PRESERVATION Juwel Rana Lecturer Department of Nutrition Food



























- Slides: 32

PRESERVATION Juwel Rana Lecturer Department of Nutrition & Food Engg. Daffodil International University.

FOOD PRESERVATION Food preservation is the process of treating and handling food to stop or slow down Food spoilage, loss of quality, edibility or nutritional value and thus allow for longer food storage. Preservation usually involves preventing the growth of bacteria, fungi (such as yeasts), and other microorganisms as well as retarding the oxidation of fats which cause rancidity. Food preservation is the process through which physical or chemical agents are used to prevent microbial spoilage.

PRINCIPLES OF FOOD PRESERVATION 1. Prevention or delay of microbial decomposition By keeping out microorganism (Asepsis) By removal of microorganism (Filtration) By hindering the growth and activity of microorganism ( Low temperature, drying, anaerobic conditions, chemicals) By killing the microorganism ( Heat of radiation) 2. Prevention or delay the self-decomposition of the food a) By destruction or inactivation of enzymes (Blanching) b) By prevention or delay of chemical reactions (prevention of oxidation by antioxidant) 3. Prevent the damage cause by insects, animals, mechanical causes etc.

ADVANTAGES OF FOOD PRESERVATION

ADVANTAGES OF FOOD PRESERVATION increased shelf-life decreased hazards from microbial pathogen decreased spoilage (microbial, enzymatic) inactivation of anti-nutritional factors ensured round the year availability of seasonal foods perishable foods that can be transported to far-off distances from the site of production increased availability of convenience foods (e. g. Ready-to-serve beverages, Instant mixes etc. ) increased variety of foods, some with enhanced sensory properties and nutritional attributes. preservation in some cases produces a different form of the products which are of great importance in various cuisines. E. g. raisins, squash and wines made from grapes.

METHODS OF FOOD PRESERVATION: 1) 2) 3) 4) 5) 6) 7) 8) Preservation by high temperature Preservation by low temperature Preservation by chemicals Preservation by Drying Preservation by Sugar Preservation by Salt Preservation by carbonation Irradiation etc.

PRESERVATION BY HIGH TEMPERATURE A) Pasteurization: Pasteurization is the process of heating a liquid or a food at boiling temperature or slightly below boiling temperature for a sufficient length of time to kill the microorganisms (pathogenic bacteria) which cause spoilage, is called pasteurization. Times and temperatures depend on: 1. The type of food and 2. The final result one wants to achieve, such as retaining a food’s nutrients, color, texture, and flavor.

OBJECTIVES OF PASTEURIZATION: The use of pasteurization to kill pathogenic bacteria has helped reduce the transmission of diseases, such as typhoid fever, tuberculosis, scarlet fever, polio, and dysentery

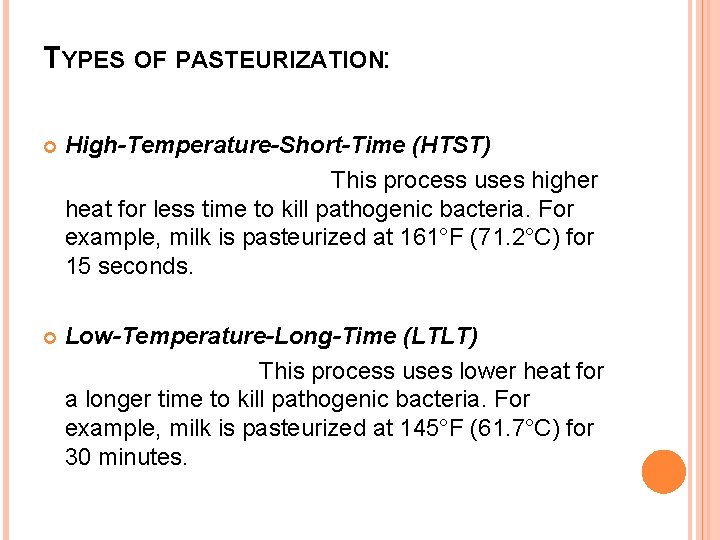
TYPES OF PASTEURIZATION: High-Temperature-Short-Time (HTST) This process uses higher heat for less time to kill pathogenic bacteria. For example, milk is pasteurized at 161°F (71. 2°C) for 15 seconds. Low-Temperature-Long-Time (LTLT) This process uses lower heat for a longer time to kill pathogenic bacteria. For example, milk is pasteurized at 145°F (61. 7°C) for 30 minutes.

OTHER TYPES OF MILK PASTEURIZATION Ultra-High-Temperature (UHT) Pasteurization - Typically involves heating milk or cream to 270° to 302°F (132° to 150°C) for 1 to 3 seconds. The milk is then packaged in sterile, hermetically-sealed (airtight) containers and can be stored without refrigeration for up to 90 days. After opening, spoilage times for UHT products are similar to those of conventionally pasteurized products.

METHODS OF PASTEURIZATION: Flash Pasteurization Steam Pasteurization Irradiation Pasteurization ******Home work******

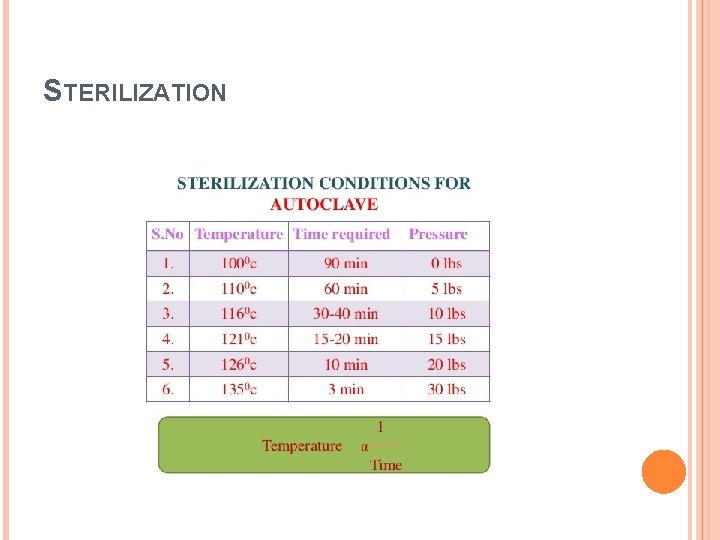
PRESERVATION BY HIGH TEMPERATURE B) Sterilization: Sterilization refers to a process that eliminates, removes, kills, or deactivates all forms of life and other biological agents (such as fungi, bacteria, viruses, spore forms, Plasmodium, etc. ) present in a food. Sterilization process maintain a temperature of 121° C for at least 20 -30 minutes by using saturated steam under at least 15 psi of pressure.

STERILIZATION

PRESERVATION BY LOW TEMPERATURE A) B) C) Cellar storage temperature (15°C) Chilling (0 to 5°C): Freezing (-18 to -40°C):

CHILLING: Chilling (0 to 5°C): Foods kept at this temperature slow down the microbial activities and chemical changes resulting in spoilage. Mechanical refrigerator or cold storage is used for this purpose. Examples of this include meats, poultry, eggs, fish, fresh milk and milk products, fruits, vegetables, etc. which can be preserved for 2 -7 days by refrigeration.

CHILLING: Advantages of Chilling Chilled foods taste closer to fresh foods than frozen, canned or dried foods. There is little loss of flavour, colour, texture, shape or nutritional value. Allows consumers to have good quality, ready- prepared food without having to shop every day. Disadvantages of Chilling: Only a temporary, short term method of preservation. Specialist storage equipment is needed – a fridge kept at below 5° C. Commercial stores must be below 2°C.

PRESERVATION BY LOW TEMPERATURE A) B) C) Cellar storage temperature (15°C) Chilling (0 to 5°C): Freezing (-18 to -40°C):

CHILLING Advantages of Chilling Chilled foods taste closer to fresh foods than frozen, canned or dried foods. There is little loss of flavour, colour, texture, shape or nutritional value. Allows consumers to have good quality, ready- prepared food without having to shop every day. Disadvantages of Chilling: Only a temporary, short term method of preservation. Specialist storage equipment is needed – a fridge kept at below 5° C. Commercial stores must be below 2°C.

FREEZING: Freezing (-18 to -40°C): In freezing, water in food turns into ice and hence, makes the water unavailable for reactions. q Most perishable foods like poultry, meats, fish, icecreams, peas, vegetables, juice concentrates, etc. can be preserved for several months at this temperature. q In vegetables, enzyme action may still produce undesirable effects on flavour and texture during freezing. q Heating, like blanching, therefore, must destroy the enzymes before the vegetables are frozen.

ADVANTAGES OF FREEZING: Long term method of preservation (weeks & months rather than days) Little change in flavour or structure compared to canned or dried food Very little nutritional loss – most vitamin B and C is retained.

DISADVANTAGES OF FREEZING: Quick & controlled freezing is needed or large ice crystals will form and break the cell walls of some foods. Specialist storage equipment is needed – a freezer kept at below -18° C. Long term stores must be below 2° C. Flavours of garlic, spices and herbs often become stronger during freezing. This needs to be taken into account when designing food. Cell damage can occur in soft fruits such as strawberries and the colloidal structure of some food products, such as sauces, can collapse when frozen.

METHODS OF FREEZING: A) slow Freezing. ( Large crystal, -15 to -29 C) B) Quick Freezing. (Small crystal, -18 to -40 C)

THE MAIN ADVANTAGES OF QUICK FREEZING OVER SLOW FREEZING: Small ice crystals formed as a result less damage to the cell structure or texture of the food The freezing period being much shorter, less time is allowed for the diffusion of salts and the separation of water in the form of ice. The product is quickly cooled below the temperature, at which bacterial, mould, and yeast growth occur, thus preventing decomposition of foods during freezing. The fourth and very practical reason in favor of quick freezing over 'slow-freezing', is the inherent speed and greater output and hence, higher capacity for commercial freezing plants with the resultant cost reduction.

PRESERVATION BY CHEMICALS According to the British Food and Drug Act of 1928, preservatives is any substances which is capable of inhibiting, retarding or arresting the growth of microorganisms without necessarily destroying them or prevent deterioration of quality during manufacture and distribution. Chemical preservatives Interfere with the cell membranes of microorganisms and stop their enzyme activity or then genetic mechanisms.

SULPHUR DIOXIDE: Sulphur dioxide is widely used throughout the world in the preservation of fruit pulp and juices, squash cordial nectar and molasses. At p. H values less than 4. 0 the antimicrobial activity reaches its maximum. It has good preserving action against bacteria and moulds. It has good preserving action on inhibits enzymes. It acts as an antioxidant and bleaching agent. It also retard the development of nonenzymatic or discoloration (after killing the enzyme) of the product. It is generally used in the form of its salt such as sulphite, bisulphite and metabisulphite. They conserve colour, act as antioxidants and control microbial growth.

BENZOIC ACID: Benzoic acid in the form of its sodium salt such as sodium benzoate. Sodium benzoate is a common preservative in acid or acidified foods such as fruit juices, syrups, jams and jellies, sauerkraut, pickles, preserves, fruit cocktails, etc. Yeasts are inhibited by benzoate to a greater extent than are moulds and bacteria. It does not stop lactic acid and acetic acid fermentation.

PRESERVATION BY DRYING: The principle behind drying is that sufficient moisture is removed, which is essential for growth of microorganisms and for enzyme activity. Removal of moisture increases the storage life of the product due to reduced water activity. If the moisture content is reduced to 1 to 5 per cent then the product can be stored for more than a year. The processing should be done in such a way that the food value, natural flavour and characteristic cooking quality of the fresh material are retained after drying.

PRESERVATION BY SUGAR Principle of Preservation by Sugar: v Sugar in high concentrations acts as a preservative due to osmosis. v Sugar attracts all available water and water is transferred from the microorganisms into the concentrated sugar syrup. v The microflora is dehydrated and cannot multiply further. v The concentration of sugar in sugar preserved products must be 65 per cent or more, which does not allow microorganisms to grow. v Lower concentrations may be effective but for short duration. v The critical concentration of sugar required to prevent microbial growth varies with the type of microorganisms and the presence of other food constituents.

PRESERVATION BY SUGAR (CONT) Some of the most popular preserves with sugar are jelly, jam and marmalade. Pectin, a natural component of fruits, forms a gel only in the presence of sugar and acid. Sugar prevents spoilage of jams, jellies, and preserves even after the container is opened. With the use of sugar, the water activity cannot be reduced below 0. 845. This level of water activity is sufficient to inhibit mesophillic bacteria and yeast but does not check mould attack. Most of these products are made of acid fruits. When foods low in acid are used, they are usually combined with some acid fruit.

PRESERVATION BY SALT: Principle of preservation by Salt: Salt acts as preservative when its concentration is increased above 12 per cent. Salt levels of about 18 to 25 per cent in solution generally will prevent all growth of microorganisms in foods. However, this level is rarely tolerated in foods except in the case of certain briny condiments. Salt exerts its preservative action by plasmolysis of microbial cells due to high osmotic pressure. Salt drawing moisture from microbes, ionizing to yield chloride ion, which is harmful to microorganisms, reducing the solubility of oxygen to water, sensitizing the cells against carbon dioxide and interfering with the action of proteolytic enzymes.

PRESERVATION BY CARBONATION Carbonation is the process by which carbon dioxide gas is dissolved in food under pressure. The principle behind this is that by eliminating oxygen, carbon dioxide inhibits bacterial growth. E. g. carbonated beverages (soft drinks), therefore, contain a natural preservative.

IRRADIATION: Food irradiation is low temperature sterilizing technique as in this case, sterilization can be effected at room temperature. Foods are exposed to high-energy rays called gamma rays or by fast-moving electrons, which kill bacteria, fungi and insects. In some cases, irradiation delays fruit ripening. A major advantage of irradiation is that it can be done after the food is packaged and sealed. It has been used in pasteurizing or sterilizing perishable foods such as meat, fish and fruits and extending their storage lives for long periods. It is also used for sprouting inhibition in onions, potatoes etc. Cobalt-60 or Cesium-137 or electrons producing machines are the principal sources of ionizing radiations used for food irradiation.
 Lecturer's name
Lecturer's name Physician associate lecturer
Physician associate lecturer Spe distinguished lecturer
Spe distinguished lecturer Good morning teacher good morning teacher
Good morning teacher good morning teacher Photography lecturer
Photography lecturer Lecturer in charge
Lecturer in charge Designation lecturer
Designation lecturer Designation of lecturer
Designation of lecturer Why himalayan rivers are pernnial in nature
Why himalayan rivers are pernnial in nature Lecturer name
Lecturer name Pearson lecturer resources
Pearson lecturer resources 140000/120
140000/120 Lector vs lecturer
Lector vs lecturer Lecturer in charge
Lecturer in charge Cfa lecturer handbook
Cfa lecturer handbook Lecturer asad ali
Lecturer asad ali National center for home food preservation
National center for home food preservation Preservation of food by high temperature
Preservation of food by high temperature High temperature food processing
High temperature food processing Mochahlama
Mochahlama Preservation by heating
Preservation by heating Eni harmayani
Eni harmayani Food preservation definition
Food preservation definition Methods of freezing food
Methods of freezing food Burial food preservation
Burial food preservation National center for home preservation
National center for home preservation Advantages of canning
Advantages of canning Purpose of food preservation
Purpose of food preservation Food preservation by high temperature
Food preservation by high temperature Sterilization
Sterilization Application of hurdle technology
Application of hurdle technology Food preservation history
Food preservation history Arkansas child nutrition unit
Arkansas child nutrition unit