Chemical Kinetics Chemical Kinetics Thermodynamics does a reaction

![A B time D[A] rate = Dt D[B] rate = Dt 13. 1 A B time D[A] rate = Dt D[B] rate = Dt 13. 1](https://slidetodoc.com/presentation_image_h/03b58aa0f40d9b7a1c4539d116b8095d/image-3.jpg)
![rate a [Br 2] rate = k [Br 2] rate = rate constant k= rate a [Br 2] rate = k [Br 2] rate = rate constant k=](https://slidetodoc.com/presentation_image_h/03b58aa0f40d9b7a1c4539d116b8095d/image-5.jpg)
![Run # Initial [A] ([A]0) Initial [B] ([B]0) Initial Rate (v 0) 1 1. Run # Initial [A] ([A]0) Initial [B] ([B]0) Initial Rate (v 0) 1 1.](https://slidetodoc.com/presentation_image_h/03b58aa0f40d9b7a1c4539d116b8095d/image-9.jpg)
![[NO(g) ] (mol dm-3) [Cl 2(g) ] (mol dm-3) Initial Rate (mol dm-3 s-1) [NO(g) ] (mol dm-3) [Cl 2(g) ] (mol dm-3) Initial Rate (mol dm-3 s-1)](https://slidetodoc.com/presentation_image_h/03b58aa0f40d9b7a1c4539d116b8095d/image-10.jpg)
![First-Order Reactions D[A] rate = Dt ln[A] - ln[A]0 = - kt rate = First-Order Reactions D[A] rate = Dt ln[A] - ln[A]0 = - kt rate =](https://slidetodoc.com/presentation_image_h/03b58aa0f40d9b7a1c4539d116b8095d/image-13.jpg)
![First-order reaction A product # of half-lives 1 [A] = [A]0/n 2 4 3 First-order reaction A product # of half-lives 1 [A] = [A]0/n 2 4 3](https://slidetodoc.com/presentation_image_h/03b58aa0f40d9b7a1c4539d116b8095d/image-17.jpg)
![Second-Order Reactions D[A] rate = Dt 1 1 = kt [A]0 [A] rate = Second-Order Reactions D[A] rate = Dt 1 1 = kt [A]0 [A] rate =](https://slidetodoc.com/presentation_image_h/03b58aa0f40d9b7a1c4539d116b8095d/image-19.jpg)
![Zero-Order Reactions D[A] rate = Dt [A] - [A]0 = kt rate = k Zero-Order Reactions D[A] rate = Dt [A] - [A]0 = kt rate = k](https://slidetodoc.com/presentation_image_h/03b58aa0f40d9b7a1c4539d116b8095d/image-20.jpg)
![Rate Laws and Elementary Steps Unimolecular reaction A products rate = k [A] Bimolecular Rate Laws and Elementary Steps Unimolecular reaction A products rate = k [A] Bimolecular](https://slidetodoc.com/presentation_image_h/03b58aa0f40d9b7a1c4539d116b8095d/image-27.jpg)
![Write the rate law for this reaction. Rate = k [HBr] [O 2] List Write the rate law for this reaction. Rate = k [HBr] [O 2] List](https://slidetodoc.com/presentation_image_h/03b58aa0f40d9b7a1c4539d116b8095d/image-31.jpg)
- Slides: 34

Chemical Kinetics

Chemical Kinetics Thermodynamics – does a reaction take place? Kinetics – how fast does a reaction proceed? Reaction rate is the change in the concentration of a reactant or a product with time (M/s). A B D[A] rate = Dt D[A] = change in concentration of A over time period Dt D[B] rate = Dt D[B] = change in concentration of B over time period Dt Because [A] decreases with time, D[A] is negative. 13. 1
![A B time DA rate Dt DB rate Dt 13 1 A B time D[A] rate = Dt D[B] rate = Dt 13. 1](https://slidetodoc.com/presentation_image_h/03b58aa0f40d9b7a1c4539d116b8095d/image-3.jpg)
A B time D[A] rate = Dt D[B] rate = Dt 13. 1

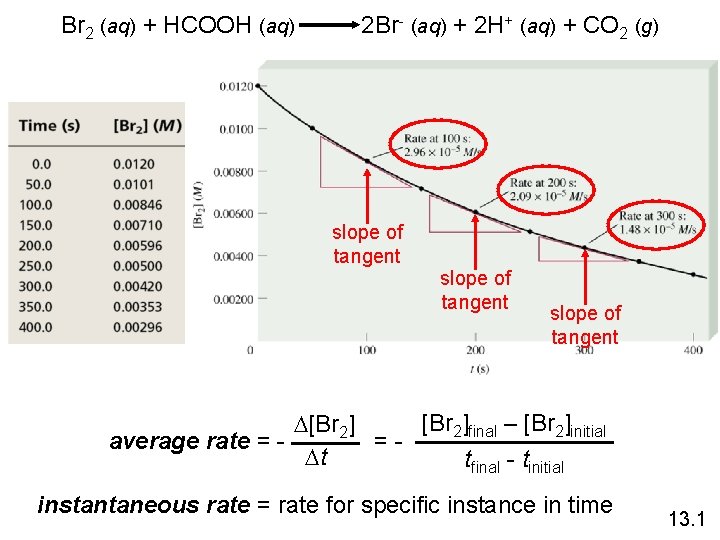
Br 2 (aq) + HCOOH (aq) 2 Br- (aq) + 2 H+ (aq) + CO 2 (g) slope of tangent [Br 2]final – [Br 2]initial D[Br 2] average rate = =Dt tfinal - tinitial instantaneous rate = rate for specific instance in time 13. 1
![rate a Br 2 rate k Br 2 rate rate constant k rate a [Br 2] rate = k [Br 2] rate = rate constant k=](https://slidetodoc.com/presentation_image_h/03b58aa0f40d9b7a1c4539d116b8095d/image-5.jpg)
rate a [Br 2] rate = k [Br 2] rate = rate constant k= [Br 2] = 3. 50 x 10 -3 s-1 13. 1

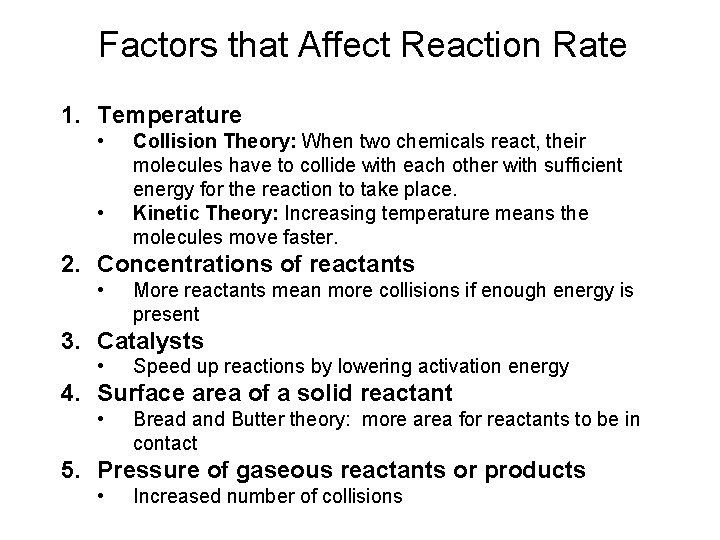
Factors that Affect Reaction Rate 1. Temperature • • Collision Theory: When two chemicals react, their molecules have to collide with each other with sufficient energy for the reaction to take place. Kinetic Theory: Increasing temperature means the molecules move faster. 2. Concentrations of reactants • More reactants mean more collisions if enough energy is present 3. Catalysts • Speed up reactions by lowering activation energy 4. Surface area of a solid reactant • Bread and Butter theory: more area for reactants to be in contact 5. Pressure of gaseous reactants or products • Increased number of collisions

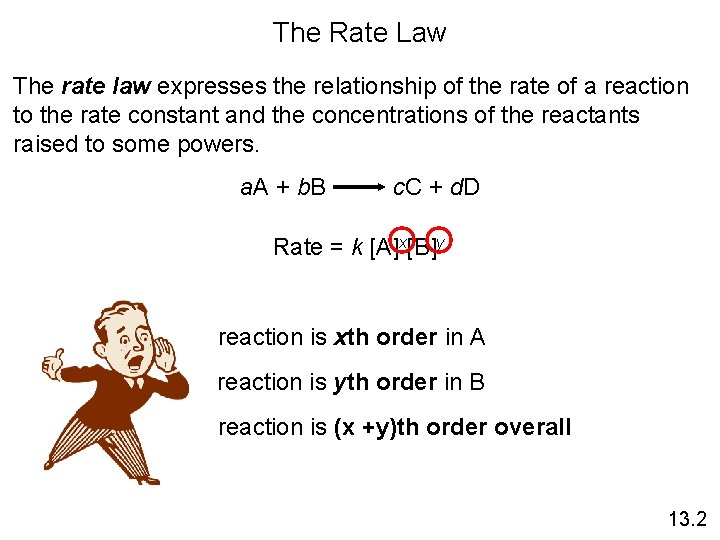
The Rate Law The rate law expresses the relationship of the rate of a reaction to the rate constant and the concentrations of the reactants raised to some powers. a. A + b. B c. C + d. D Rate = k [A]x[B]y reaction is xth order in A reaction is yth order in B reaction is (x +y)th order overall 13. 2

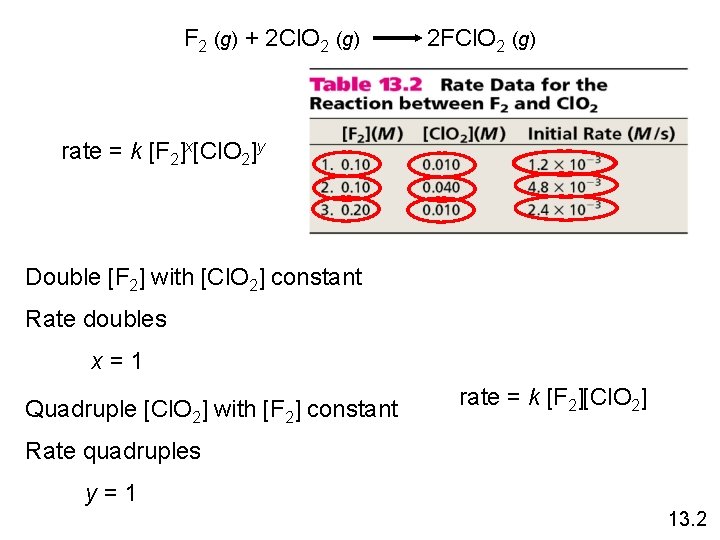
F 2 (g) + 2 Cl. O 2 (g) 2 FCl. O 2 (g) rate = k [F 2]x[Cl. O 2]y Double [F 2] with [Cl. O 2] constant Rate doubles x=1 Quadruple [Cl. O 2] with [F 2] constant rate = k [F 2][Cl. O 2] Rate quadruples y=1 13. 2
![Run Initial A A0 Initial B B0 Initial Rate v 0 1 1 Run # Initial [A] ([A]0) Initial [B] ([B]0) Initial Rate (v 0) 1 1.](https://slidetodoc.com/presentation_image_h/03b58aa0f40d9b7a1c4539d116b8095d/image-9.jpg)
Run # Initial [A] ([A]0) Initial [B] ([B]0) Initial Rate (v 0) 1 1. 00 M 1. 25 x 10 -2 M/s 2 1. 00 M 2. 5 x 10 -2 M/s 3 2. 00 M 2. 5 x 10 -2 M/s What is the order with respect to A? 0 What is the order with respect to B? 1 What is the overall order of the reaction? 1
![NOg mol dm3 Cl 2g mol dm3 Initial Rate mol dm3 s1 [NO(g) ] (mol dm-3) [Cl 2(g) ] (mol dm-3) Initial Rate (mol dm-3 s-1)](https://slidetodoc.com/presentation_image_h/03b58aa0f40d9b7a1c4539d116b8095d/image-10.jpg)
[NO(g) ] (mol dm-3) [Cl 2(g) ] (mol dm-3) Initial Rate (mol dm-3 s-1) 0. 250 1. 43 x 10 -6 0. 250 0. 500 2. 86 x 10 -6 0. 500 1. 14 x 10 -5 What is the order with respect to Cl 2? 1 What is the order with respect to NO? 2 What is the overall order of the reaction? 3

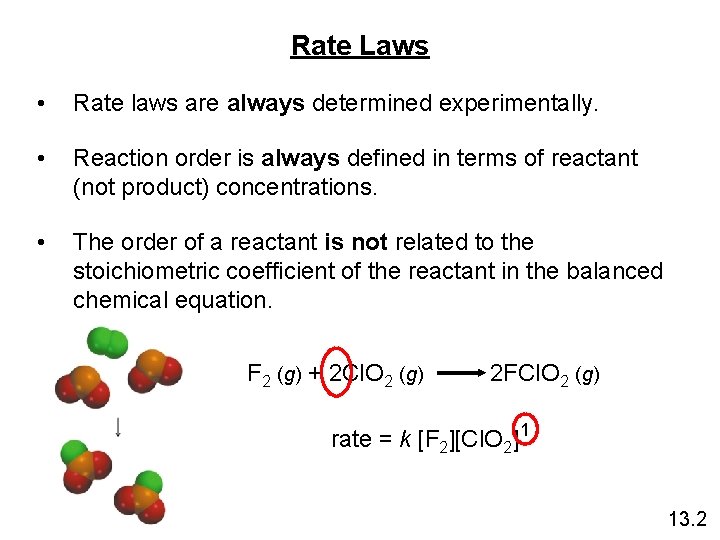
Rate Laws • Rate laws are always determined experimentally. • Reaction order is always defined in terms of reactant (not product) concentrations. • The order of a reactant is not related to the stoichiometric coefficient of the reactant in the balanced chemical equation. F 2 (g) + 2 Cl. O 2 (g) 2 FCl. O 2 (g) rate = k [F 2][Cl. O 2] 1 13. 2

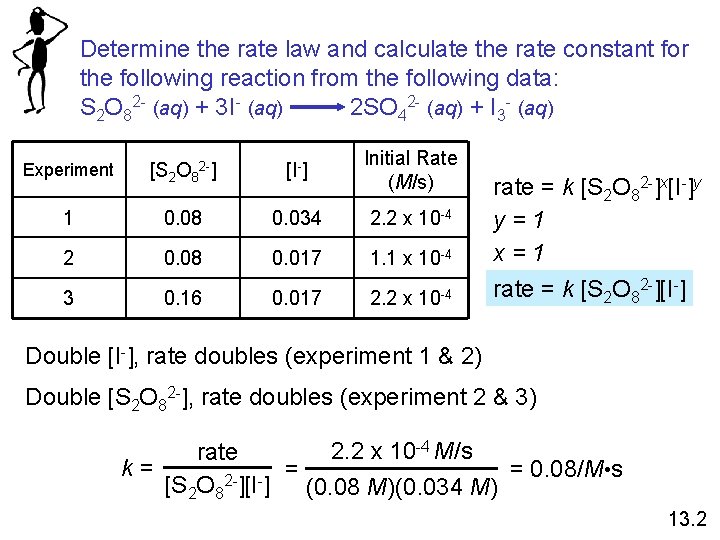
Determine the rate law and calculate the rate constant for the following reaction from the following data: S 2 O 82 - (aq) + 3 I- (aq) 2 SO 42 - (aq) + I 3 - (aq) Experiment [S 2 O 82 -] [I-] Initial Rate (M/s) 1 0. 08 0. 034 2. 2 x 10 -4 2 0. 08 0. 017 1. 1 x 10 -4 3 0. 16 0. 017 2. 2 x 10 -4 rate = k [S 2 O 82 -]x[I-]y y=1 x=1 rate = k [S 2 O 82 -][I-] Double [I-], rate doubles (experiment 1 & 2) Double [S 2 O 82 -], rate doubles (experiment 2 & 3) 2. 2 x 10 -4 M/s rate k= = = 0. 08/M • s 2[S 2 O 8 ][I ] (0. 08 M)(0. 034 M) 13. 2
![FirstOrder Reactions DA rate Dt lnA lnA0 kt rate First-Order Reactions D[A] rate = Dt ln[A] - ln[A]0 = - kt rate =](https://slidetodoc.com/presentation_image_h/03b58aa0f40d9b7a1c4539d116b8095d/image-13.jpg)
First-Order Reactions D[A] rate = Dt ln[A] - ln[A]0 = - kt rate = k [A] = [A]0 e-kt [A] is the concentration of A at any time t [A]0 is the concentration of A at time t=0 13. 3

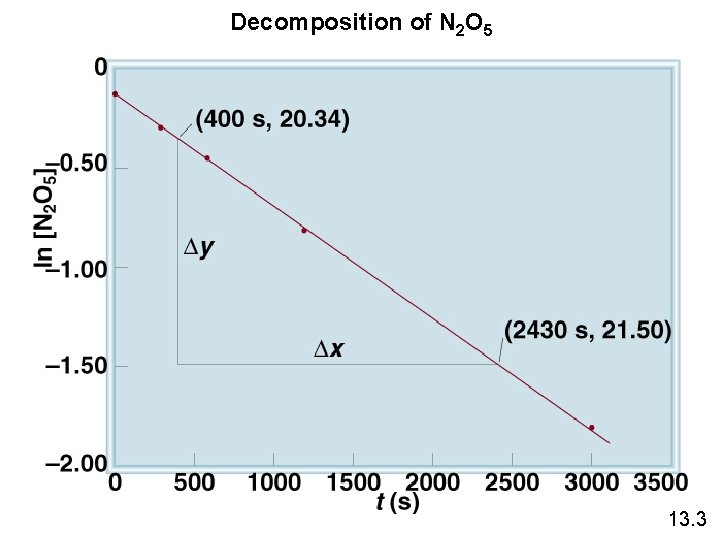
Decomposition of N 2 O 5 13. 3

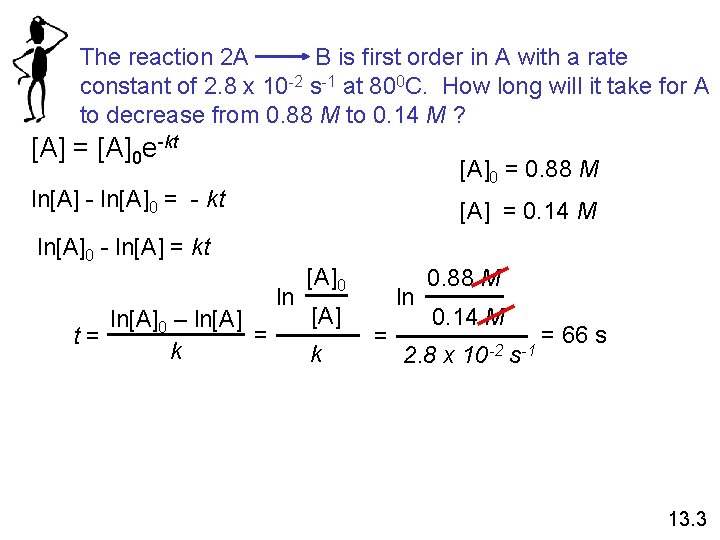
The reaction 2 A B is first order in A with a rate constant of 2. 8 x 10 -2 s-1 at 800 C. How long will it take for A to decrease from 0. 88 M to 0. 14 M ? [A] = [A]0 e-kt [A]0 = 0. 88 M ln[A] - ln[A]0 = - kt [A] = 0. 14 M ln[A]0 - ln[A] = kt ln[A]0 – ln[A] = t= k ln [A]0 [A] k ln = 0. 88 M 0. 14 M 2. 8 x 10 -2 s-1 = 66 s 13. 3

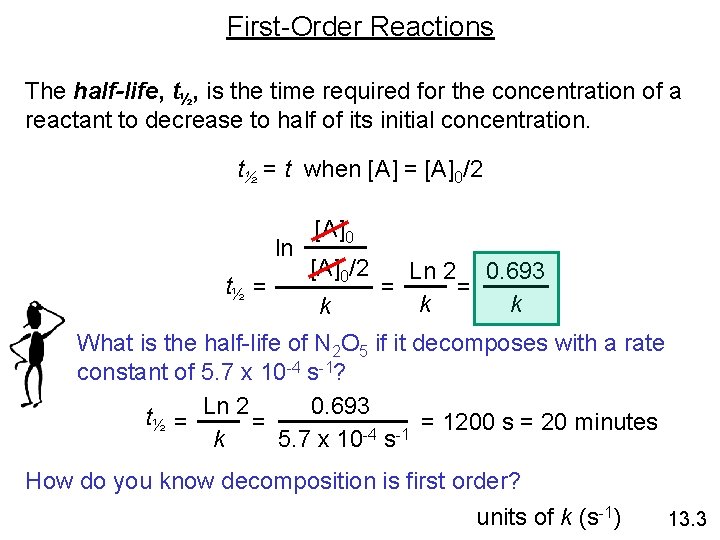
First-Order Reactions The half-life, t½, is the time required for the concentration of a reactant to decrease to half of its initial concentration. t½ = t when [A] = [A]0/2 ln t½ = [A]0/2 k Ln 2 0. 693 = = k k What is the half-life of N 2 O 5 if it decomposes with a rate constant of 5. 7 x 10 -4 s-1? 0. 693 t½ = Ln 2 = = 1200 s = 20 minutes -4 -1 k 5. 7 x 10 s How do you know decomposition is first order? units of k (s-1) 13. 3
![Firstorder reaction A product of halflives 1 A A0n 2 4 3 First-order reaction A product # of half-lives 1 [A] = [A]0/n 2 4 3](https://slidetodoc.com/presentation_image_h/03b58aa0f40d9b7a1c4539d116b8095d/image-17.jpg)
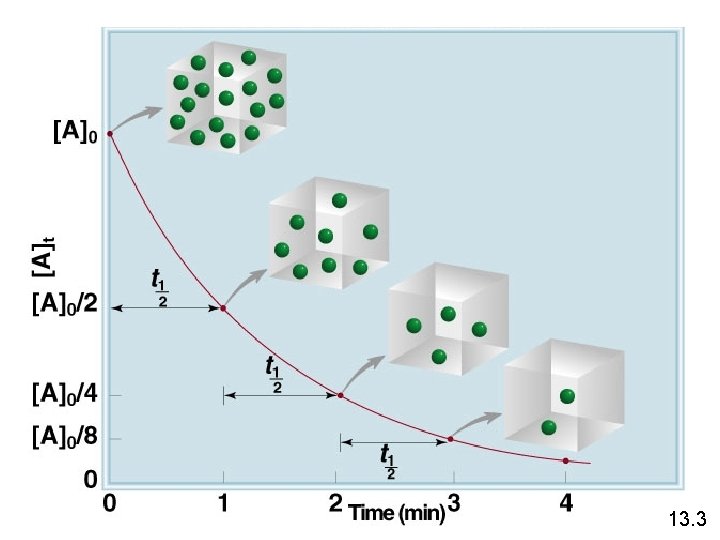
First-order reaction A product # of half-lives 1 [A] = [A]0/n 2 4 3 8 4 16 2 13. 3

13. 3
![SecondOrder Reactions DA rate Dt 1 1 kt A0 A rate Second-Order Reactions D[A] rate = Dt 1 1 = kt [A]0 [A] rate =](https://slidetodoc.com/presentation_image_h/03b58aa0f40d9b7a1c4539d116b8095d/image-19.jpg)
Second-Order Reactions D[A] rate = Dt 1 1 = kt [A]0 [A] rate = k [A]2 [A] is the concentration of A at any time t [A]0 is the concentration of A at time t=0 Half life for second order t½ = t when [A] = [A]0/2 1 t½ = k[A]0 13. 3
![ZeroOrder Reactions DA rate Dt A A0 kt rate k Zero-Order Reactions D[A] rate = Dt [A] - [A]0 = kt rate = k](https://slidetodoc.com/presentation_image_h/03b58aa0f40d9b7a1c4539d116b8095d/image-20.jpg)
Zero-Order Reactions D[A] rate = Dt [A] - [A]0 = kt rate = k [A]0 = k [A] is the concentration of A at any time t [A]0 is the concentration of A at time t=0 Half life for zero order t½ = t when [A] = [A]0/2 [A]0 t½ = 2 k 13. 3

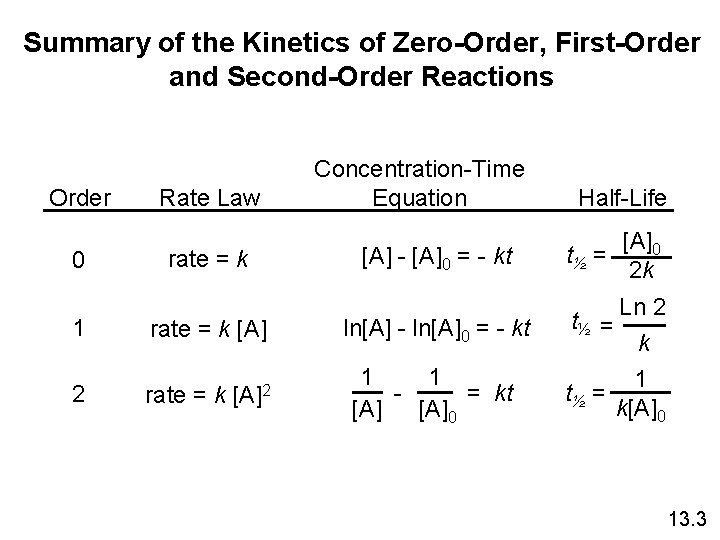
Summary of the Kinetics of Zero-Order, First-Order and Second-Order Reactions Order 0 Rate Law rate = k 1 rate = k [A] 2 [A]2 rate = k Concentration-Time Equation [A] - [A]0 = - kt ln[A] - ln[A]0 = - kt 1 1 = kt [A]0 Half-Life t½ = [A]0 2 k Ln 2 k 1 t½ = k[A]0 13. 3

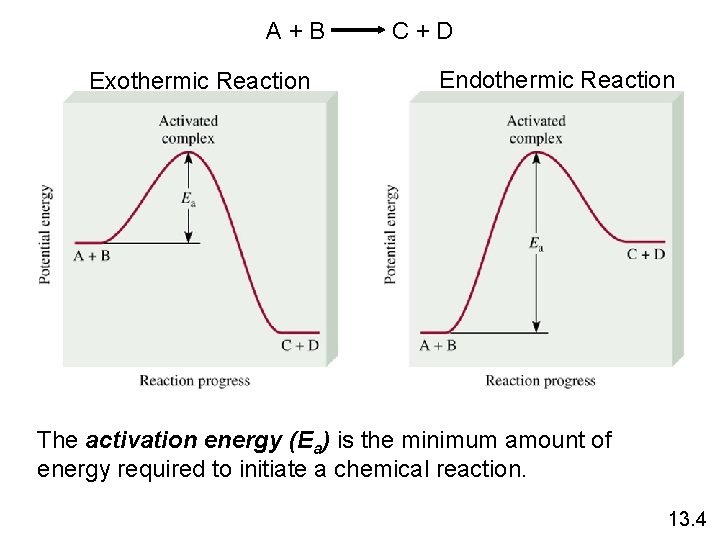
A+B Exothermic Reaction C+D Endothermic Reaction The activation energy (Ea) is the minimum amount of energy required to initiate a chemical reaction. 13. 4

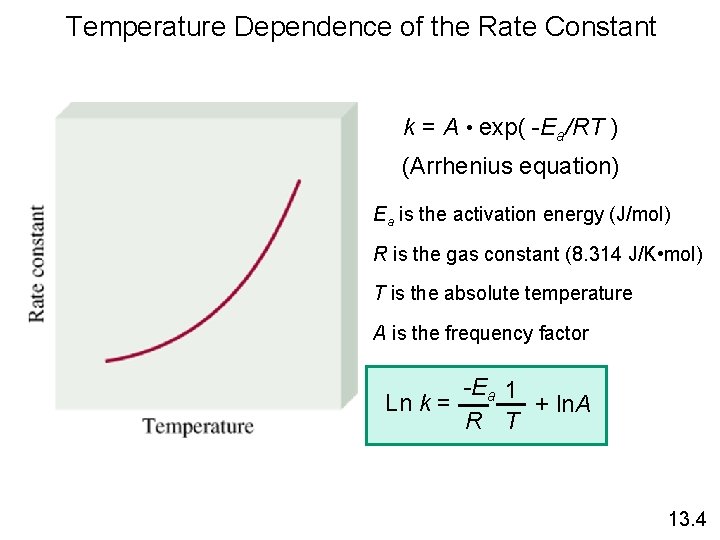
Temperature Dependence of the Rate Constant k = A • exp( -Ea/RT ) (Arrhenius equation) Ea is the activation energy (J/mol) R is the gas constant (8. 314 J/K • mol) T is the absolute temperature A is the frequency factor -Ea 1 Ln k = + ln. A R T 13. 4

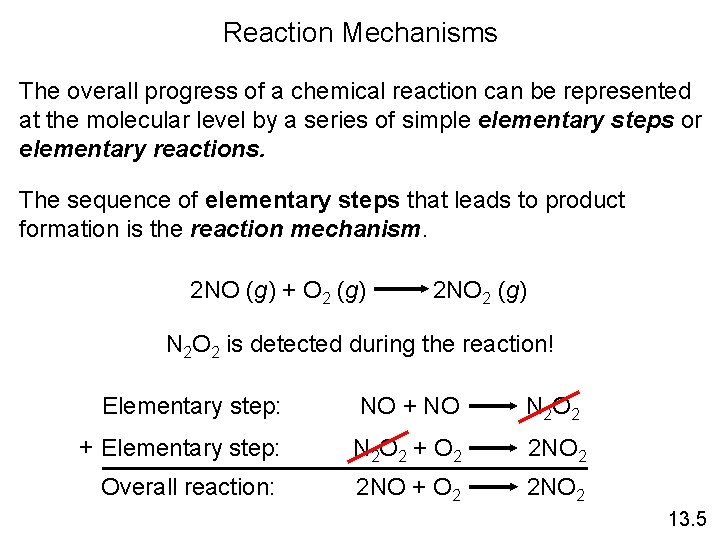
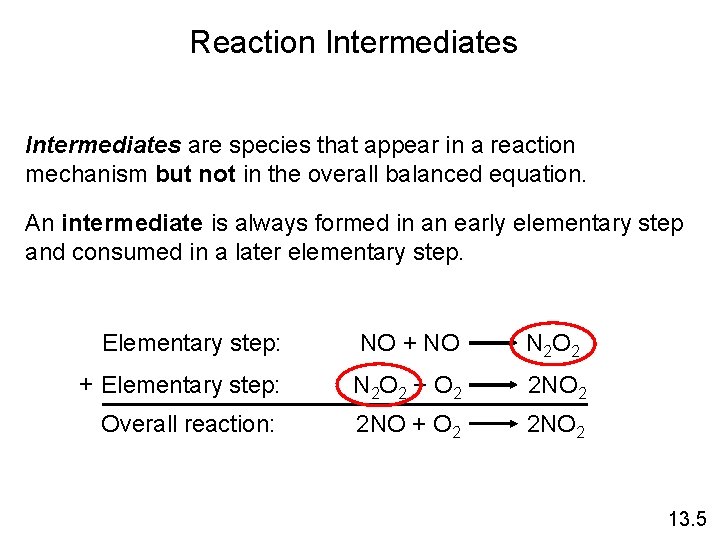
Reaction Mechanisms The overall progress of a chemical reaction can be represented at the molecular level by a series of simple elementary steps or elementary reactions. The sequence of elementary steps that leads to product formation is the reaction mechanism. 2 NO (g) + O 2 (g) 2 NO 2 (g) N 2 O 2 is detected during the reaction! Elementary step: NO + NO N 2 O 2 + Elementary step: N 2 O 2 + O 2 2 NO 2 Overall reaction: 2 NO + O 2 2 NO 2 13. 5

Reaction Intermediates are species that appear in a reaction mechanism but not in the overall balanced equation. An intermediate is always formed in an early elementary step and consumed in a later elementary step. Elementary step: NO + NO N 2 O 2 + Elementary step: N 2 O 2 + O 2 2 NO 2 Overall reaction: 2 NO + O 2 2 NO 2 13. 5

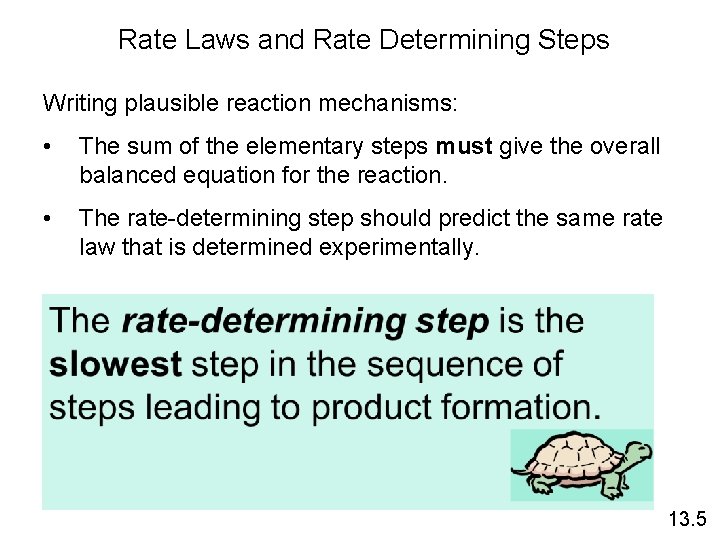
Rate Laws and Rate Determining Steps Writing plausible reaction mechanisms: • The sum of the elementary steps must give the overall balanced equation for the reaction. • The rate-determining step should predict the same rate law that is determined experimentally. 13. 5
![Rate Laws and Elementary Steps Unimolecular reaction A products rate k A Bimolecular Rate Laws and Elementary Steps Unimolecular reaction A products rate = k [A] Bimolecular](https://slidetodoc.com/presentation_image_h/03b58aa0f40d9b7a1c4539d116b8095d/image-27.jpg)
Rate Laws and Elementary Steps Unimolecular reaction A products rate = k [A] Bimolecular reaction A+B products rate = k [A][B] Bimolecular reaction A+A products rate = k [A]2 13. 5

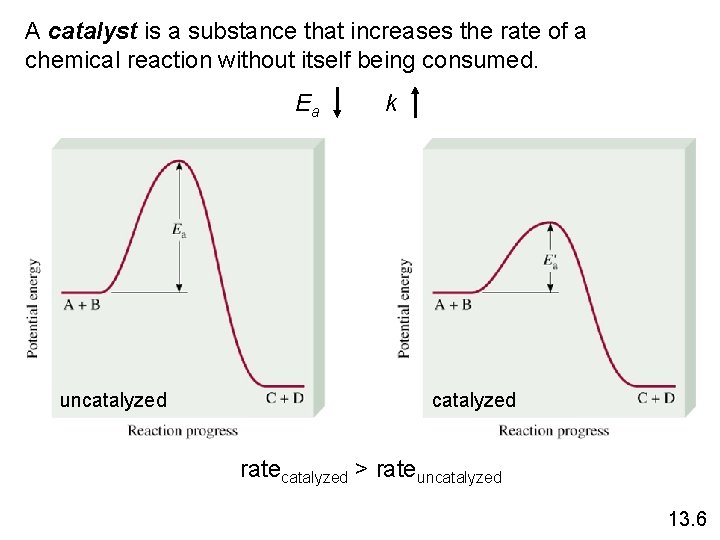
A catalyst is a substance that increases the rate of a chemical reaction without itself being consumed. Ea uncatalyzed k catalyzed ratecatalyzed > rateuncatalyzed 13. 6

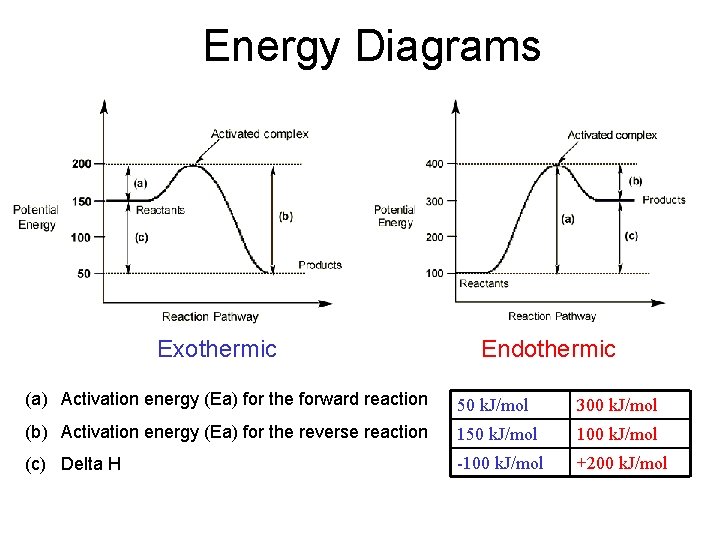
Energy Diagrams Exothermic Endothermic (a) Activation energy (Ea) for the forward reaction 50 k. J/mol 300 k. J/mol (b) Activation energy (Ea) for the reverse reaction 150 k. J/mol 100 k. J/mol (c) Delta H -100 k. J/mol +200 k. J/mol

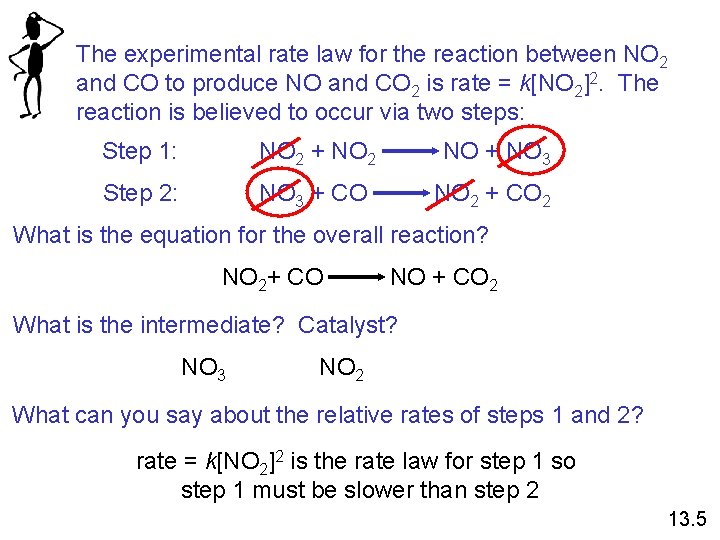
The experimental rate law for the reaction between NO 2 and CO to produce NO and CO 2 is rate = k[NO 2]2. The reaction is believed to occur via two steps: Step 1: NO 2 + NO 2 NO + NO 3 Step 2: NO 3 + CO NO 2 + CO 2 What is the equation for the overall reaction? NO 2+ CO NO + CO 2 What is the intermediate? Catalyst? NO 3 NO 2 What can you say about the relative rates of steps 1 and 2? rate = k[NO 2]2 is the rate law for step 1 so step 1 must be slower than step 2 13. 5
![Write the rate law for this reaction Rate k HBr O 2 List Write the rate law for this reaction. Rate = k [HBr] [O 2] List](https://slidetodoc.com/presentation_image_h/03b58aa0f40d9b7a1c4539d116b8095d/image-31.jpg)
Write the rate law for this reaction. Rate = k [HBr] [O 2] List all intermediates in this reaction. HOOBr, HOBr List all catalysts in this reaction. None

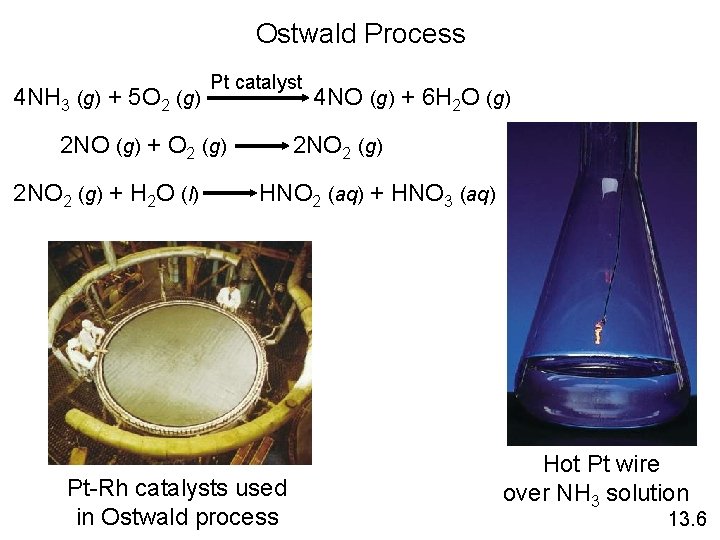
Ostwald Process 4 NH 3 (g) + 5 O 2 (g) Pt catalyst 2 NO (g) + O 2 (g) 2 NO 2 (g) + H 2 O (l) 4 NO (g) + 6 H 2 O (g) 2 NO 2 (g) HNO 2 (aq) + HNO 3 (aq) Pt-Rh catalysts used in Ostwald process Hot Pt wire over NH 3 solution 13. 6

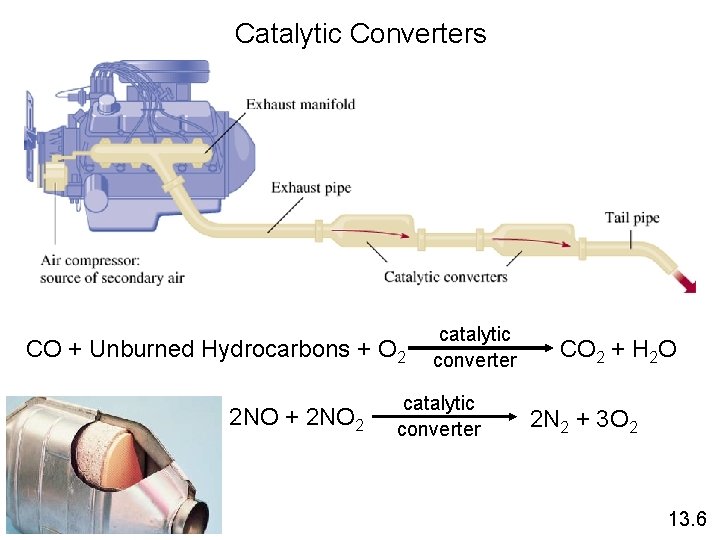
Catalytic Converters CO + Unburned Hydrocarbons + O 2 2 NO + 2 NO 2 catalytic converter CO 2 + H 2 O 2 N 2 + 3 O 2 13. 6

Enzyme Catalysis 13. 6
 Half life formula
Half life formula Kinetics reaction
Kinetics reaction Thermodynamics enthalpy of reaction and hess's law
Thermodynamics enthalpy of reaction and hess's law Definition of chemical kinetics in chemistry
Definition of chemical kinetics in chemistry Chemical reactions grade 11
Chemical reactions grade 11 Half life kinetics
Half life kinetics Chemical kinetics definition
Chemical kinetics definition Chemical kinetics experiment
Chemical kinetics experiment Applications of chemical kinetics
Applications of chemical kinetics What is steady state kinetics
What is steady state kinetics What is used up in and stops a chemical reaction?
What is used up in and stops a chemical reaction? Chemical engineering thermodynamics 8th solution chapter 6
Chemical engineering thermodynamics 8th solution chapter 6 Chemical engineering thermodynamics 8th solution chapter 3
Chemical engineering thermodynamics 8th solution chapter 3 Chemical engineering thermodynamics 8th solution chapter 4
Chemical engineering thermodynamics 8th solution chapter 4 Chemical equilibrium
Chemical equilibrium Chapter 6
Chapter 6 Chemical engineering thermodynamics 8th solution chapter 2
Chemical engineering thermodynamics 8th solution chapter 2 Chemical engineering thermodynamics 8th solution chapter 10
Chemical engineering thermodynamics 8th solution chapter 10 熱力
熱力 Rate reaction equation
Rate reaction equation Addition reaction and substitution reaction
Addition reaction and substitution reaction Leukoerythroblastic reaction vs leukemoid reaction
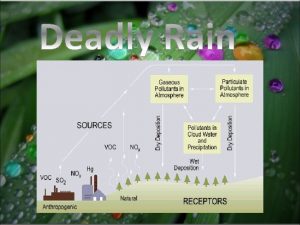
Leukoerythroblastic reaction vs leukemoid reaction Conclusion of acid rain
Conclusion of acid rain Word equation examples
Word equation examples Tyoes of chemical reactions
Tyoes of chemical reactions What is the reaction at the anode in a breathalyzer?
What is the reaction at the anode in a breathalyzer? In a chemical reaction stoichiometry refers to
In a chemical reaction stoichiometry refers to Stability measurement instruments
Stability measurement instruments Which chemical reaction switches 2 elements
Which chemical reaction switches 2 elements Percent yield chemistry definition
Percent yield chemistry definition Why is pyrrole aromatic
Why is pyrrole aromatic Rancidity chemical reaction
Rancidity chemical reaction Factors affecting the rate of chemical reaction temperature
Factors affecting the rate of chemical reaction temperature What is a combustion reaction example
What is a combustion reaction example Incomplete combustion of benzene
Incomplete combustion of benzene