Chemical Kinetics Honors Unit 11 Chemical Kinetics KINETICS




![Rate Laws & Orders of Reactions Rate Law for a reaction: Rate = k[A]m[B]n[C]p Rate Laws & Orders of Reactions Rate Law for a reaction: Rate = k[A]m[B]n[C]p](https://slidetodoc.com/presentation_image_h2/7574b61386f2bc8c8e519496e3dbd5df/image-12.jpg)
![Significance of Rate Laws Rate of rxn = k[CH 3 CHO]2 Here the rate Significance of Rate Laws Rate of rxn = k[CH 3 CHO]2 Here the rate](https://slidetodoc.com/presentation_image_h2/7574b61386f2bc8c8e519496e3dbd5df/image-18.jpg)

![Example #2: Experiment 1 2 3 4 5 6 [NO] 0. 0010 0. 0020 Example #2: Experiment 1 2 3 4 5 6 [NO] 0. 0010 0. 0020](https://slidetodoc.com/presentation_image_h2/7574b61386f2bc8c8e519496e3dbd5df/image-21.jpg)
![Example #2: Experiment 1 2 3 4 5 6 [NO] 0. 0010 0. 0020 Example #2: Experiment 1 2 3 4 5 6 [NO] 0. 0010 0. 0020](https://slidetodoc.com/presentation_image_h2/7574b61386f2bc8c8e519496e3dbd5df/image-22.jpg)




- Slides: 37

Chemical Kinetics Honors Unit 11

Chemical Kinetics § KINETICS = The study of reaction rates and the mechanism (the way the reaction proceeds) n n Some rxns. are FAST (Ex. Lighting a match) Others are SLOW (Ex. Forming diamond, Rusting) ***Only kinetics will tell us how fast the reaction happens!

Reaction Rate n

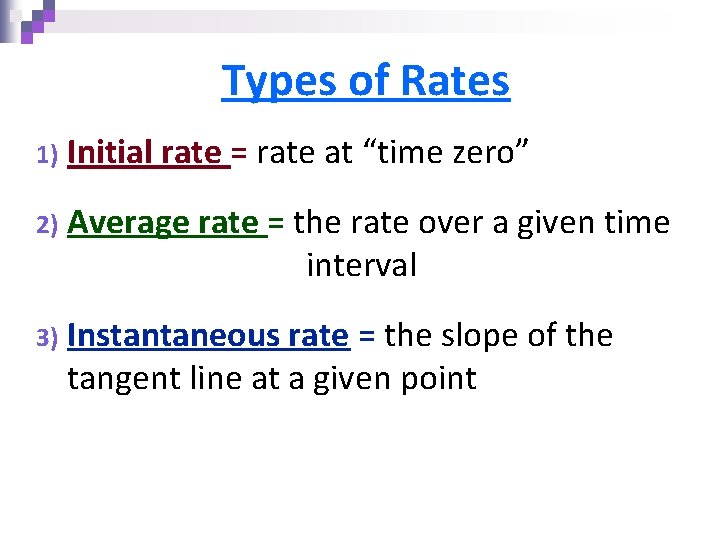
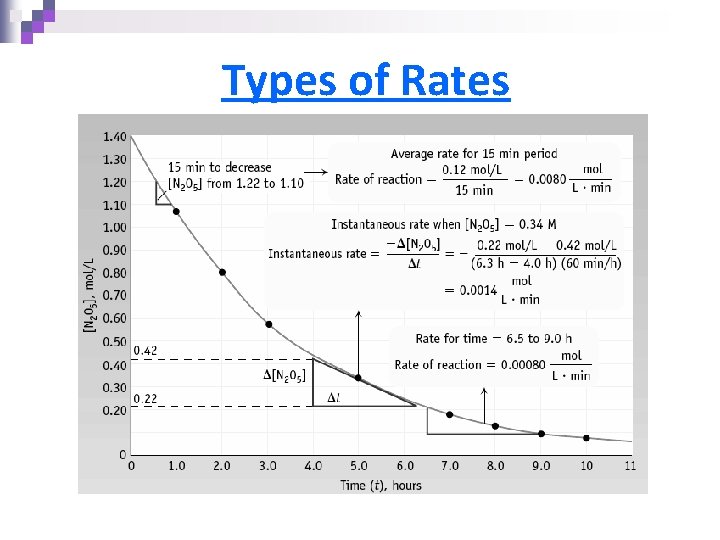
Types of Rates 1) Initial rate = rate at “time zero” 2) Average rate = the rate over a given time interval 3) Instantaneous rate = the slope of the tangent line at a given point

Types of Rates

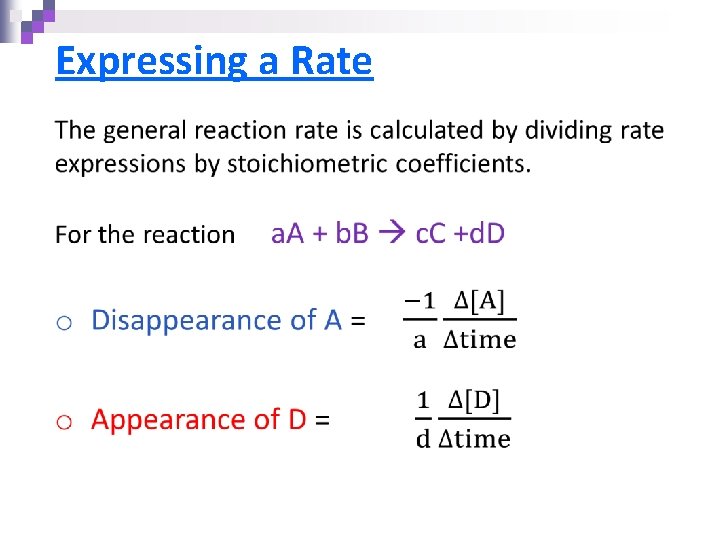
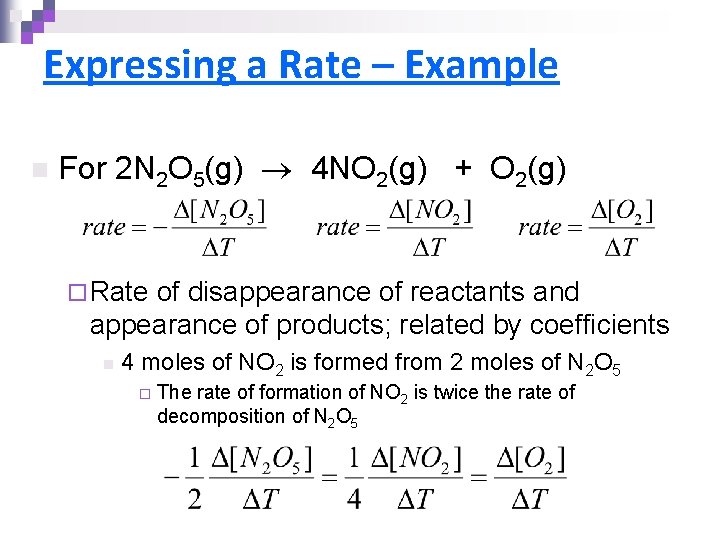
Expressing a Rate

Expressing a Rate – Example n For 2 N 2 O 5(g) 4 NO 2(g) + O 2(g) ¨ Rate of disappearance of reactants and appearance of products; related by coefficients n 4 moles of NO 2 is formed from 2 moles of N 2 O 5 ¨ The rate of formation of NO 2 is twice the rate of decomposition of N 2 O 5

Collision Theory of Reactants § Reactions occur when molecules collide to exchange or rearrange atoms § Effective collisions occur when molecules have correct energy and orientation

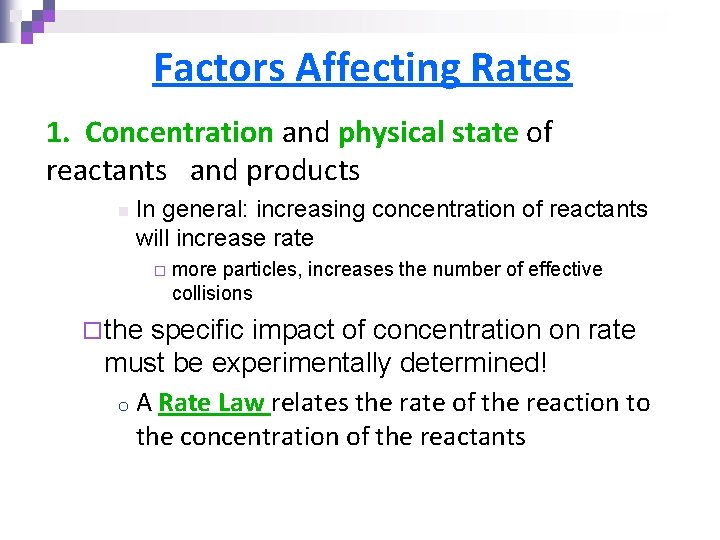
Factors Affecting Rates 1. Concentration and physical state of reactants and products n In general: increasing concentration of reactants will increase rate ¨ ¨ the more particles, increases the number of effective collisions specific impact of concentration on rate must be experimentally determined! o A Rate Law relates the rate of the reaction to the concentration of the reactants

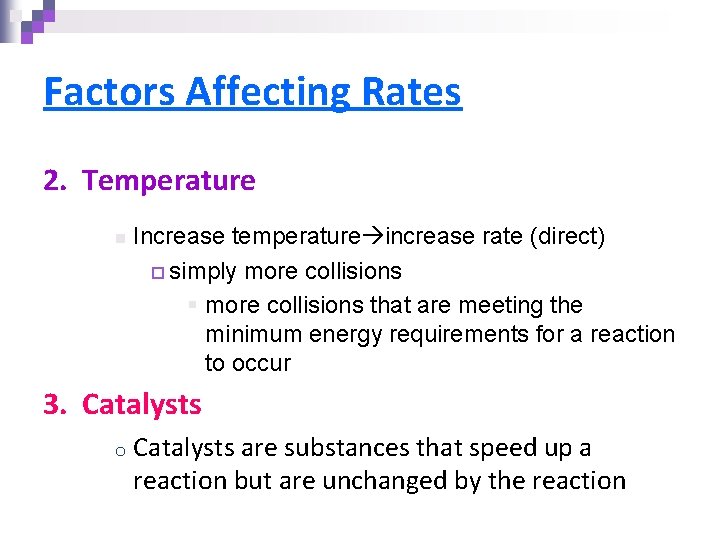
Factors Affecting Rates 2. Temperature n Increase temperature increase rate (direct) ¨ simply more collisions § more collisions that are meeting the minimum energy requirements for a reaction to occur 3. Catalysts o Catalysts are substances that speed up a reaction but are unchanged by the reaction

Writing Rate Laws For a. A + b. B c. C + d. D The rate law is: Rate = k[A]m[B]n § k is the rate constant § The exponents must be determined by performing an experiment. § They are NOT derived from the stoichiometry coefficients in an overall chemical equation!!
![Rate Laws Orders of Reactions Rate Law for a reaction Rate kAmBnCp Rate Laws & Orders of Reactions Rate Law for a reaction: Rate = k[A]m[B]n[C]p](https://slidetodoc.com/presentation_image_h2/7574b61386f2bc8c8e519496e3dbd5df/image-12.jpg)
Rate Laws & Orders of Reactions Rate Law for a reaction: Rate = k[A]m[B]n[C]p The exponents m, n, and p: § Are the reaction order § Can be 0, 1, 2, or fractions (may be other whole numbers in fictional examples) § Must be determined by experimentation Overall Order = sum of m, n, and p

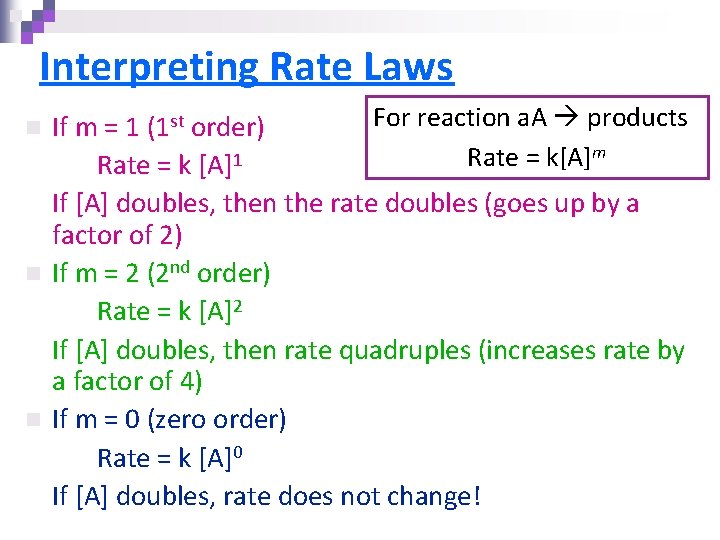
Interpreting Rate Laws n n n For reaction a. A products If m = 1 (1 st order) m 1 Rate = k[A] Rate = k [A] If [A] doubles, then the rate doubles (goes up by a factor of 2) If m = 2 (2 nd order) Rate = k [A]2 If [A] doubles, then rate quadruples (increases rate by a factor of 4) If m = 0 (zero order) Rate = k [A]0 If [A] doubles, rate does not change!

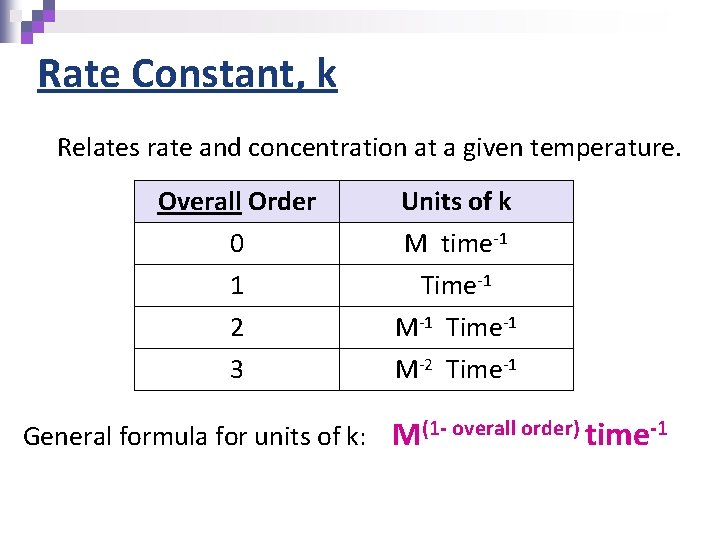
Rate Constant, k Relates rate and concentration at a given temperature. Overall Order 0 1 Units of k M time-1 Time-1 2 3 M-1 Time-1 M-2 Time-1 General formula for units of k: M(1 - overall order) time-1

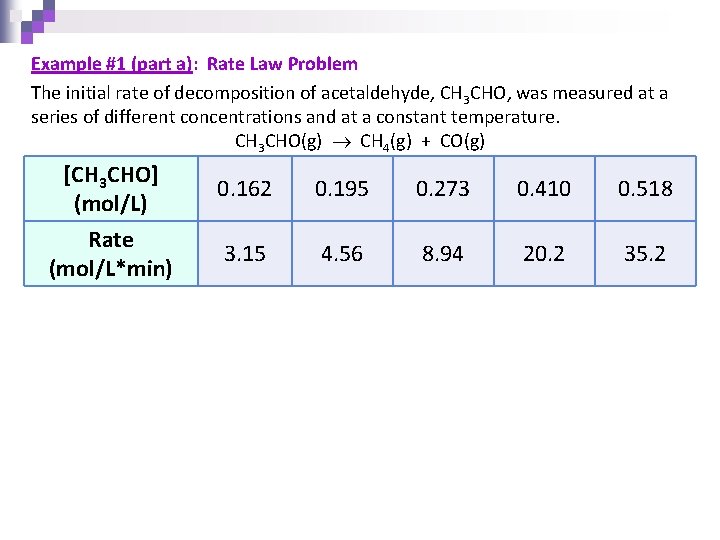
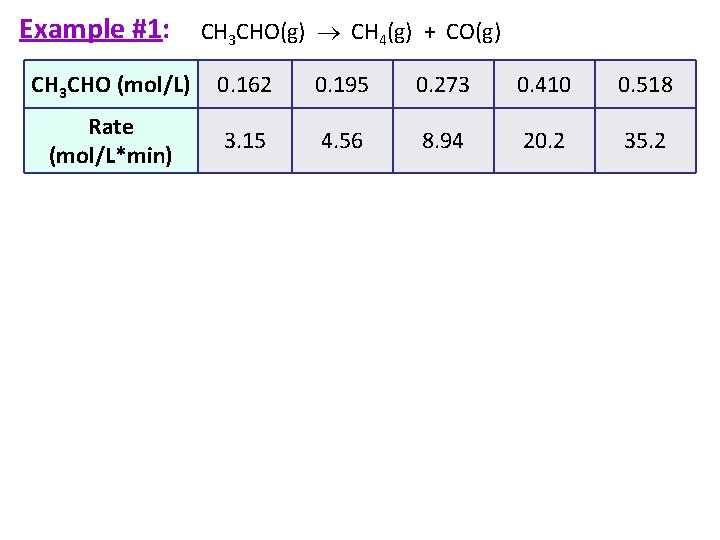
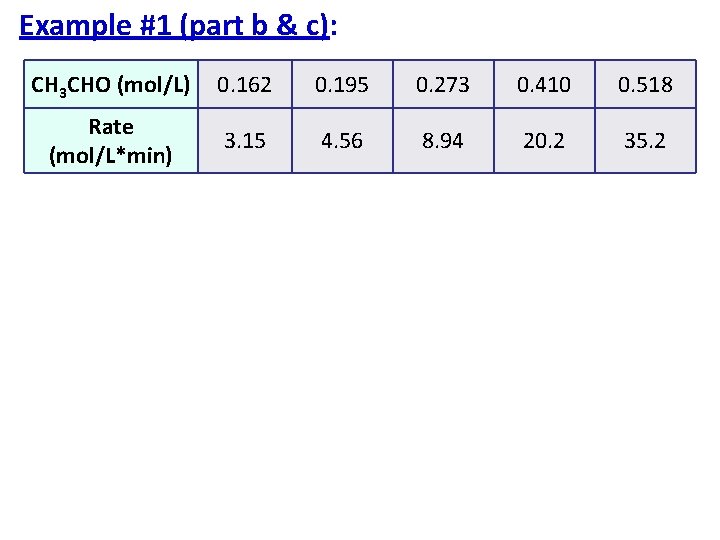
Example #1 (part a): Rate Law Problem The initial rate of decomposition of acetaldehyde, CH 3 CHO, was measured at a series of different concentrations and at a constant temperature. CH 3 CHO(g) CH 4(g) + CO(g) [CH 3 CHO] (mol/L) 0. 162 0. 195 0. 273 0. 410 0. 518 Rate (mol/L*min) 3. 15 4. 56 8. 94 20. 2 35. 2

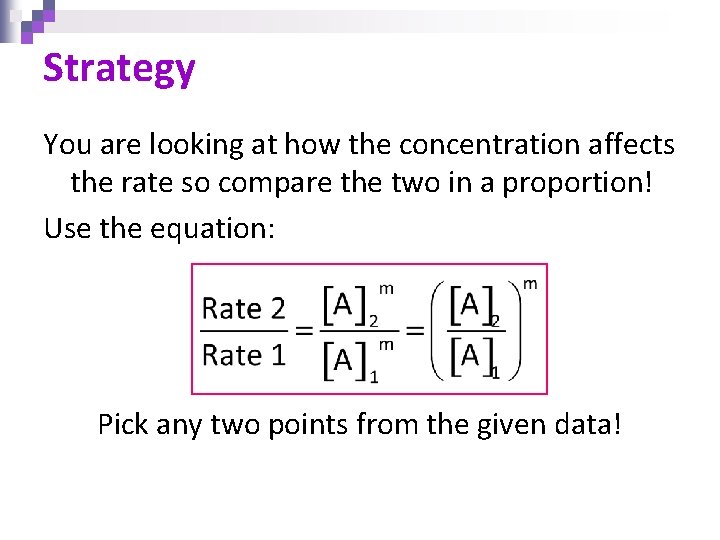
Strategy You are looking at how the concentration affects the rate so compare the two in a proportion! Use the equation: Pick any two points from the given data!

Example #1: CH 3 CHO(g) CH 4(g) + CO(g) CH 3 CHO (mol/L) 0. 162 0. 195 0. 273 0. 410 0. 518 Rate (mol/L*min) 3. 15 4. 56 8. 94 20. 2 35. 2
![Significance of Rate Laws Rate of rxn kCH 3 CHO2 Here the rate Significance of Rate Laws Rate of rxn = k[CH 3 CHO]2 Here the rate](https://slidetodoc.com/presentation_image_h2/7574b61386f2bc8c8e519496e3dbd5df/image-18.jpg)
Significance of Rate Laws Rate of rxn = k[CH 3 CHO]2 Here the rate goes up by FOUR when the initial concentration doubles. Therefore, the reaction is SECOND order overall.

Example #1 (part b & c): CH 3 CHO (mol/L) 0. 162 0. 195 0. 273 0. 410 0. 518 Rate (mol/L*min) 3. 15 4. 56 8. 94 20. 2 35. 2

Example #2: The data below is for the reaction of nitrogen (II) oxide with hydrogen at 800 o. C. 2 NO(g) + 2 H 2(g) N 2(g) + 2 H 2 O(g) (a) Determine the order of the reaction with respect to both reactants and write the rate law (b) calculate the value of the rate constant, and (c) determine the rate of formation of product when [NO]=0. 0024 M and [H 2]=0. 0042 M.
![Example 2 Experiment 1 2 3 4 5 6 NO 0 0010 0 0020 Example #2: Experiment 1 2 3 4 5 6 [NO] 0. 0010 0. 0020](https://slidetodoc.com/presentation_image_h2/7574b61386f2bc8c8e519496e3dbd5df/image-21.jpg)
Example #2: Experiment 1 2 3 4 5 6 [NO] 0. 0010 0. 0020 0. 0030 0. 0040 [H 2] 0. 0040 0. 0010 0. 0020 0. 0030 0. 12 0. 48 1. 08 0. 48 0. 96 1. 44
![Example 2 Experiment 1 2 3 4 5 6 NO 0 0010 0 0020 Example #2: Experiment 1 2 3 4 5 6 [NO] 0. 0010 0. 0020](https://slidetodoc.com/presentation_image_h2/7574b61386f2bc8c8e519496e3dbd5df/image-22.jpg)
Example #2: Experiment 1 2 3 4 5 6 [NO] 0. 0010 0. 0020 0. 0030 0. 0040 [H 2] 0. 0040 0. 0010 0. 0020 0. 0030 0. 12 0. 48 1. 08 0. 48 0. 96 1. 44

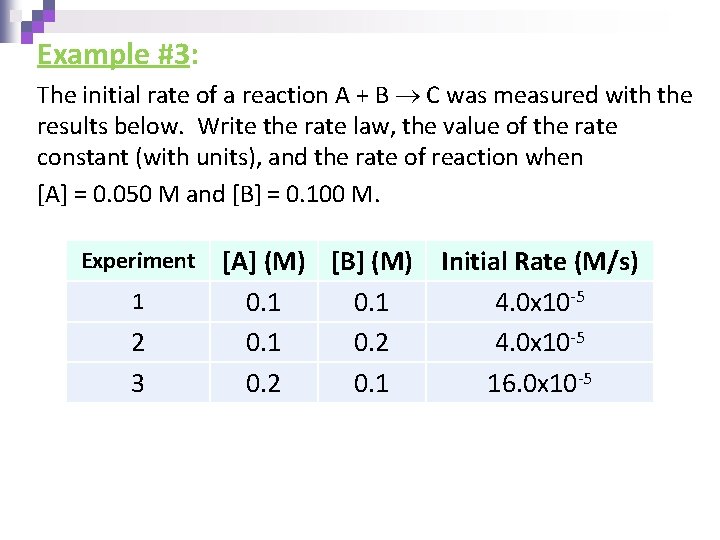
Example #3: The initial rate of a reaction A + B C was measured with the results below. Write the rate law, the value of the rate constant (with units), and the rate of reaction when [A] = 0. 050 M and [B] = 0. 100 M. Experiment 1 2 3 [A] (M) [B] (M) 0. 1 0. 2 0. 1 Initial Rate (M/s) 4. 0 x 10 -5 16. 0 x 10 -5

Example #3:

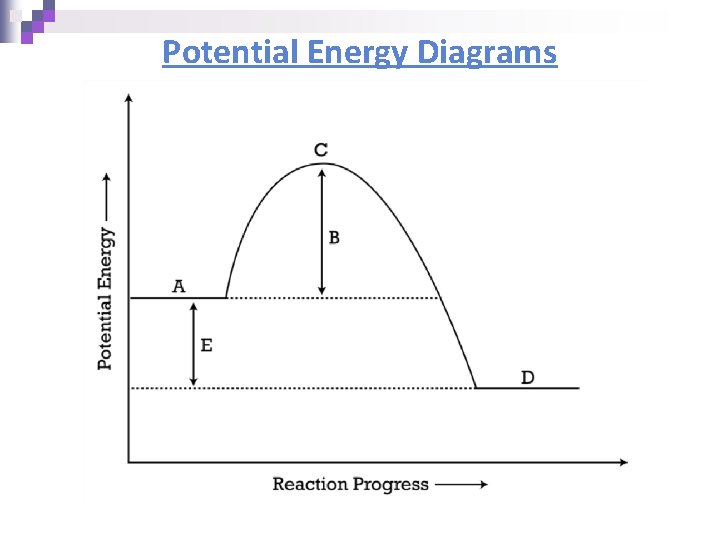
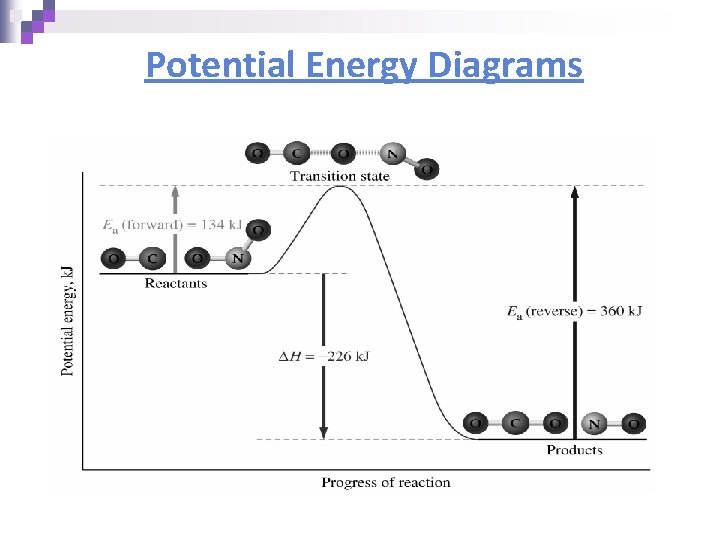
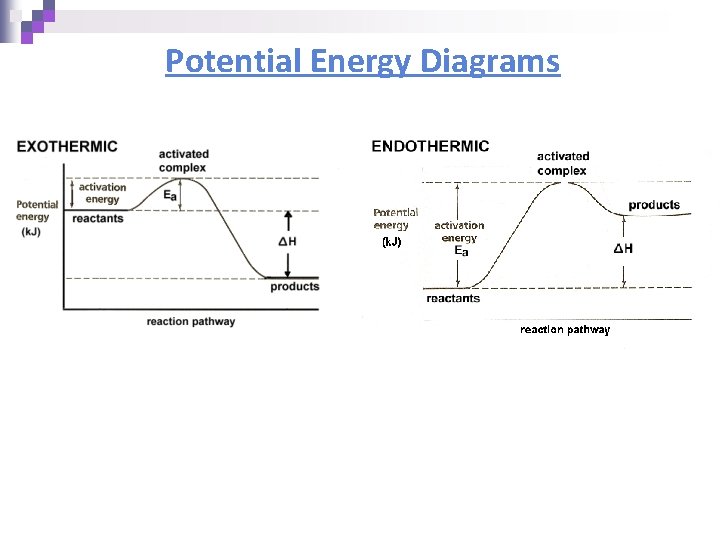
Potential Energy Diagrams n Molecules need a minimum amount of energy for a reaction to take place. ¨ Activation energy (Ea) – the minimum amount of energy that the reacting species must possess to undergo a specific reaction n Activated complex - a short-lived molecule formed when reactants collide; it can return to reactants or form products. ¨ Formation depends on the activation energy & the correct geometry (orientation)

Potential Energy Diagrams

Potential Energy Diagrams

Potential Energy Diagrams

Catalyzed Pathway Catalysts lower activation energy!!!

Reaction Mechanisms Mechanism – how reactants are converted to products at the molecular level Most reactions DO NOT occur in a single step! They occur as a series of elementary steps (a single step in a reaction).

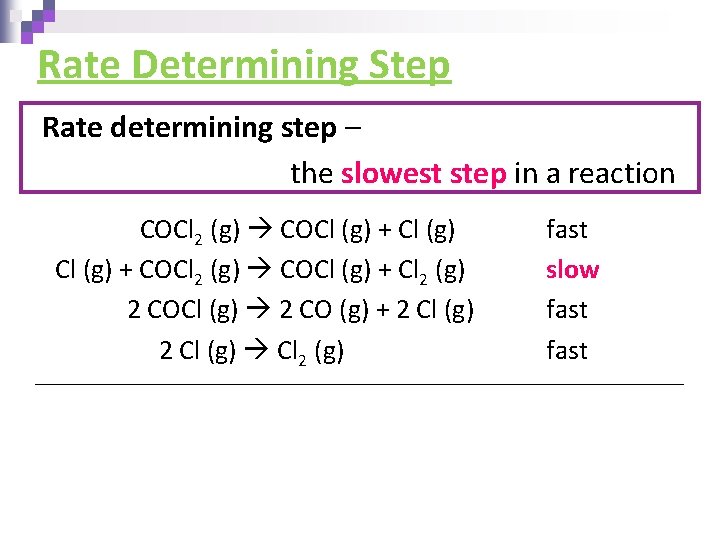
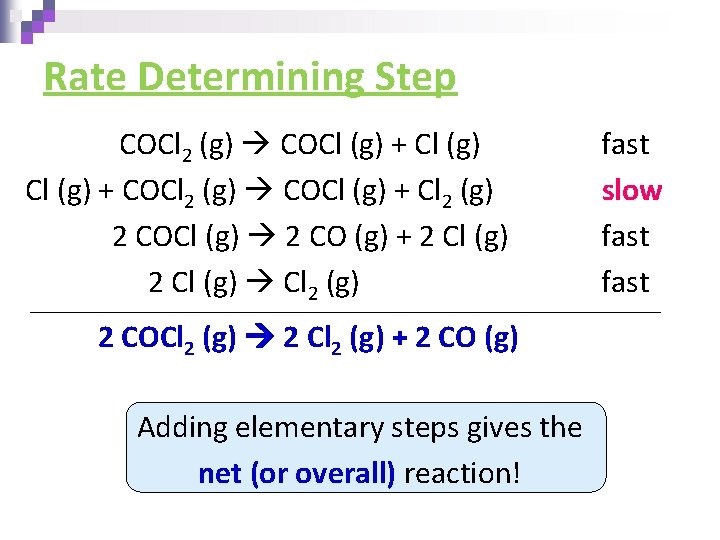
Rate Determining Step Rate determining step – the slowest step in a reaction COCl 2 (g) COCl (g) + COCl 2 (g) COCl (g) + Cl 2 (g) 2 COCl (g) 2 CO (g) + 2 Cl (g) Cl 2 (g) fast slow fast

Rate Determining Step COCl 2 (g) COCl (g) + COCl 2 (g) COCl (g) + Cl 2 (g) 2 COCl (g) 2 CO (g) + 2 Cl (g) Cl 2 (g) 2 COCl 2 (g) 2 Cl 2 (g) + 2 CO (g) Adding elementary steps gives the net (or overall) reaction! fast slow fast

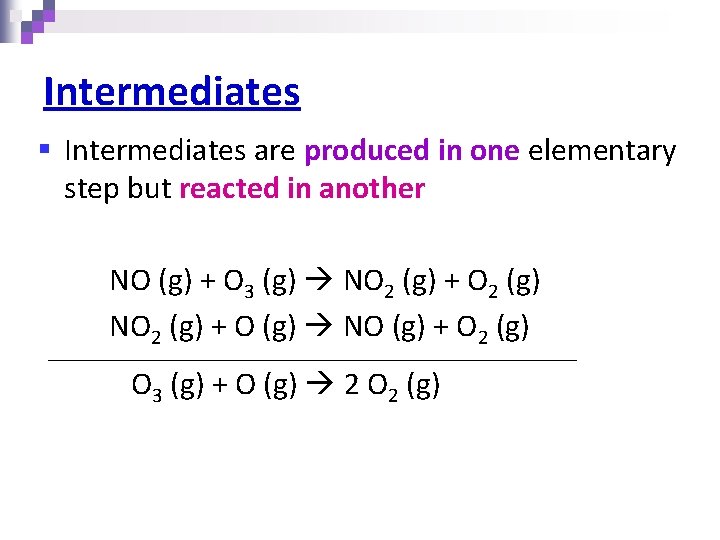
Intermediates § Intermediates are produced in one elementary step but reacted in another NO (g) + O 3 (g) NO 2 (g) + O 2 (g) NO 2 (g) + O (g) NO (g) + O 2 (g) O 3 (g) + O (g) 2 O 2 (g)

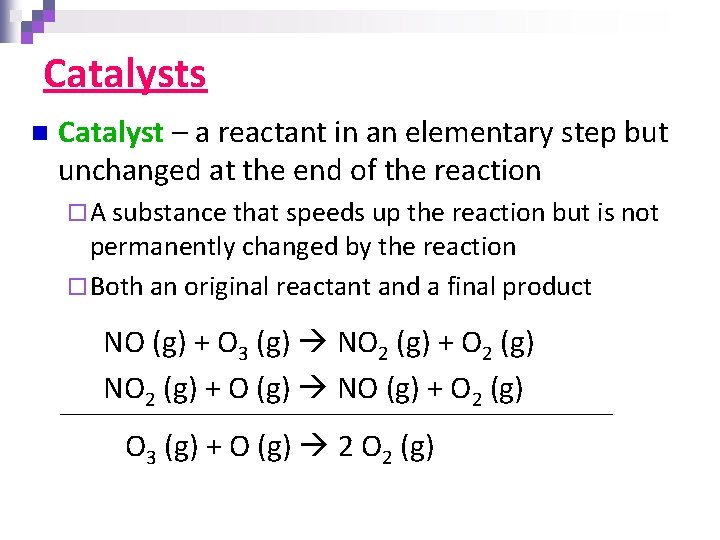
Catalysts n Catalyst – a reactant in an elementary step but unchanged at the end of the reaction ¨ A substance that speeds up the reaction but is not permanently changed by the reaction ¨ Both an original reactant and a final product NO (g) + O 3 (g) NO 2 (g) + O 2 (g) NO 2 (g) + O (g) NO (g) + O 2 (g) O 3 (g) + O (g) 2 O 2 (g)

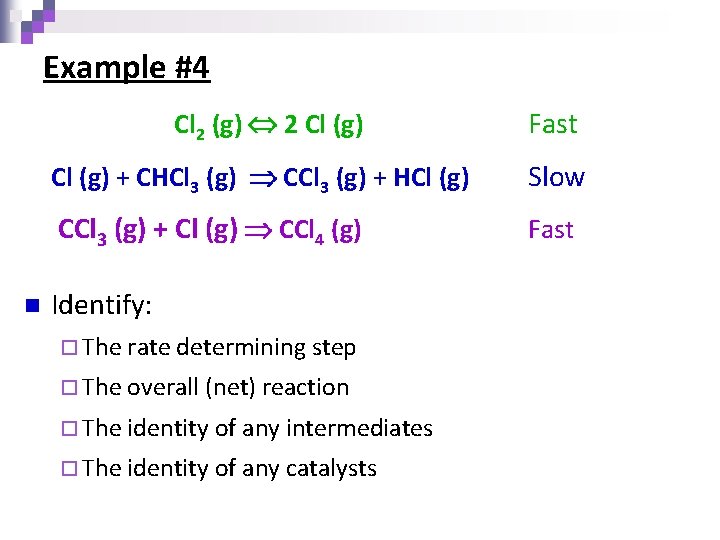
Example #4 Cl 2 (g) 2 Cl (g) n Fast Cl (g) + CHCl 3 (g) CCl 3 (g) + HCl (g) Slow CCl 3 (g) + Cl (g) CCl 4 (g) Fast Identify: ¨ The rate determining step ¨ The overall (net) reaction ¨ The identity of any intermediates ¨ The identity of any catalysts

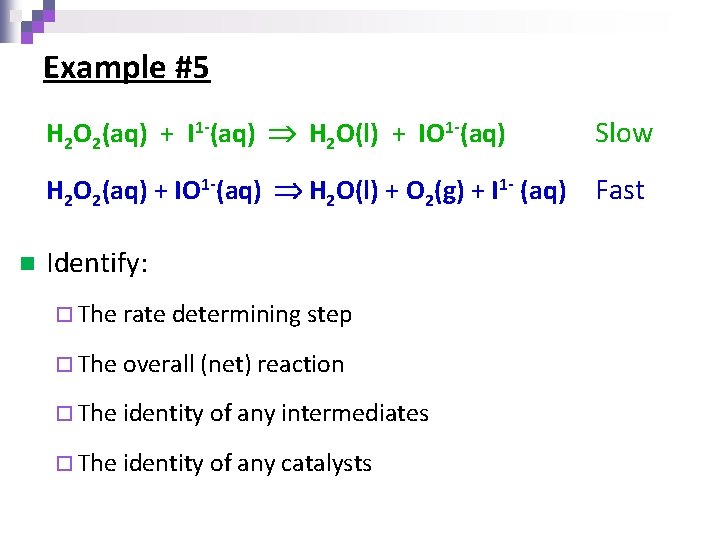
Example #5 n H 2 O 2(aq) + I 1 -(aq) H 2 O(l) + IO 1 -(aq) Slow H 2 O 2(aq) + IO 1 -(aq) H 2 O(l) + O 2(g) + I 1 - (aq) Fast Identify: ¨ The rate determining step ¨ The overall (net) reaction ¨ The identity of any intermediates ¨ The identity of any catalysts

Example #6 n O 3 (g) + Cl (g) O 2 (g) + Cl. O (g) Slow Cl. O (g) + O (g) Cl (g) + O 2 (g) Fast Identify: ¨ The rate determining step ¨ The overall (net) reaction ¨ The identity of any intermediates ¨ The identity of any catalysts
 Chemistry grade 11 unit 4 chemical kinetics
Chemistry grade 11 unit 4 chemical kinetics Honors biology unit 4 test
Honors biology unit 4 test Chemistry unit 4 review answers
Chemistry unit 4 review answers English 2 honors vocabulary unit 1
English 2 honors vocabulary unit 1 Definition of chemical kinetics in chemistry
Definition of chemical kinetics in chemistry Half life kinetics
Half life kinetics Chemical kinetics definition
Chemical kinetics definition Chemical kinetics experiment
Chemical kinetics experiment Applications of chemical kinetics
Applications of chemical kinetics Steady state approximation examples
Steady state approximation examples Creighton honors program
Creighton honors program Aces james scholar
Aces james scholar Deped order no. 36, s. 2016
Deped order no. 36, s. 2016 Duels ucsb
Duels ucsb Honors physics semester 1 review
Honors physics semester 1 review Honors biology ecology test
Honors biology ecology test Uncle sam's toolbox honors
Uncle sam's toolbox honors Quadrilateral test review
Quadrilateral test review Honors earth science
Honors earth science Kuei honors chemistry
Kuei honors chemistry Mrs blueprint
Mrs blueprint Hilton honors military program
Hilton honors military program Honors project
Honors project Honors biology properties of water lab
Honors biology properties of water lab Honors geometry parallel lines and transversals worksheet
Honors geometry parallel lines and transversals worksheet Tulane honors program
Tulane honors program Second quarter exam
Second quarter exam Honors precalculus chapter 1 test
Honors precalculus chapter 1 test Vyi physics
Vyi physics Smc scholars classes
Smc scholars classes Math 3 honors
Math 3 honors Crystalline solid and amorphous solid
Crystalline solid and amorphous solid Honors chemistry summer assignment
Honors chemistry summer assignment Stlucieschools skyward
Stlucieschools skyward Mahurin honors college
Mahurin honors college Bond order formula
Bond order formula Honors algebra 2 final exam
Honors algebra 2 final exam Umes honors program
Umes honors program