Thermodynamics Energy Changes and Matter Thermodynamics Thermodynamics is











- Slides: 15

Thermodynamics Energy Changes and Matter

Thermodynamics • Thermodynamics- is the study of energy, and energy changes in matter. • Remember a Fun “Mini Lab” on Endothermic and Exothermic ? • A plastic baggie with Ca. Cl 2 became very warm when phenol red (or water) was added. EXOTHERMIC REACTION • A plastic baggie with Na. HCO 3 became very cool when phenol red (or water) was added. ENDOTHERMIC REACTION

Phase Changes • The three commons states (Phases) of matter are Solid, Liquid, Gas. • The average kinetic energy of the atoms in the substance determine its state of matter. • Melting- the physical change of a substance from a solid to a liquid. This phase change absorbs energy from the surroundings. • Freezing – the physical change of a substance from a liquid to a solid. This phase change releases energy into the surroundings.

Heat of Fusion • Heat of Fusion- is the amount of energy that must be added to one kilogram of a substance to change that substance from a solid to a liquid. • (please note the same amount of energy is release if a one kilogram sample of that substance freezes. ) • Example: The Heat of Fusion of Water is 334, 000 Joules/ kilogram so if you have one kilogram of water at 0 OC solid (ice) and you add 334, 000 joules of energy you will then have one kilogram of liquid water at 0 o. C.

Phase Change • Vaporization- is the physical change of a liquid to a gas. This phase change absorbs energy from the surroundings. • Condensation-is the physical change of a gas to a liquid. This phase change releases energy into the surroundings.

Heat of Vaporization • Heat of Vaporization- is the amount of energy needed to change one kilogram of a substance from a liquid to gas. • (please note the amount of energy needed to change a gas to liquid is the same amount of energy that would be released) • Example: The Heat of Vaporization of Water is 2, 260, 000 Joules/ kilogram so if you have one kilogram of water at 100 OC liquid and you add 2, 260, 000 joules of energy you will then have one kilogram of water vapor at 100 o. C.

Phase Change • Sublimation- is the physical change of solid matter directly to the gaseous state. This phase change absorbs energy from the surroundings. Think of snow on a sunny day or dry ice (solid CO 2) which goes directly to a gas. Dry ice is often used to make special effects at light show such as a concert. • Deposition-is the physical change of a gas directly to a solid. This phase change releases energy into the surroundings. Think of “old” fashioned freezers that ice over inside. Water vapor from the air gets depositied as ice on the internal frame of the ice box!

Specific Heat Capacity • Specific Heat Capacity- is the amount of energy needed change one kilogram of a substance by one degree Celsius. Example: The Specific Heat Capacity of water at room temperature is 4180 Joules/kg*C q = mass x C x ∆Temp q is Energy or Heat or ∆ H, measured in joules. C is Specific Heat Capacity for that unique substance. ∆Temp =Final T – Initial T

S. H. C. Calculations • Miss King turned on a hot plate to make some Hot Chocolate. She placed 500 grams of water into the container. The starting temp of the H 2 O is 20 o. C and the water heated to 99 o. C. How much energy did the hot plate “give” to the water? Reminder the S. H. C. for water is 4180 J/kg*K • q = m x C x ∆ Temp • q=. 500 kg x 4180 J/kg*K x 79 K • q= 165110 joules of energy or 165. 110 kilojoules

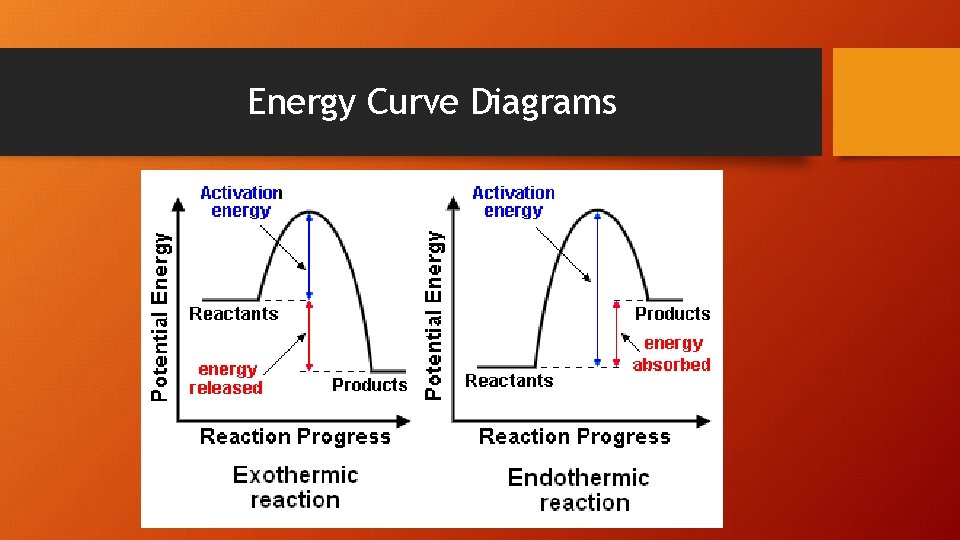
Energy Curve Diagrams

∆H= ∑∆Hf Products - ∑∆Hf Reactants • • ∆H= (+) value is endothermic ∆H= (-) value is exothermic ∆H= remember elements have a zero ∆Hf value ∆H= remember to multiply the ∆Hf by the mole coefficient!

Phase Change Calculations are FUN! • Follow the phase change graph step by step and let is lead you through the calculations. • Decide how many steps are involved in the phase change and do a “q” calculation for each step. Then add the “q” from all the steps together! Ta Da! Success!

Phase Change Example • Please calculate the amount of energy absorbed if 750 gram of H 2 O at room temp 22 o. C is heated to steam at 104 o. C. • Use your graph to visualize the change…… • There are three steps! 22 o. C up to 100 o. C liquid to 100 o. C steam. Steam 100 o. C-104 o. C.

ANSWER • • 1. q=. 750 kg x 4180 j/kg*K x 78 K 244530 j 2. q=. 750 kg x 2, 260, 000 j/kg 169500 j 3. q=. 750 kg x 2070 j/kg*K x 4 K 6210 j Total==============420240 joules

Thermodynamics Labs are FUN! • The labs we will cover are: • Heat of Fusion (scientifically calculation the amount of energy needed to melt ice!) • Calorimetry lab we combust food items and estimate the calorie content of the food! • Hess’s Law lab We will record the energy released in a chemical change and compare that number to the same reaction by a different pathway! And maybe more!!!!!
 Ecological succession
Ecological succession Elizabeth mulroney
Elizabeth mulroney Chemical changes example
Chemical changes example Properties and changes of matter worksheet
Properties and changes of matter worksheet Definition of substance
Definition of substance Matter-properties and changes answer key
Matter-properties and changes answer key Properties of and changes in matter grade 5
Properties of and changes in matter grade 5 Eating food physical or chemical change
Eating food physical or chemical change Physical changes examples
Physical changes examples 5 phases of matter
5 phases of matter Change in state of matter
Change in state of matter Two types of changes
Two types of changes R constant
R constant 6 common phase changes
6 common phase changes States of matter concept map
States of matter concept map Grey vs white matter
Grey vs white matter





























