 23. 0 k. J Use this equation with pressure, and you should get the same answer, w = 23. 0 k. J 30

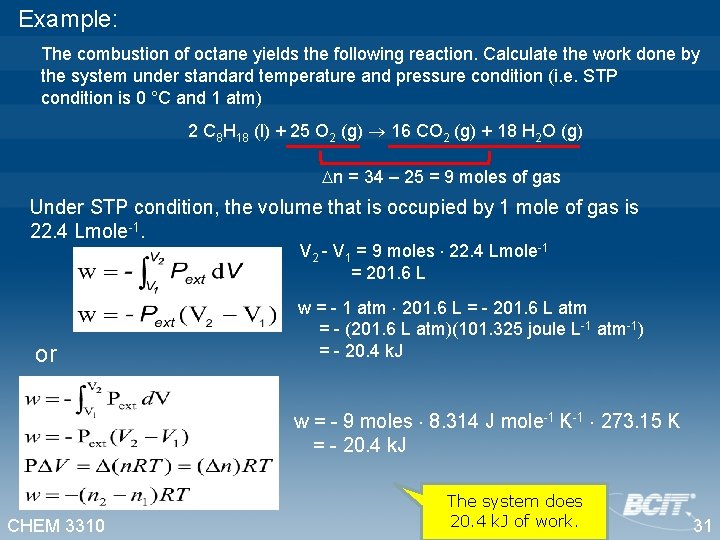
Example: The combustion of octane yields the following reaction. Calculate the work done by the system under standard temperature and pressure condition (i. e. STP condition is 0 °C and 1 atm) 2 C 8 H 18 (l) + 25 O 2 (g) 16 CO 2 (g) + 18 H 2 O (g) n = 34 – 25 = 9 moles of gas Under STP condition, the volume that is occupied by 1 mole of gas is 22. 4 Lmole-1. V 2 - V 1 = 9 moles 22. 4 Lmole-1 = 201. 6 L or w = - 1 atm 201. 6 L = - 201. 6 L atm = - (201. 6 L atm)(101. 325 joule L-1 atm-1) = - 20. 4 k. J w = - 9 moles 8. 314 J mole-1 K-1 273. 15 K = - 20. 4 k. J CHEM 3310 The system does 20. 4 k. J of work. 31

Summary: Internal energy, E: All the energy of the system (chemical, potential, kinetic, etc. ) Thermal energy: The part of the internal energy that changes temperature Gases: Possess translational, vibrational, rotational motions, all contributing to thermal energy Ways to change internal energy of gas system: • Heat, q • Work, w • Both heat and work State: The values of P, V, T, E of the system Change of State: By altering P, V, T, and E CHEM 3310 32