CHEM 3310 Chemical Kinetics The Zeroth Order Integrated



- Slides: 16

CHEM 3310 Chemical Kinetics The Zeroth Order Integrated Rate Equation

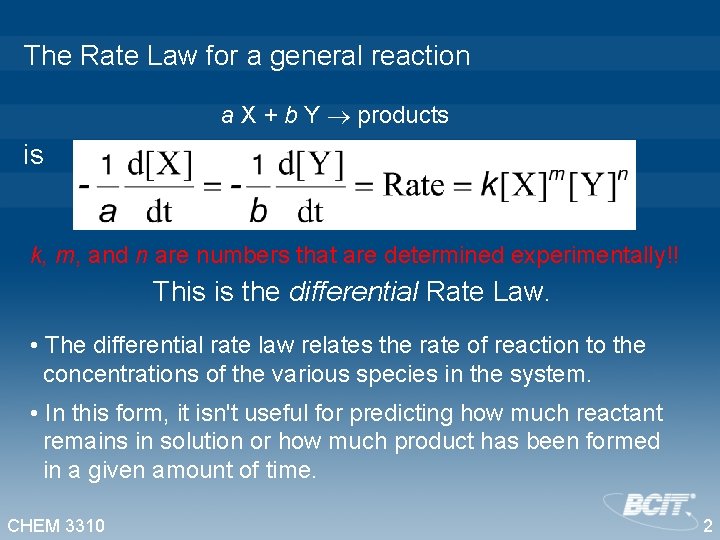
The Rate Law for a general reaction a X + b Y products is k, m, and n are numbers that are determined experimentally!! This is the differential Rate Law. • The differential rate law relates the rate of reaction to the concentrations of the various species in the system. • In this form, it isn't useful for predicting how much reactant remains in solution or how much product has been formed in a given amount of time. CHEM 3310 2

We use the integrated form of the rate law for: • Testing to see whether a set of data follows 0 th, 1 st, or 2 nd order. • Predicting how much reactant remains in solution or how much product has been formed in a given amount of time. Let’s see what the integrated form of the rate law looks like for: Zeroth, First, and Second Order Rate Laws. CHEM 3310 3

Zeroth Order Rate Law CHEM 3310 4

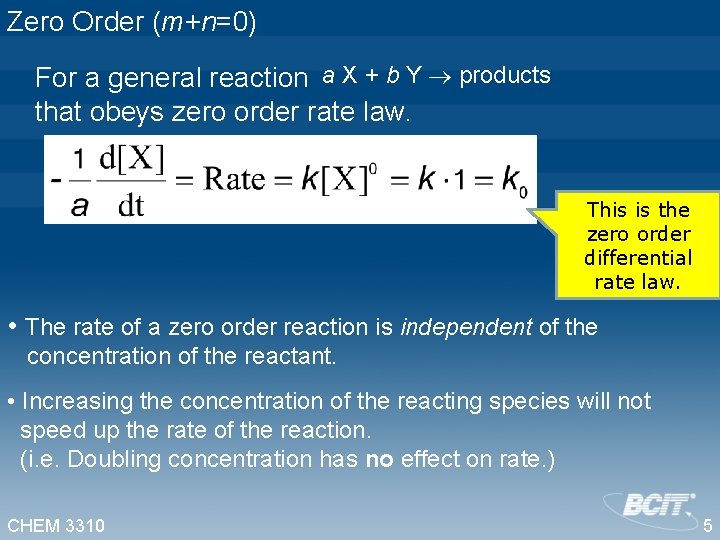
Zero Order (m+n=0) For a general reaction a X + b Y products that obeys zero order rate law. This is the zero order differential rate law. • The rate of a zero order reaction is independent of the concentration of the reactant. • Increasing the concentration of the reacting species will not speed up the rate of the reaction. (i. e. Doubling concentration has no effect on rate. ) CHEM 3310 5

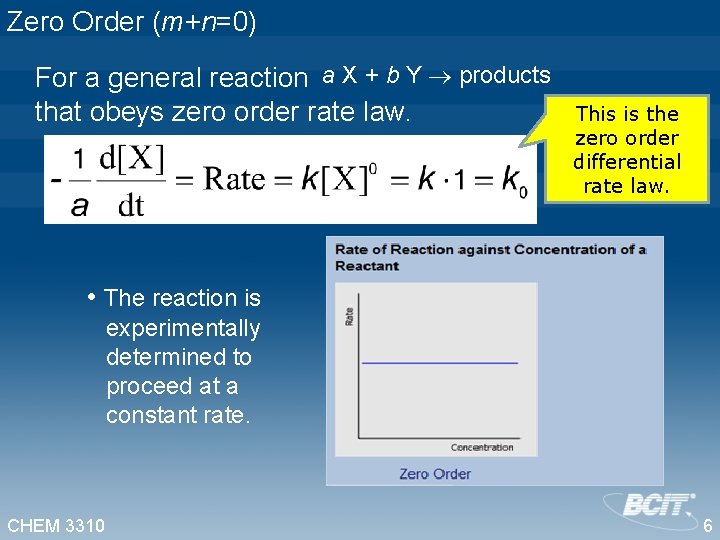
Zero Order (m+n=0) For a general reaction a X + b Y products that obeys zero order rate law. This is the zero order differential rate law. • The reaction is experimentally determined to proceed at a constant rate. CHEM 3310 6

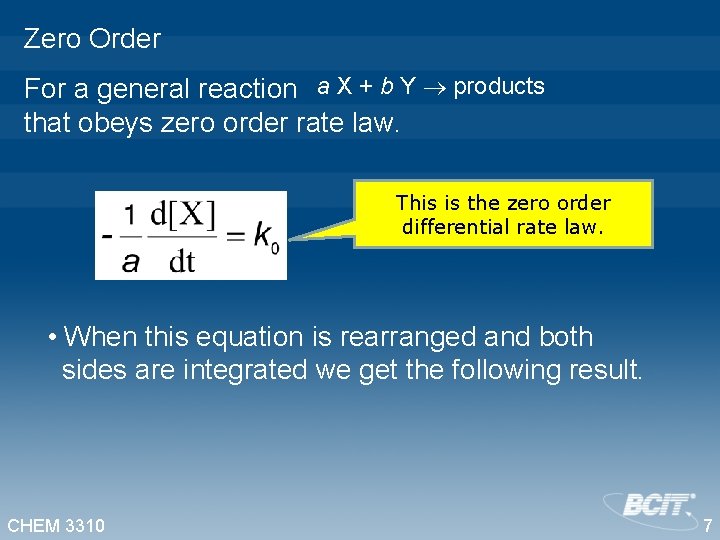
Zero Order For a general reaction a X + b Y products that obeys zero order rate law. This is the zero order differential rate law. • When this equation is rearranged and both sides are integrated we get the following result. CHEM 3310 7

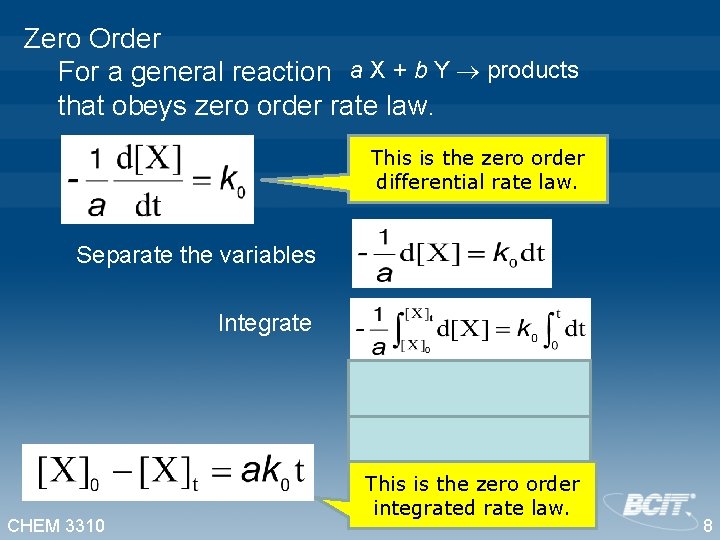
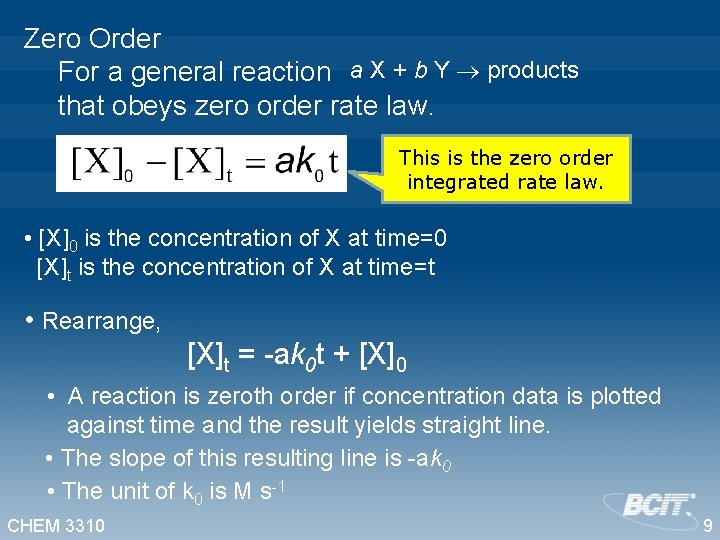
Zero Order For a general reaction a X + b Y products that obeys zero order rate law. This is the zero order differential rate law. Separate the variables Integrate CHEM 3310 This is the zero order integrated rate law. 8

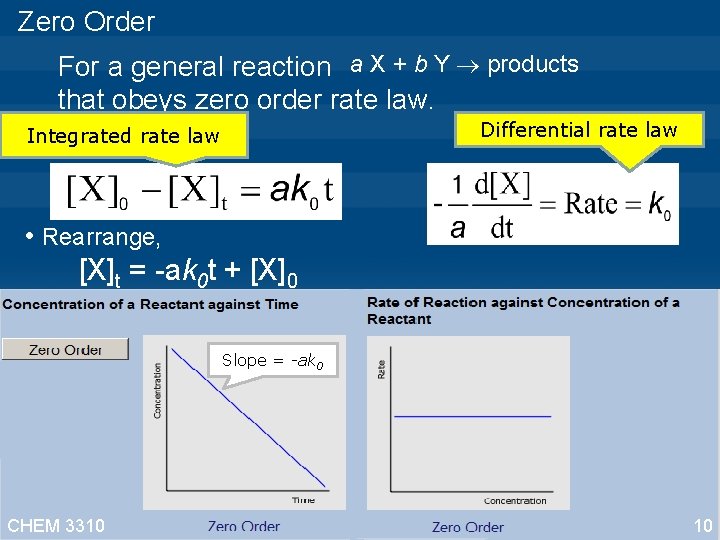
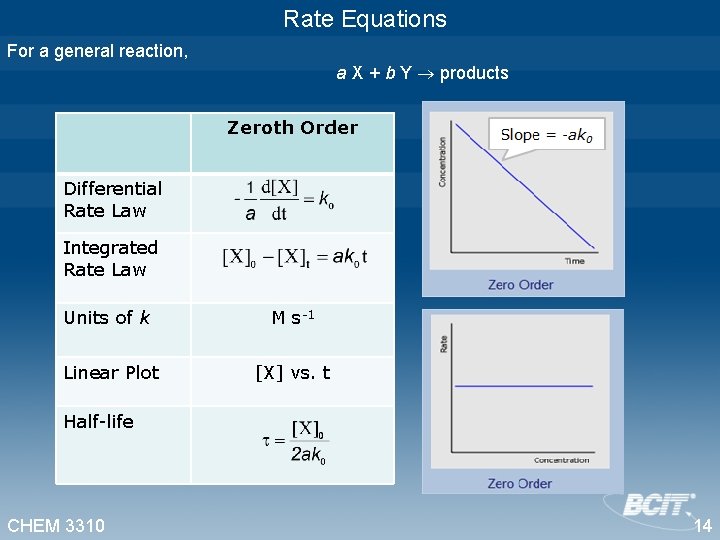
Zero Order For a general reaction a X + b Y products that obeys zero order rate law. This is the zero order integrated rate law. • [X]0 is the concentration of X at time=0 [X]t is the concentration of X at time=t • Rearrange, [X]t = -ak 0 t + [X]0 • A reaction is zeroth order if concentration data is plotted against time and the result yields straight line. • The slope of this resulting line is -ak 0 • The unit of k 0 is M s-1 CHEM 3310 9

Zero Order For a general reaction a X + b Y products that obeys zero order rate law. Differential rate law Integrated rate law • Rearrange, [X]t = -ak 0 t + [X]0 Slope = -ak 0 CHEM 3310 10

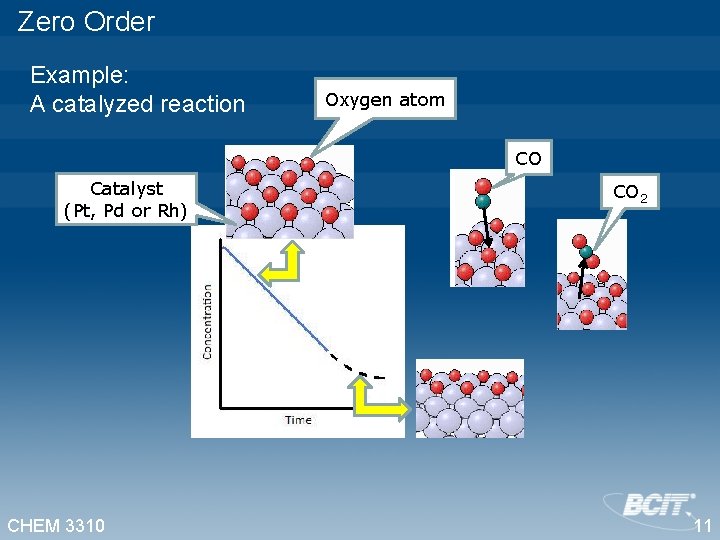
Zero Order Example: A catalyzed reaction Oxygen atom CO Catalyst (Pt, Pd or Rh) CHEM 3310 CO 2 11

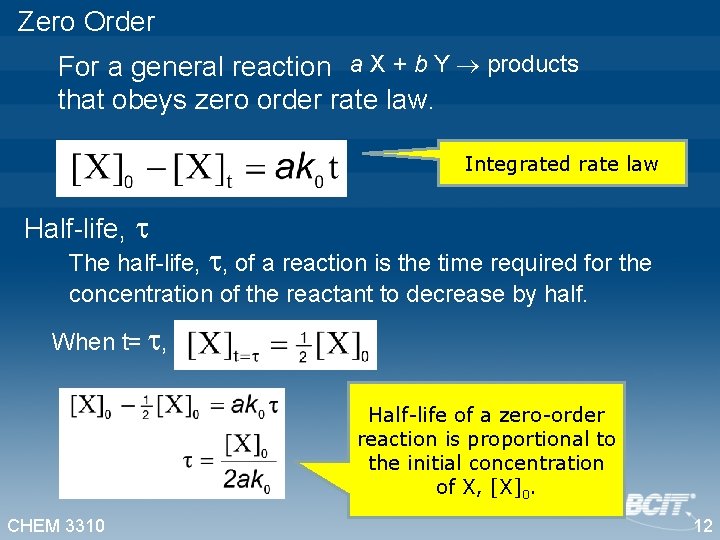
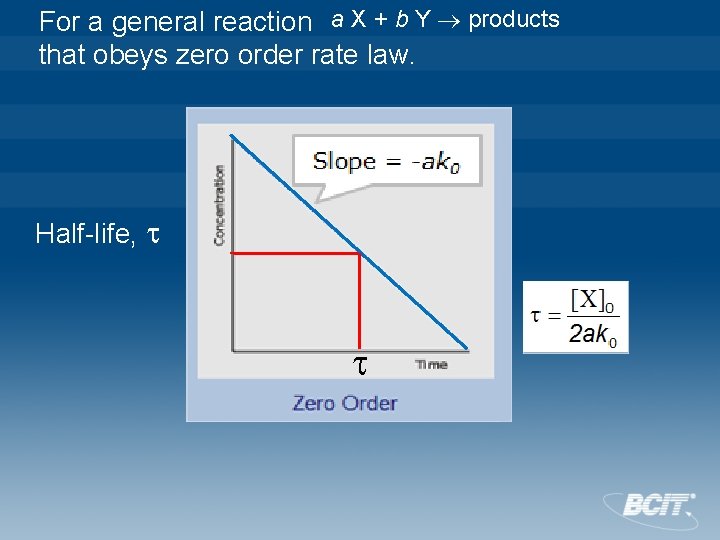
Zero Order For a general reaction a X + b Y products that obeys zero order rate law. Integrated rate law Half-life, The half-life, , of a reaction is the time required for the concentration of the reactant to decrease by half. When t= , Half-life of a zero-order reaction is proportional to the initial concentration of X, [X]0. CHEM 3310 12

For a general reaction a X + b Y products that obeys zero order rate law. Half-life,

Rate Equations For a general reaction, a X + b Y products Zeroth Order Differential Rate Law Integrated Rate Law Units of k M s-1 Linear Plot [X] vs. t Half-life CHEM 3310 14

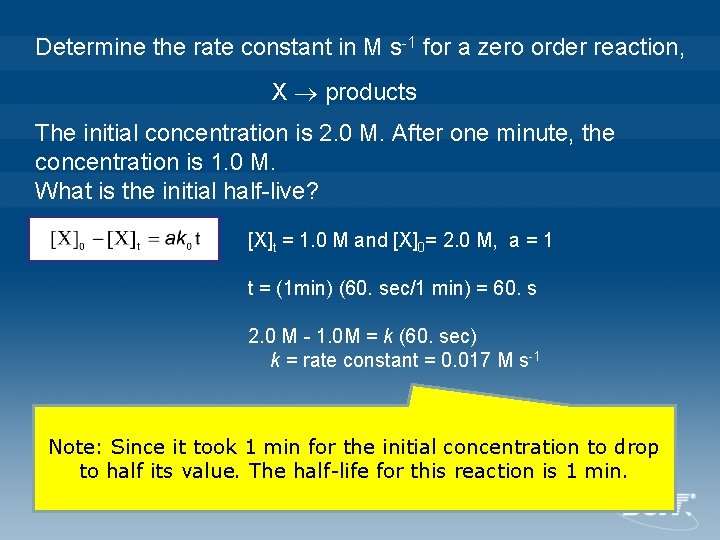
Determine the rate constant in M s-1 for a zero order reaction, X products The initial concentration is 2. 0 M. After one minute, the concentration is 1. 0 M. What is the initial half-live? [X]t = 1. 0 M and [X]0= 2. 0 M, a = 1 t = (1 min) (60. sec/1 min) = 60. s 2. 0 M - 1. 0 M = k (60. sec) k = rate constant = 0. 017 M s-1 Note: Since it took 1 min for the initial concentration to drop to half its value. The half-life for this reaction is 1 min.

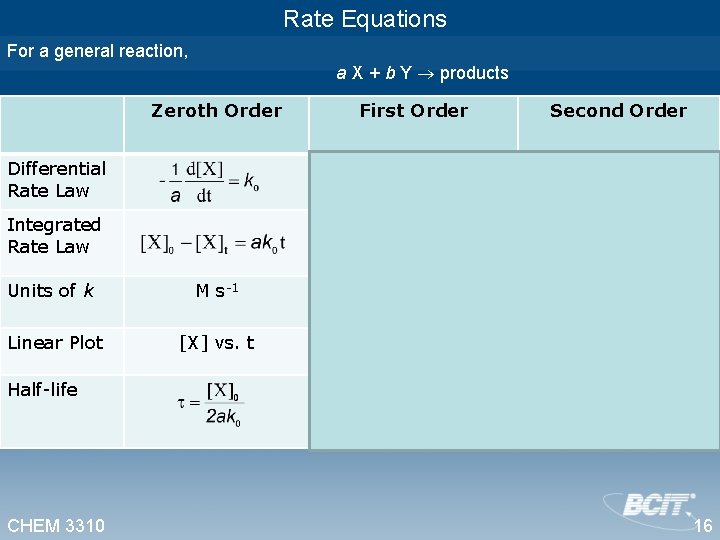
Rate Equations For a general reaction, a X + b Y products Zeroth Order First Order Second Order Units of k M s-1 M-1 s-1 Linear Plot [X] vs. t ln([X]) vs. t Differential Rate Law Integrated Rate Law Half-life CHEM 3310 16
 Zero order kinetics
Zero order kinetics Ap chem kinetics
Ap chem kinetics Difference between 1st order and zero order kinetics
Difference between 1st order and zero order kinetics Zero order elimination drugs
Zero order elimination drugs Rfc3310
Rfc3310 3310
3310 District 3310
District 3310 Ns 3310
Ns 3310 Fin 3310
Fin 3310 Thermodynamic
Thermodynamic Thermodynamics laws
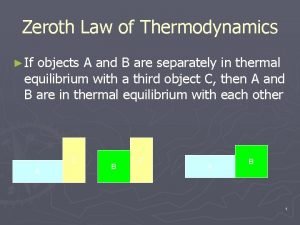
Thermodynamics laws Zeroth law of thermodynamics
Zeroth law of thermodynamics Zeroth law of thermodynamics
Zeroth law of thermodynamics Zeroth law of thermodynamics
Zeroth law of thermodynamics Molecularity of reaction
Molecularity of reaction Grade 11 chemistry unit 4
Grade 11 chemistry unit 4 Chemical kinetics half life
Chemical kinetics half life