Effects of chemical reactions Chemical reactions rearrange atoms

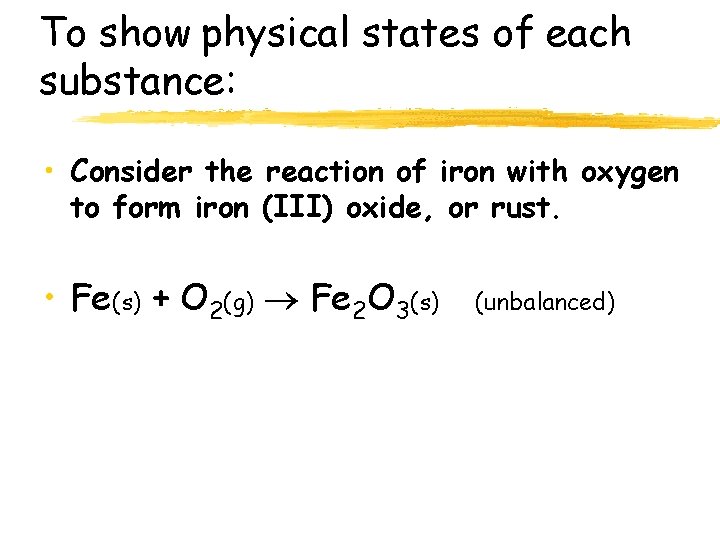





- Slides: 34


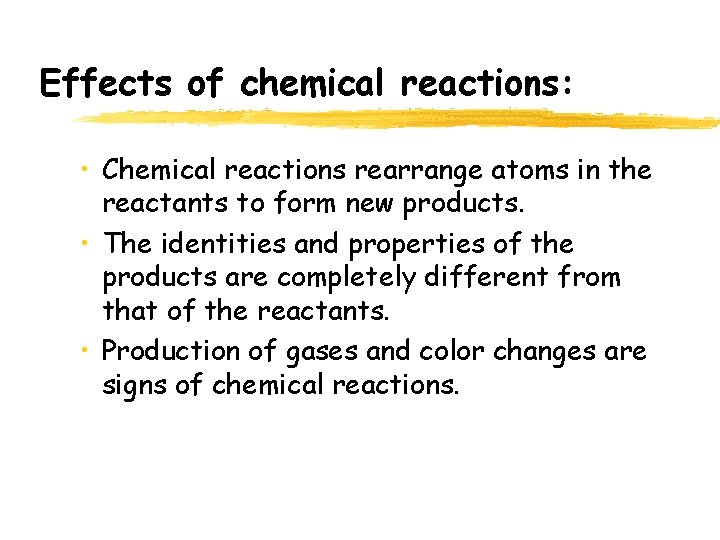
Effects of chemical reactions: • Chemical reactions rearrange atoms in the reactants to form new products. • The identities and properties of the products are completely different from that of the reactants. • Production of gases and color changes are signs of chemical reactions.

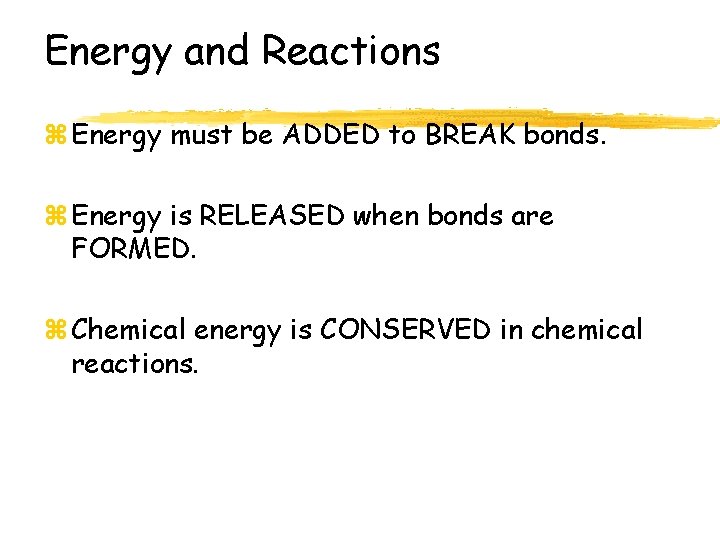
Energy and Reactions z Energy must be ADDED to BREAK bonds. z Energy is RELEASED when bonds are FORMED. z Chemical energy is CONSERVED in chemical reactions.

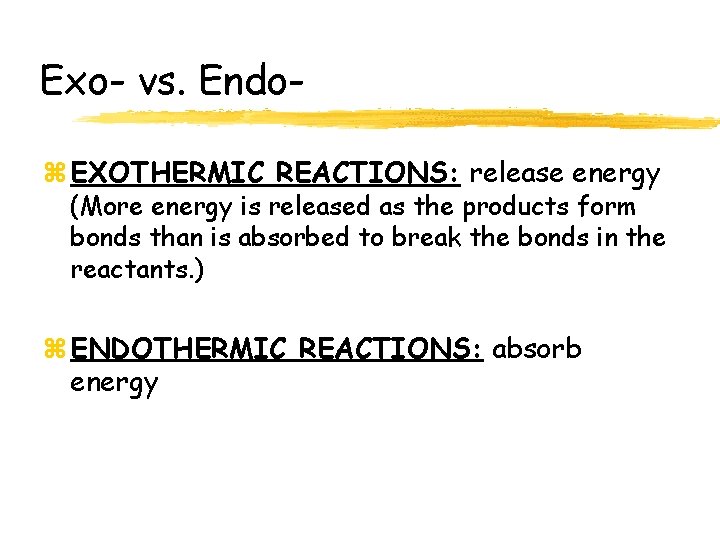
Exo- vs. Endoz EXOTHERMIC REACTIONS: release energy (More energy is released as the products form bonds than is absorbed to break the bonds in the reactants. ) z ENDOTHERMIC REACTIONS: absorb energy

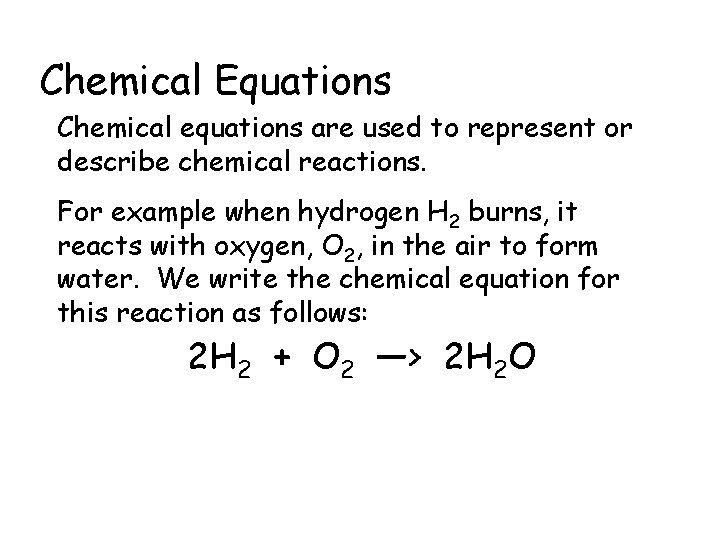
Chemical Equations Chemical equations are used to represent or describe chemical reactions. For example when hydrogen H 2 burns, it reacts with oxygen, O 2, in the air to form water. We write the chemical equation for this reaction as follows: 2 H 2 + O 2 —> 2 H 2 O

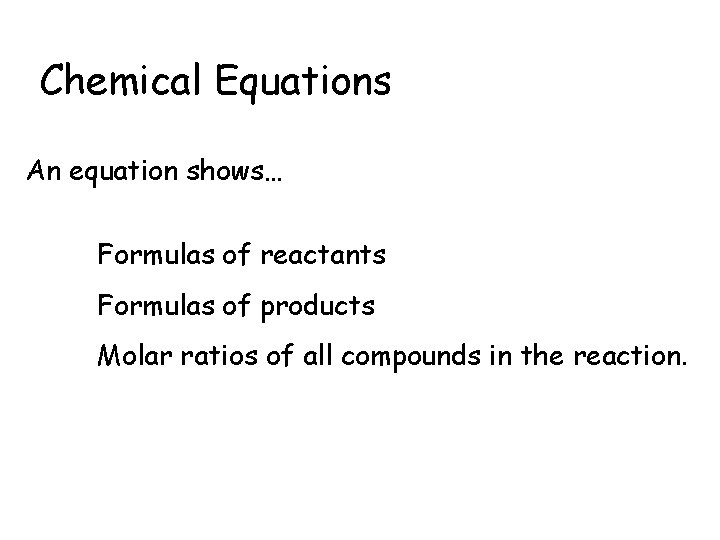
Chemical Equations An equation shows… Formulas of reactants Formulas of products Molar ratios of all compounds in the reaction.

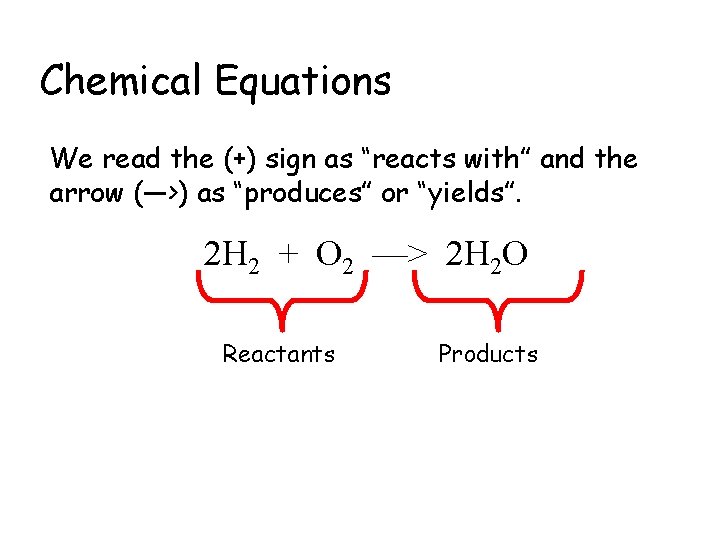
Chemical Equations We read the (+) sign as “reacts with” and the arrow (—>) as “produces” or “yields”. 2 H 2 + O 2 —> 2 H 2 O Reactants Products

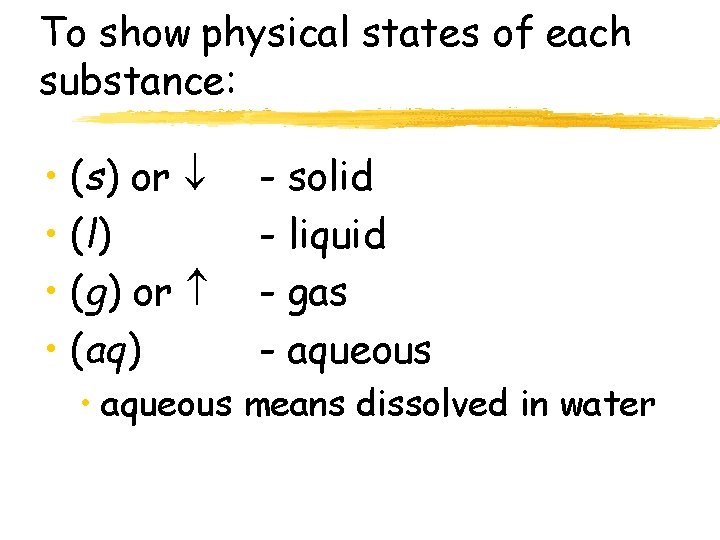
To show physical states of each substance: • (s) or • (l) • (g) or • (aq) - solid - liquid - gas - aqueous • aqueous means dissolved in water
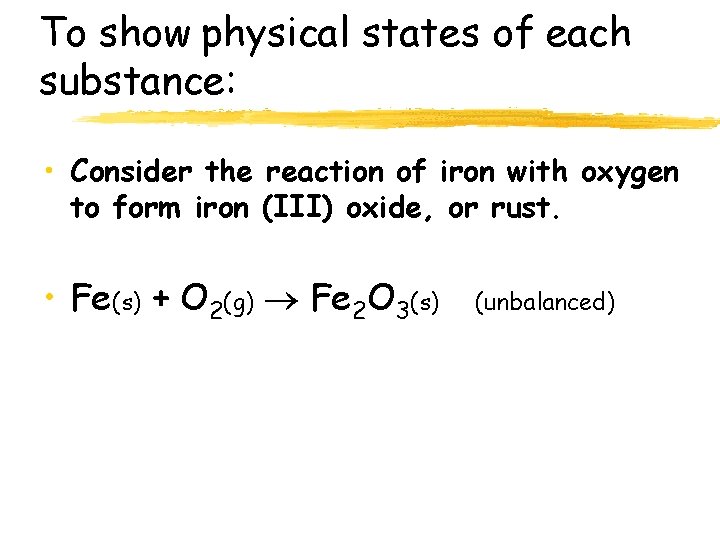
To show physical states of each substance: • Consider the reaction of iron with oxygen to form iron (III) oxide, or rust. • Fe(s) + O 2(g) Fe 2 O 3(s) (unbalanced)

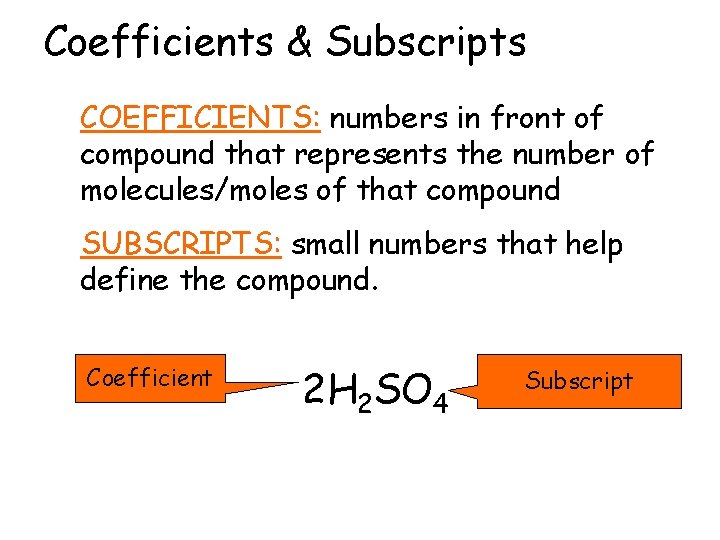
Coefficients & Subscripts COEFFICIENTS: numbers in front of compound that represents the number of molecules/moles of that compound SUBSCRIPTS: small numbers that help define the compound. Coefficient 2 H 2 SO 4 Subscript

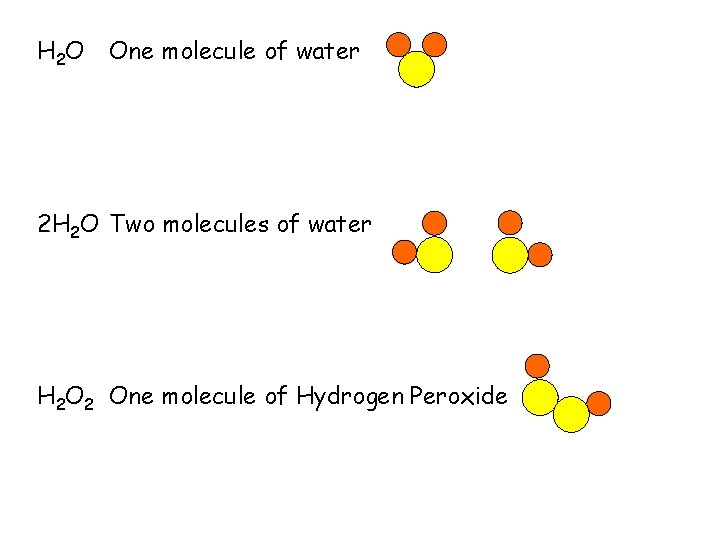
H 2 O One molecule of water 2 H 2 O Two molecules of water H 2 O 2 One molecule of Hydrogen Peroxide

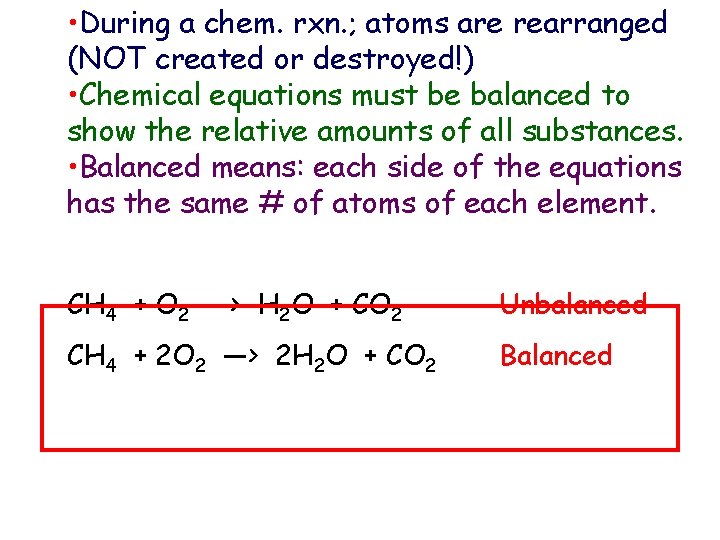
• During a chem. rxn. ; atoms are rearranged (NOT created or destroyed!) • Chemical equations must be balanced to show the relative amounts of all substances. • Balanced means: each side of the equations has the same # of atoms of each element. CH 4 + O 2 —> H 2 O + CO 2 Unbalanced CH 4 + 2 O 2 —> 2 H 2 O + CO 2 Balanced

In order to balance… • Write correct formulas for all reactants and products • Reactants Products • Count the number of atoms of each element in reactants & products. • Balance one at a time using coefficients. • Check for balance • Are the coefficients in the lowest possible ratio?

Balancing Equations NOTE: When balancing equations, you may change coefficients as much as you need to, but you may never change subscripts because you can’t change what substances are involved.

Balancing equations involves a great deal of “trial and error” at first, but there are some tricks…

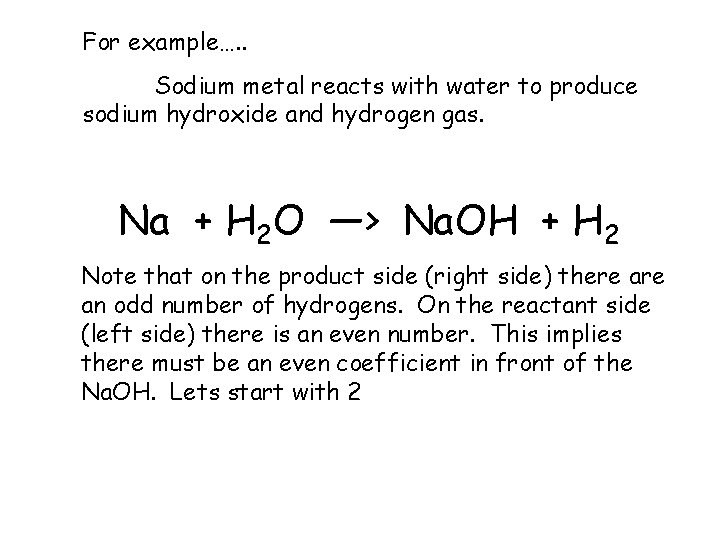
For example…. . Sodium metal reacts with water to produce sodium hydroxide and hydrogen gas. Na + H 2 O —> Na. OH + H 2 Note that on the product side (right side) there an odd number of hydrogens. On the reactant side (left side) there is an even number. This implies there must be an even coefficient in front of the Na. OH. Lets start with 2

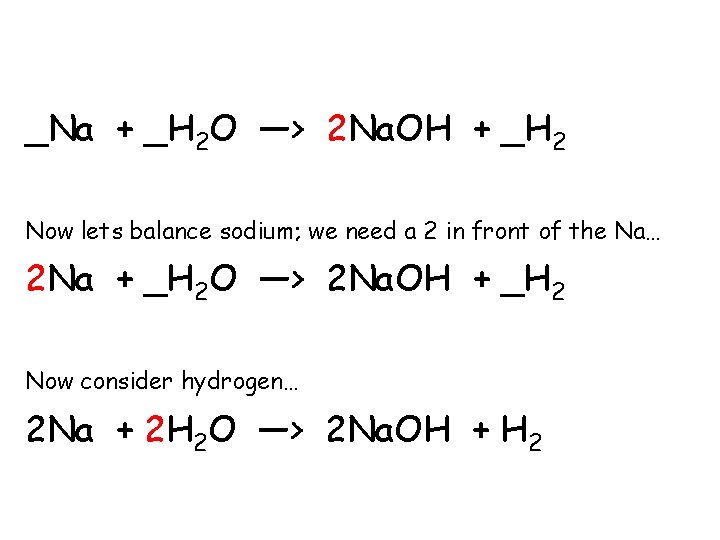
_Na + _H 2 O —> 2 Na. OH + _H 2 Now lets balance sodium; we need a 2 in front of the Na… 2 Na + _H 2 O —> 2 Na. OH + _H 2 Now consider hydrogen… 2 Na + 2 H 2 O —> 2 Na. OH + H 2

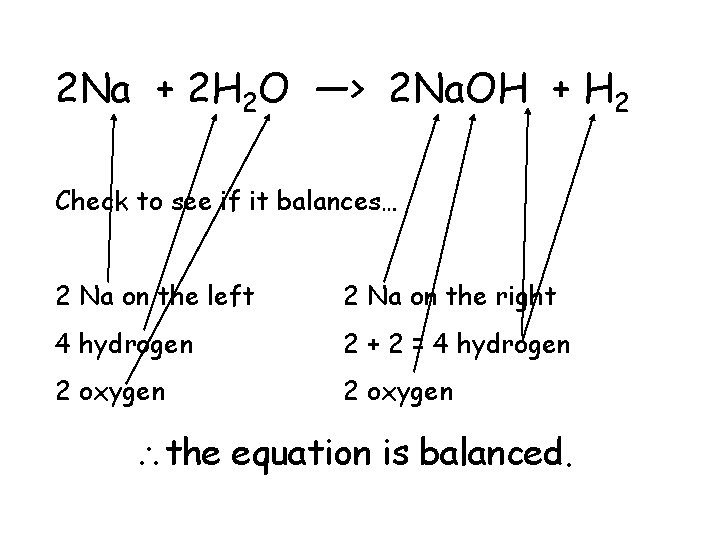
2 Na + 2 H 2 O —> 2 Na. OH + H 2 Check to see if it balances… 2 Na on the left 2 Na on the right 4 hydrogen 2 + 2 = 4 hydrogen 2 oxygen the equation is balanced.

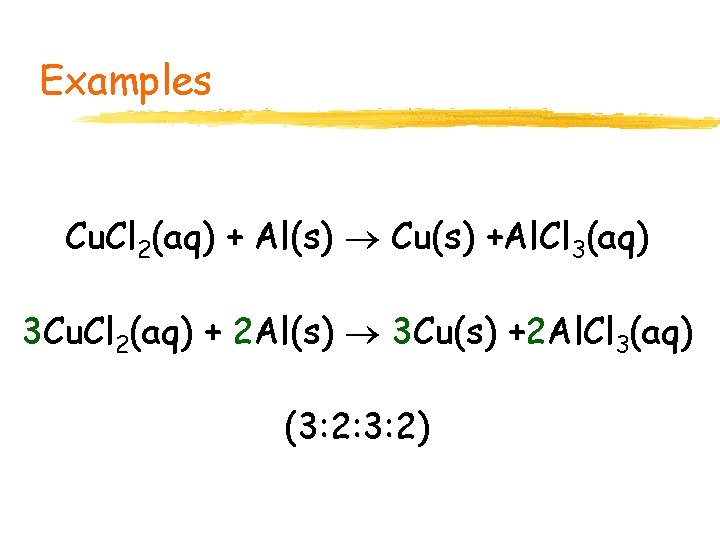
Examples Cu. Cl 2(aq) + Al(s) Cu(s) +Al. Cl 3(aq) 3 Cu. Cl 2(aq) + 2 Al(s) 3 Cu(s) +2 Al. Cl 3(aq) (3: 2: 3: 2)

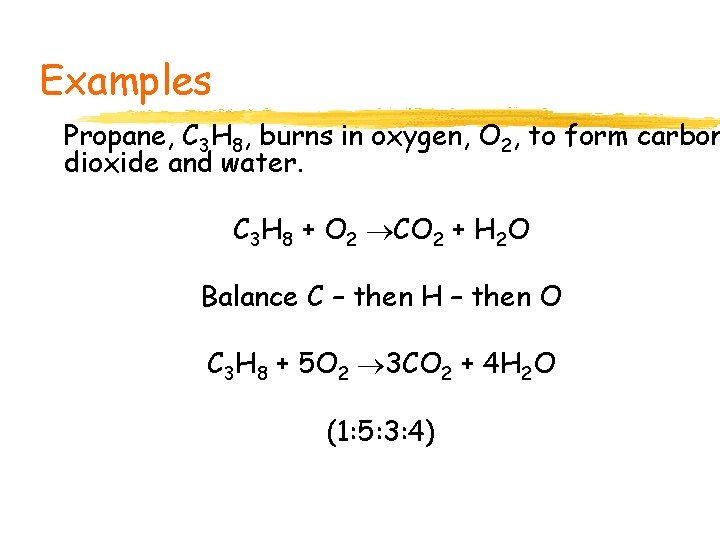
Examples Propane, C 3 H 8, burns in oxygen, O 2, to form carbon dioxide and water. C 3 H 8 + O 2 CO 2 + H 2 O Balance C – then H – then O C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O (1: 5: 3: 4)

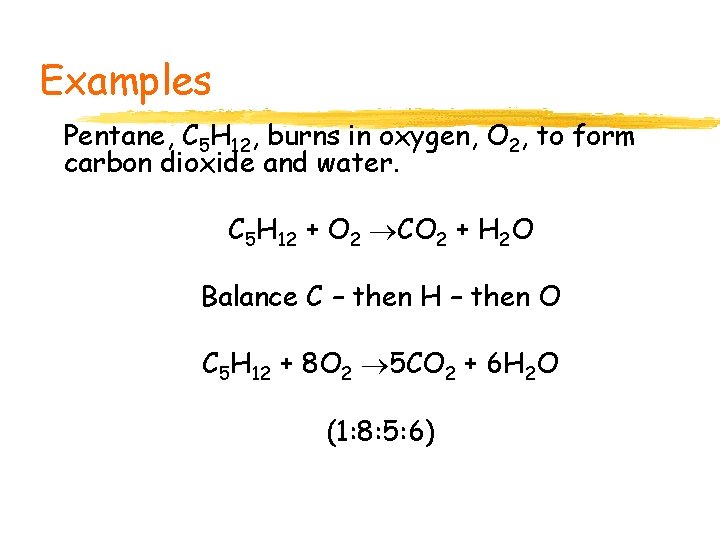
Examples Pentane, C 5 H 12, burns in oxygen, O 2, to form carbon dioxide and water. C 5 H 12 + O 2 CO 2 + H 2 O Balance C – then H – then O C 5 H 12 + 8 O 2 5 CO 2 + 6 H 2 O (1: 8: 5: 6)

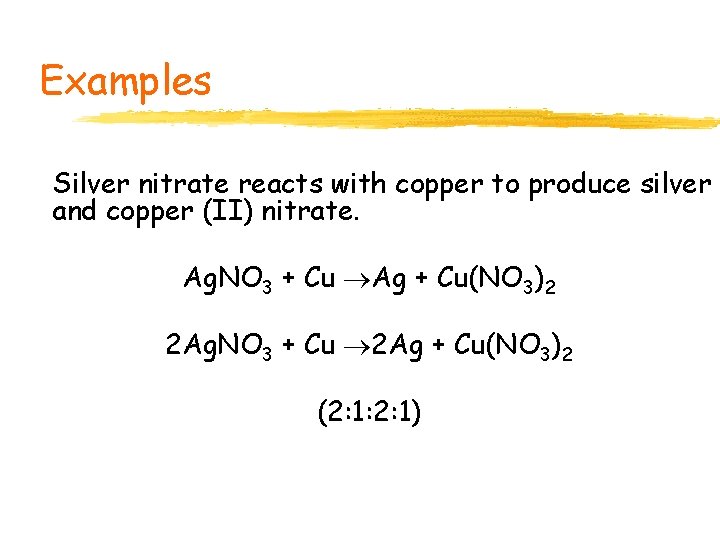
Examples Silver nitrate reacts with copper to produce silver and copper (II) nitrate. Ag. NO 3 + Cu Ag + Cu(NO 3)2 2 Ag. NO 3 + Cu 2 Ag + Cu(NO 3)2 (2: 1: 2: 1)

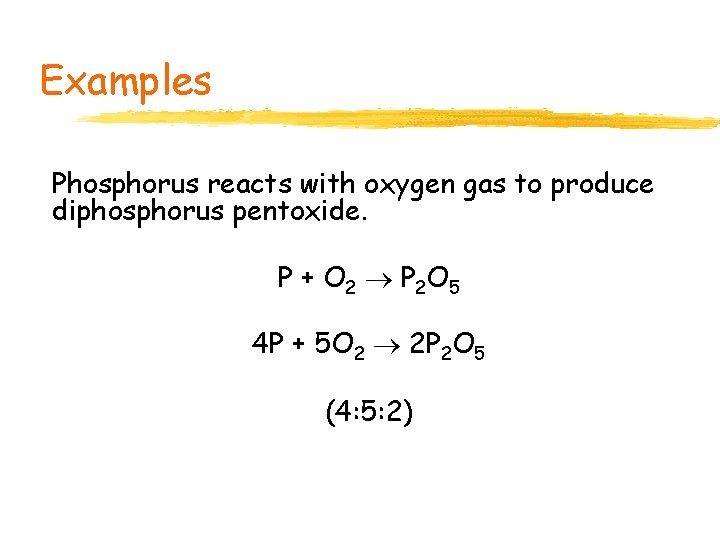
Examples Phosphorus reacts with oxygen gas to produce diphosphorus pentoxide. P + O 2 P 2 O 5 4 P + 5 O 2 2 P 2 O 5 (4: 5: 2)

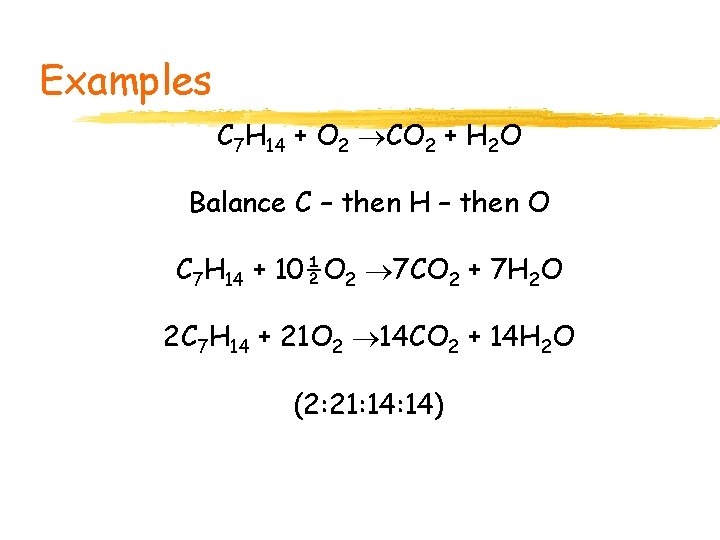
Examples C 7 H 14 + O 2 CO 2 + H 2 O Balance C – then H – then O C 7 H 14 + 10½O 2 7 CO 2 + 7 H 2 O 2 C 7 H 14 + 21 O 2 14 CO 2 + 14 H 2 O (2: 21: 14)


Types of Chemical Reactions • • • Synthesis / Combination Decomposition Single Replacement Double Replacement Combustion

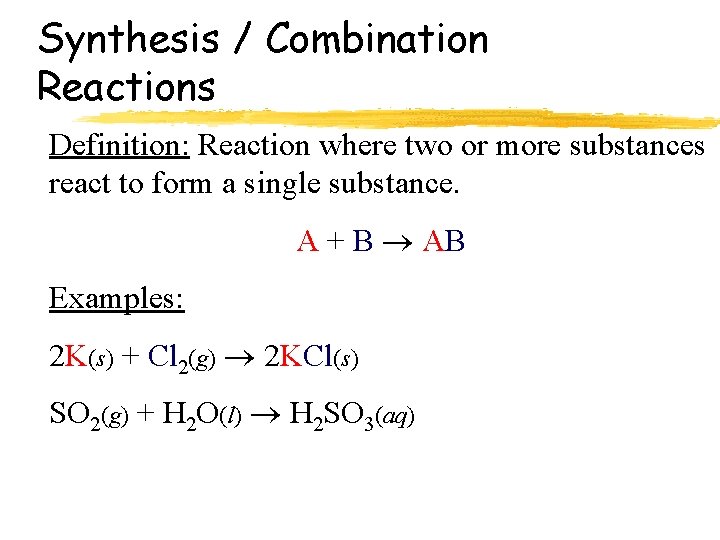
Synthesis / Combination Reactions Definition: Reaction where two or more substances react to form a single substance. A + B AB Examples: 2 K(s) + Cl 2(g) 2 KCl(s) SO 2(g) + H 2 O(l) H 2 SO 3(aq)

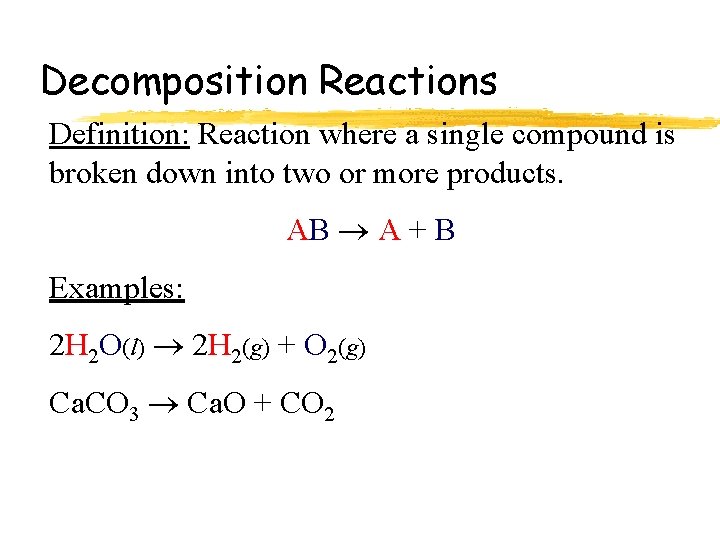
Decomposition Reactions Definition: Reaction where a single compound is broken down into two or more products. AB A + B Examples: 2 H 2 O(l) 2 H 2(g) + O 2(g) Ca. CO 3 Ca. O + CO 2

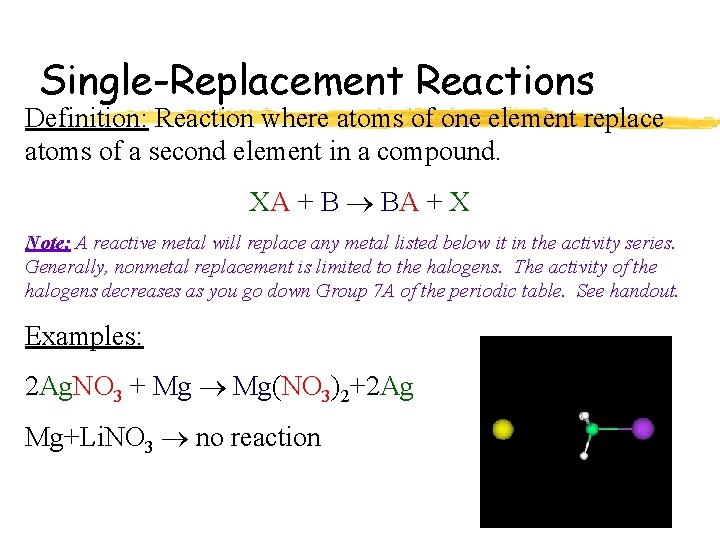
Single-Replacement Reactions Definition: Reaction where atoms of one element replace atoms of a second element in a compound. XA + B BA + X Note: A reactive metal will replace any metal listed below it in the activity series. Generally, nonmetal replacement is limited to the halogens. The activity of the halogens decreases as you go down Group 7 A of the periodic table. See handout. Examples: 2 Ag. NO 3 + Mg Mg(NO 3)2+2 Ag Mg+Li. NO 3 no reaction

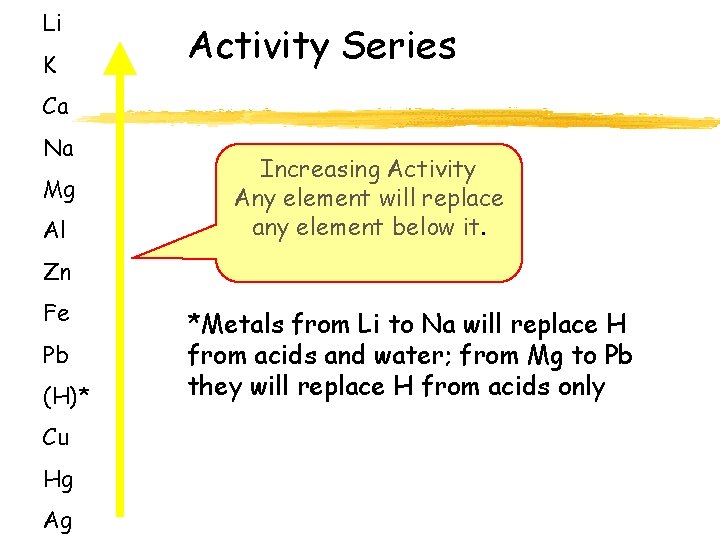
Li K Activity Series Ca Na Mg Al Increasing Activity Any element will replace any element below it. Zn Fe Pb (H)* Cu Hg Ag *Metals from Li to Na will replace H from acids and water; from Mg to Pb they will replace H from acids only

For Example… Ca + Mg. O Ca. O + Mg The Ca will replace the Mg because Ca is more active than Mg. That is to say…Ca is above Mg on the activity list.

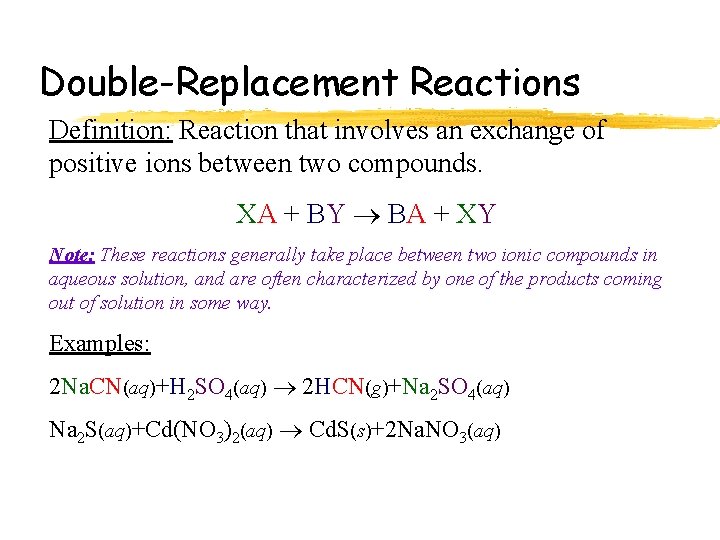
Double-Replacement Reactions Definition: Reaction that involves an exchange of positive ions between two compounds. XA + BY BA + XY Note: These reactions generally take place between two ionic compounds in aqueous solution, and are often characterized by one of the products coming out of solution in some way. Examples: 2 Na. CN(aq)+H 2 SO 4(aq) 2 HCN(g)+Na 2 SO 4(aq) Na 2 S(aq)+Cd(NO 3)2(aq) Cd. S(s)+2 Na. NO 3(aq)

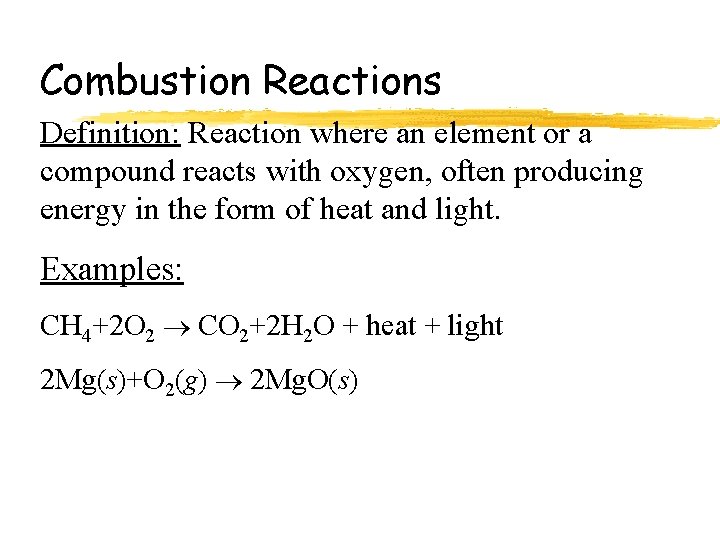
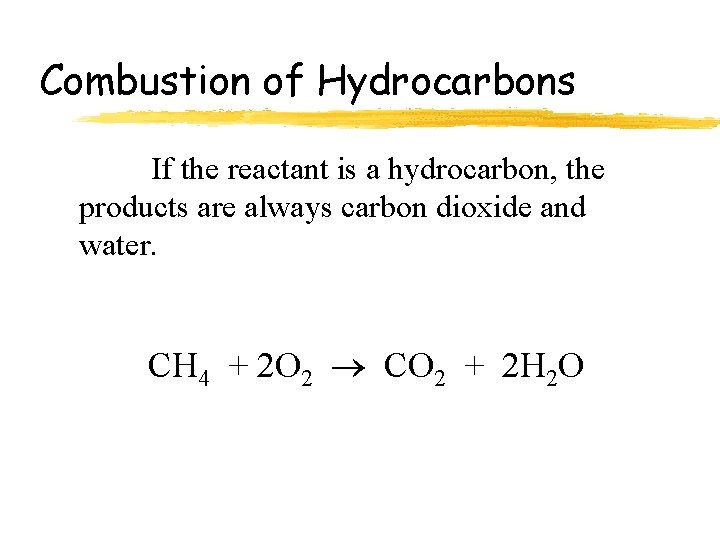
Combustion Reactions Definition: Reaction where an element or a compound reacts with oxygen, often producing energy in the form of heat and light. Examples: CH 4+2 O 2 CO 2+2 H 2 O + heat + light 2 Mg(s)+O 2(g) 2 Mg. O(s)

Combustion of Hydrocarbons If the reactant is a hydrocarbon, the products are always carbon dioxide and water. CH 4 + 2 O 2 CO 2 + 2 H 2 O
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions rearranging atoms worksheet answers
Chemical reactions rearranging atoms worksheet answers In chemical reactions atoms are rearranged
In chemical reactions atoms are rearranged Types of reactions
Types of reactions Periodic table of elements regents
Periodic table of elements regents Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Are kc and kp equal
Are kc and kp equal Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Balancing redox reactions in acidic solution
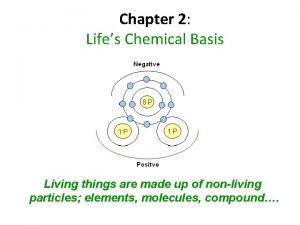
Balancing redox reactions in acidic solution Chapter 2 life's chemical basis
Chapter 2 life's chemical basis What is expanded octet
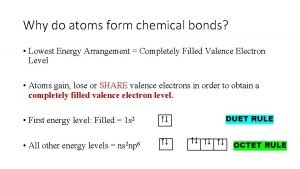
What is expanded octet Why do atoms form bonds
Why do atoms form bonds Counting atoms in chemical formulas
Counting atoms in chemical formulas Chemical properties of atoms
Chemical properties of atoms Chemical properties of atoms
Chemical properties of atoms Physcial change
Physcial change Chemical properties of atoms
Chemical properties of atoms Chemical bonds form when atoms *
Chemical bonds form when atoms * Chemical effects of electric current
Chemical effects of electric current Chemical equations worksheet
Chemical equations worksheet 5 chemical reactions
5 chemical reactions Non aqueous solvents classification
Non aqueous solvents classification Chapter 9 chemical reactions
Chapter 9 chemical reactions Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Www.biology-roots.com
Www.biology-roots.com Non examples of chemical reactions
Non examples of chemical reactions Principles of immuno chemical reactions
Principles of immuno chemical reactions Rules for chemical reactions
Rules for chemical reactions Type of chemical reactions
Type of chemical reactions Hcl and sodium hydrogen carbonate
Hcl and sodium hydrogen carbonate Predict the products of the following reactions.
Predict the products of the following reactions. Chemical reaction in bread
Chemical reaction in bread Alkyne chemistry
Alkyne chemistry Chapter 9 study guide chemical reactions
Chapter 9 study guide chemical reactions