Law of Thermodynamics Juwel Rana Lecturer Dept of










- Slides: 16

Law of Thermodynamics Juwel Rana Lecturer Dept. of Nutrition & Food Engineering Daffodil International University.

Table of Contents 1. Thermodynamics 2. Thermodynamic Systems & Surroundings 3. The Zeroth Law of Thermodynamics 4. The First Law of Thermodynamics 5. The Second Law of Thermodynamics 6. The Third Law of Thermodynamics

What is Thermodynamics? The branch of physics that deals with heat and temperature, and their relation to energy, work, radiation, and properties of matter. Laws of Thermodynamics Zeroth Law Of Thermodynamics First Law Of Thermodynamics Second Law Of Thermodynamics Third Law Of Thermodynamics

Thermodynamic Systems and Their Surroundings Thermodynamics is the branch of physics that is built upon the fundamental laws that heat and work obey. The collection of objects on which attention is being focused is called the system, while everything else in the environment is called the surroundings. Walls that permit heat flow are called diathermal walls, while walls that do not permit heat flow are called adiabatic walls. To understand thermodynamics, it is necessary to describe the state of a system.

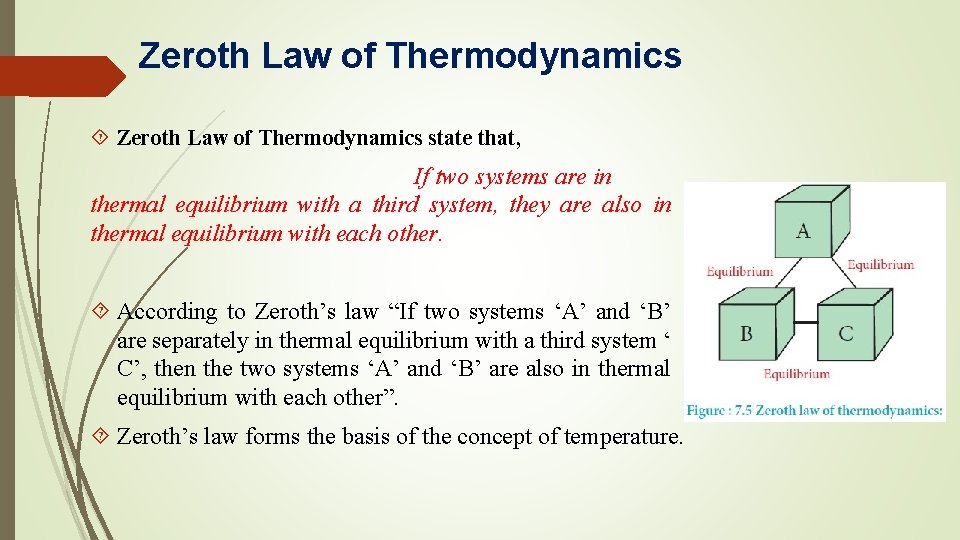
Zeroth Law of Thermodynamics state that, If two systems are in thermal equilibrium with a third system, they are also in thermal equilibrium with each other. According to Zeroth’s law “If two systems ‘A’ and ‘B’ are separately in thermal equilibrium with a third system ‘ C’, then the two systems ‘A’ and ‘B’ are also in thermal equilibrium with each other”. Zeroth’s law forms the basis of the concept of temperature.

Can any one say, example of Zeroth law of thermodynamics?

Example of Zeroth Law The thermometer may be the most well-known example of the zeroth law in action. For example, say thermostat in your bedroom reads 67 degrees Fahrenheit. This means that thermostat is in thermal equilibrium with your bedroom. However, because of the zeroth law of thermodynamics, you can assume that both the room and other objects in the room (say, a clock hanging in the wall) are also at 67 degrees Fahrenheit.

Example of Zeroth Law Similar to the above example, if you take a glass of ice water and a glass of hot water and place them on the kitchen countertop for a few hours, they will eventually reach thermal equilibrium with the room, with all 3 reaching the same temperature. If you place a package of meat in your freezer and leave it overnight, you assume that the meat has reached the same temperature as the freezer and the other items in the freezer.

First Law of Thermodynamics The first law of thermodynamics which is also known as the conservation of energy principle states that: First Law of Thermodynamics state that, Energy can neither be created nor destroyed, but it can be changed from one form to another.

First Law Of Thermodynamics Examples: A) Fans convert electrical energy to mechanical energy. B) Plants convert the radiant energy of sunlight to chemical energy through photosynthesis. We eat plants and convert the chemical energy into kinetic energy while we swim, walk, breathe and when we scroll through this page.

Second Law of Thermodynamics The second law of thermodynamics states that: Energy in the form of heat only flows from regions of higher temperature to that of lower temperature. Many individuals take this statement lightly and for granted, but it has an extensive impact and consequence. This is why it costs money to run an air conditioner. The human body obeys the second law of thermodynamics too.

Second Law Of Thermodynamics Examples sweating in a crowded room: q Assume yourself to be in a small room full of people. q You are very likely to feel warm and start sweating. Sweating is a mechanism the human body uses to cool itself. Here, the heat from your body is transferred to sweat. As the sweat absorbs more and more heat from the body it evaporates and transfers heat to the surrounding air, thereby, heating up the temperature of the room.

Third Law Of Thermodynamics state that, It is not possible to lower the temperature of any system to absolute zero in a finite number of steps. The third law of thermodynamics also refers to a state known as “absolute zero”. This is the lowest point on the Kelvin scale. So 0 Kelvin becomes the lowest temperature in the universe. It is -273. 15 o. C or -459. 7 o. F. But reality works differently; actually, no object or system can attain zero Kelvin, as per the second law of thermodynamics. Temperature mean available kinetic energy. 0 kelvin mean= 0 kinetic energy. 0 kelvin mean all goes to solid state.

Thank You

Practical Applications of Thermodynamics in Foods Thermodynamics helps us understand heat transfer, refrigeration, water activity, and mass transfer, among many other phenomena. The rates at which physical and biochemical processes occur are the realm of kinetics and hydrodynamics, but the limits and ultimate states are often determined by thermodynamics. For example, in heat exchange, there must be a finite difference between the phases exchanging energy, whether the objective is heating or cooling. The rate is determined by the magnitude of the difference (among other factors), but nothing useful will occur if the gradient of temperature is not in the correct direction. One cannot cool a food to 35˚F with a 40˚F coolant.

Practical Applications of Thermodynamics in Foods Refrigeration is one of the most important utilities in food processing. It is possible to understand because of thermodynamics. A working fluid, any one of many synthetic chemicals, ammonia, or carbon dioxide, is converted from gas to liquid and then back again by changing its pressure, which then changes the temperature at which it vaporizes or condenses. Most fluids boil or condense at higher temperatures when at higher pressure. Thus, a working fluid can be compressed to a pressure such that it will condense against ambient air, typically at 90˚F. If the pressure is reduced, by passing through a valve, a mixture of liquid and vapor results at a much lower temperature. The vapor can be returned to the compressor while the liquid is vaporized by the heat of the cooling load (evaporated). There are sophisticated modifications of this simple cycle, but air conditioners, coolers, and freezers all operate on the same fundamental principle. The thermodynamic concepts involved include phase change, heats of vaporization and of condensation (usually the same), heat exchange, and theoretical efficiency. The efficiency of a refrigeration cycle is expressed as the coefficient of performance (COP) equal to the amount of cooling provided divided by the work required in equivalent units, such as British Thermal Units (BTU). One BTU is the energy required to heat one pound of water one degree Fahrenheit. Work is often expressed in kilowatt hours (k. Wh). 3412. 14 BTU equal one k. Wh. COP is dimensionless and often has values around 4. 0. Another measure of refrigeration or cooling is the chiller ton, which is 12, 000 BTU/hr, derived from the energy removal necessary to freeze one ton of ice in a day.
 Lecturer's name or lecturer name
Lecturer's name or lecturer name Jeannie watkins
Jeannie watkins Spe distinguished lecturer
Spe distinguished lecturer Good afternoon student
Good afternoon student Photography lecturer
Photography lecturer Lecturer in charge
Lecturer in charge Designation lecturer
Designation lecturer Designation of lecturer
Designation of lecturer Guest lecturer in geography
Guest lecturer in geography Lecturer name
Lecturer name Pearson lecturer resources
Pearson lecturer resources Spe distinguished lecturer
Spe distinguished lecturer Lector vs lecturer
Lector vs lecturer Lecturer in charge
Lecturer in charge Cfa lecturer handbook
Cfa lecturer handbook Lecturer asad ali
Lecturer asad ali Steady flow energy equation thermodynamics
Steady flow energy equation thermodynamics































