Chemical Kinetics Reaction Rates Chemical Kinetics the study






- Slides: 12

Chemical Kinetics (Reaction Rates) Chemical Kinetics: the study of how fast or slow a reaction takes place

Chemical Reactions occur at different rates! There are slow reactions like a copper roof oxidizing (turning green) over a period of several years. When you have a lot of bonds to break the reaction will be slow. Covalent bonds are hard to break.

There are fast reactions: which require the least number of bonds to be broken. Fast reaction involve ions. Precipitation reactions are also fast. Pb 2+ (aq) + 2 I 1 - (aq) --> Pb. I 2 (s) Neutralization reactions are also fast. H 2 SO 4(aq) + 2 KOH (aq) --> 2 H 2 O (l) + K 2 SO 4 (s)

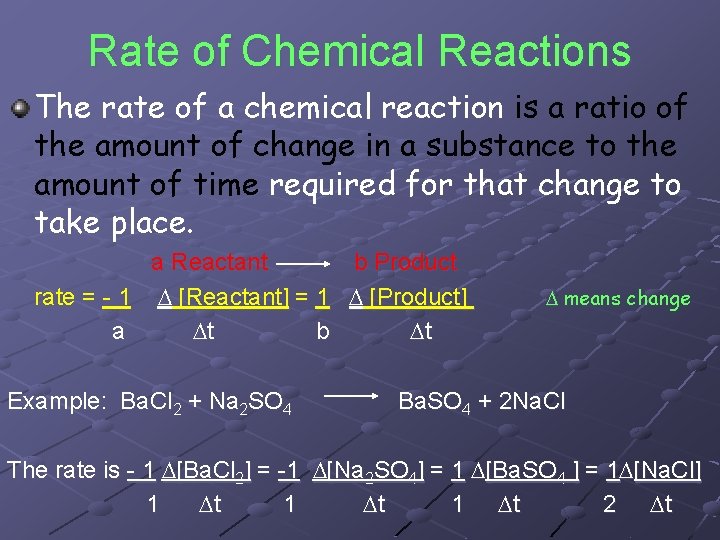
Rate of Chemical Reactions The rate of a chemical reaction is a ratio of the amount of change in a substance to the amount of time required for that change to take place. a Reactant b Product rate = - 1 D [Reactant] = 1 D [Product] a Dt b Dt Example: Ba. Cl 2 + Na 2 SO 4 D means change Ba. SO 4 + 2 Na. Cl The rate is - 1 D[Ba. Cl 2] = -1 D[Na 2 SO 4] = 1 D[Ba. SO 4 ] = 1 D[Na. Cl] 1 Dt 2 Dt

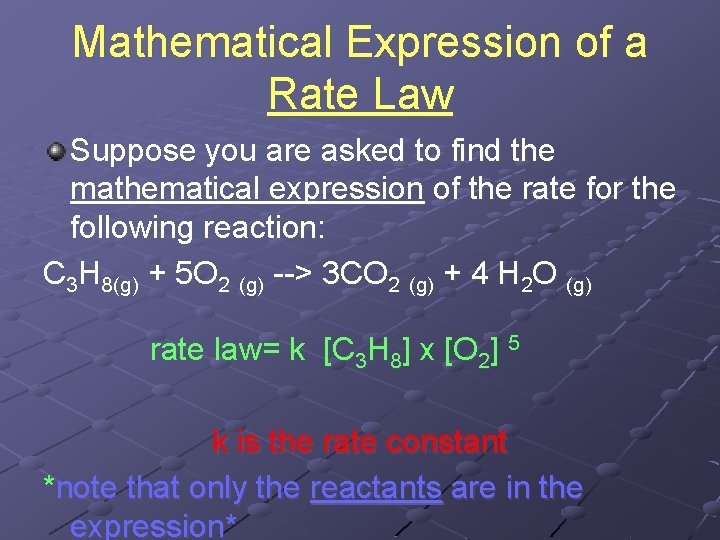
Mathematical Expression of a Rate Law Suppose you are asked to find the mathematical expression of the rate for the following reaction: C 3 H 8(g) + 5 O 2 (g) --> 3 CO 2 (g) + 4 H 2 O (g) rate law= k [C 3 H 8] x [O 2] 5 k is the rate constant *note that only the reactants are in the expression*

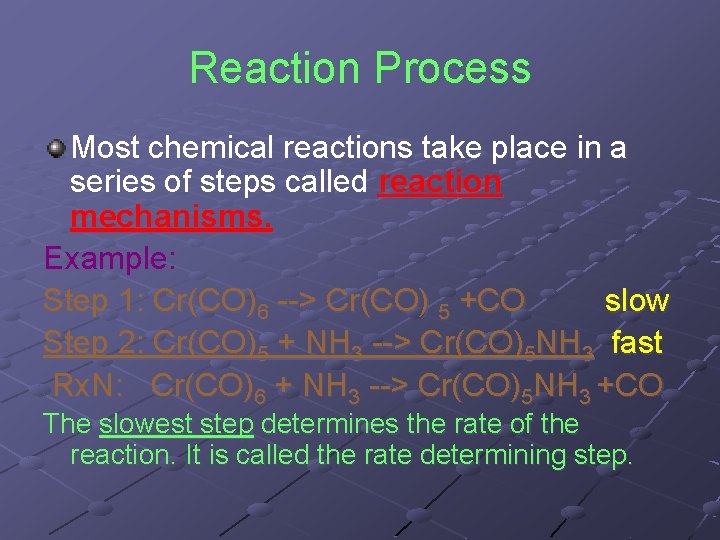
Reaction Process Most chemical reactions take place in a series of steps called reaction mechanisms. Example: Step 1: Cr(CO)6 --> Cr(CO) 5 +CO slow Step 2: Cr(CO)5 + NH 3 --> Cr(CO)5 NH 3 fast Rx. N: Cr(CO)6 + NH 3 --> Cr(CO)5 NH 3 +CO The slowest step determines the rate of the reaction. It is called the rate determining step.

Collision Theory and Activation Energy

The collision theory states that for a product to be made the reactants must collide with enough energy and with the right geometry. If the molecules collide with enough energy, then the atoms will rearrange and form a new product. This is called an effective collision.

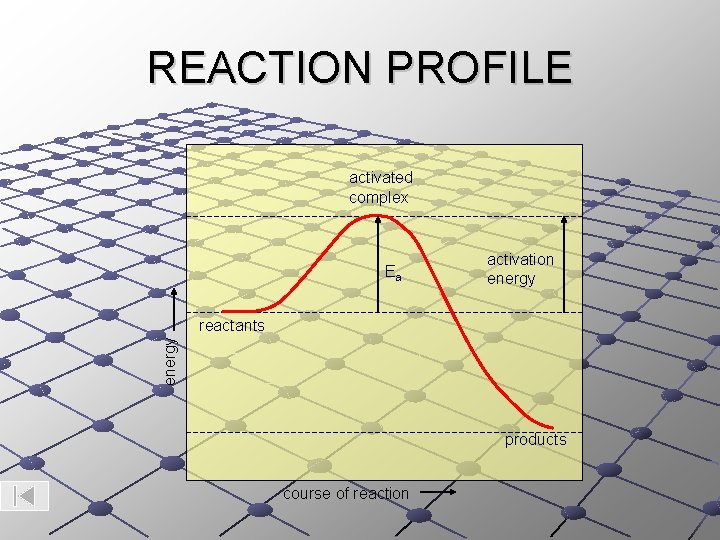
REACTION PROFILE activated complex Ea activation energy reactants products course of reaction

ACTIVATED COMPLEX: is the temporary, unstable arrangement of particles present at the highest point in a chemical reaction profile. ACTIVATION ENERGY (Ea): is the minimum energy with which particles must collide in order for the collision to be effective (resulting in the formation of a NEW substance)

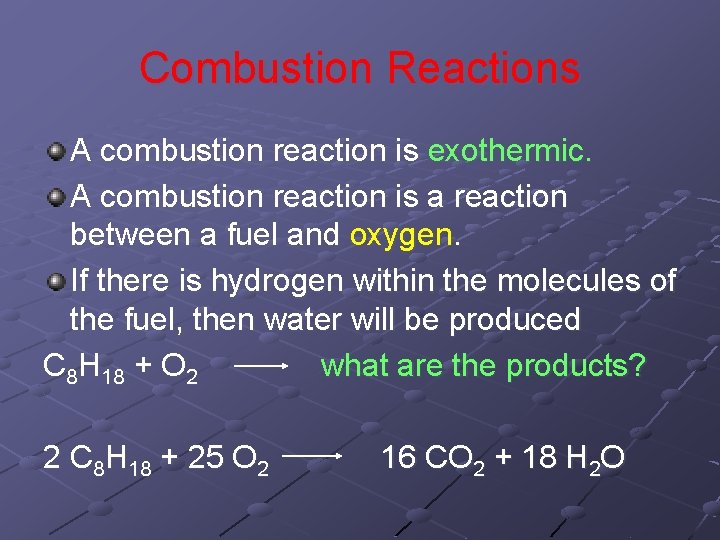
Combustion Reactions A combustion reaction is exothermic. A combustion reaction is a reaction between a fuel and oxygen. If there is hydrogen within the molecules of the fuel, then water will be produced C 8 H 18 + O 2 what are the products? 2 C 8 H 18 + 25 O 2 16 CO 2 + 18 H 2 O

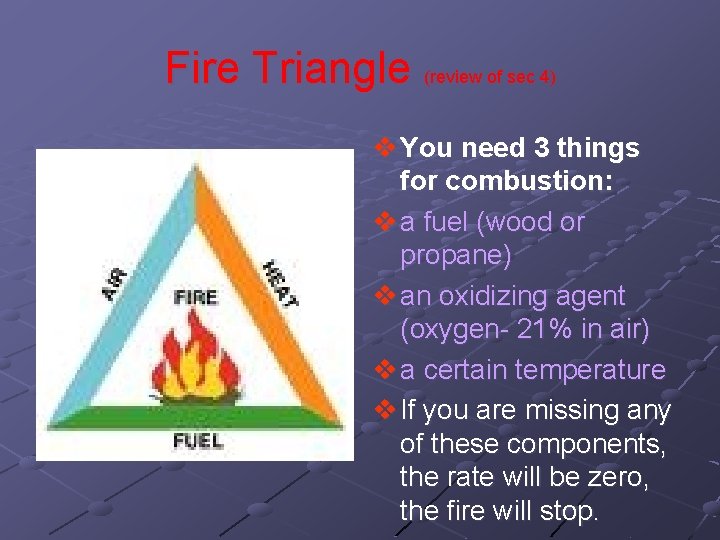
Fire Triangle (review of sec 4) v You need 3 things for combustion: v a fuel (wood or propane) v an oxidizing agent (oxygen- 21% in air) v a certain temperature v If you are missing any of these components, the rate will be zero, the fire will stop.
 Unit ratio
Unit ratio Equivalent ratios
Equivalent ratios Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates Half time equation
Half time equation Kinetics reaction
Kinetics reaction Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Reaction rates and equilibrium worksheet answers chapter 19
Reaction rates and equilibrium worksheet answers chapter 19 Section 4 reaction rates and equilibrium
Section 4 reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Expressing reaction rates
Expressing reaction rates






















