Kinetics Reaction Rates Reaction Rates Collision theory Potential





- Slides: 14

Kinetics Reaction Rates

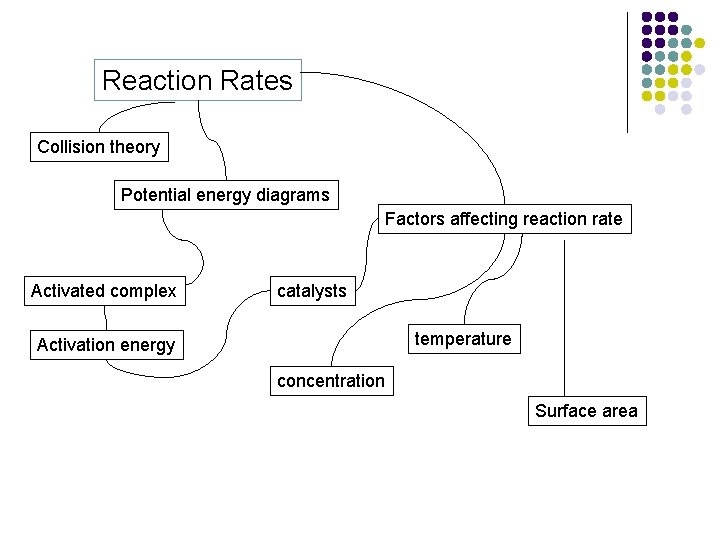
Reaction Rates Collision theory Potential energy diagrams Factors affecting reaction rate Activated complex catalysts temperature Activation energy concentration Surface area

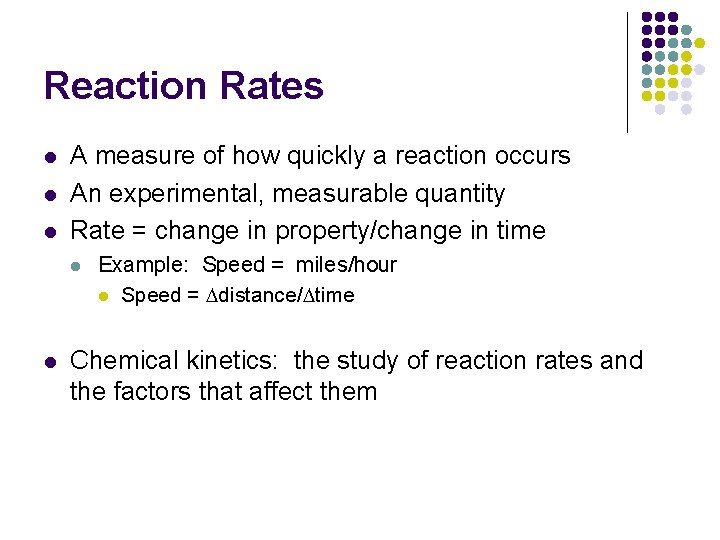
Reaction Rates l l l A measure of how quickly a reaction occurs An experimental, measurable quantity Rate = change in property/change in time l l Example: Speed = miles/hour l Speed = Ddistance/Dtime Chemical kinetics: the study of reaction rates and the factors that affect them

What could we measure for a reaction? Easily measured properties include: l Change in mass of a solid l Change in concentration l Temperature changes l p. H changes l Gas volume changes l Color changes l We must also measure changes in TIME!

Writing Rate Expressions l l l For a general reaction a. A + b. B c. C + d. D General form: We need to modify the rate expression to compensate for stoichiometry l The reaction has only one rate for a given set of conditions l Convention: all reaction rates are positive

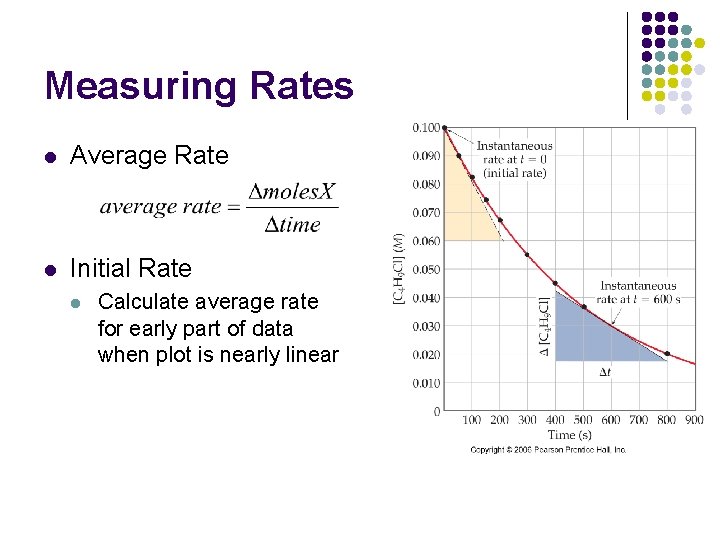
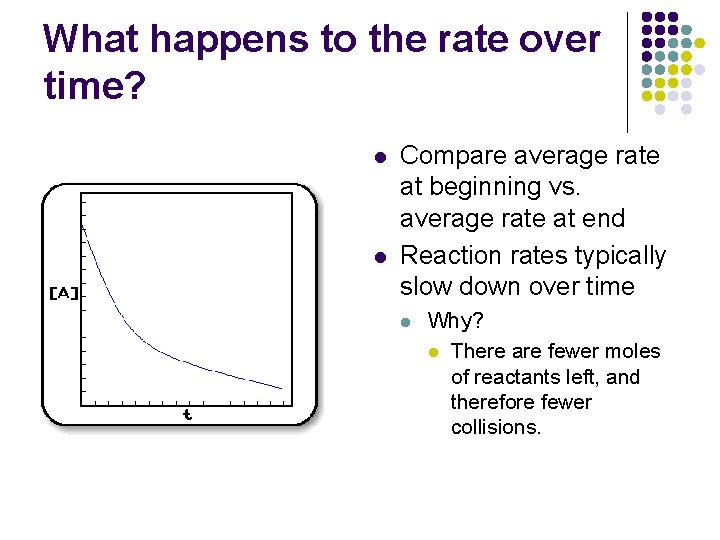
Measuring Rates l Average Rate l Initial Rate l Calculate average rate for early part of data when plot is nearly linear

What happens to the rate over time? l l Compare average rate at beginning vs. average rate at end Reaction rates typically slow down over time l Why? l There are fewer moles of reactants left, and therefore fewer collisions.

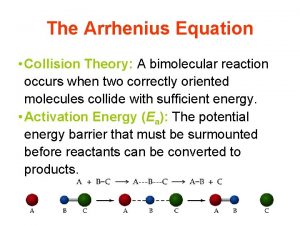
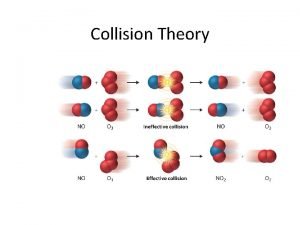
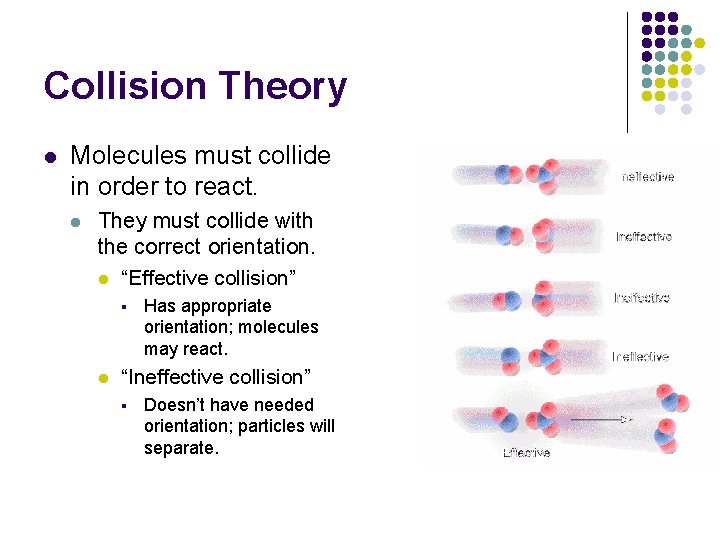
Collision Theory l Molecules must collide in order to react. l They must collide with the correct orientation. l “Effective collision” § l Has appropriate orientation; molecules may react. “Ineffective collision” § Doesn’t have needed orientation; particles will separate.

Collision Theory, cont. l Molecules must collide in order to react. l They must have enough energy to react. l Activation Energy, Ea § The minimum energy that reactants must have for the reaction to occur

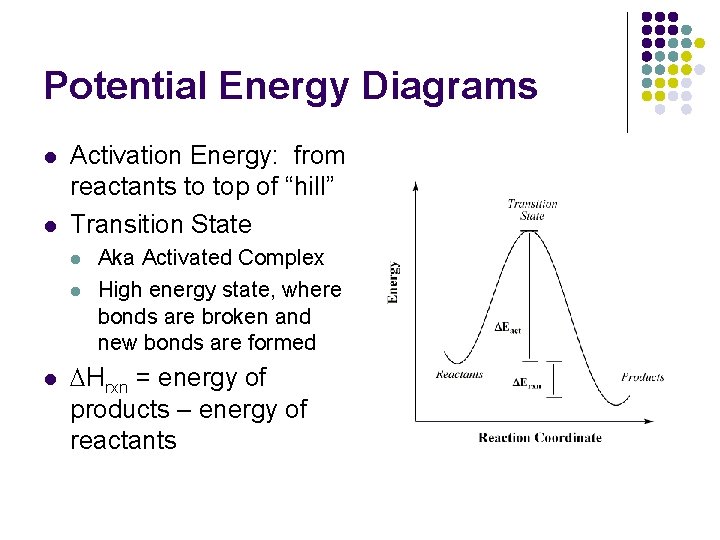
Potential Energy Diagrams l l Activation Energy: from reactants to top of “hill” Transition State l l l Aka Activated Complex High energy state, where bonds are broken and new bonds are formed DHrxn = energy of products – energy of reactants

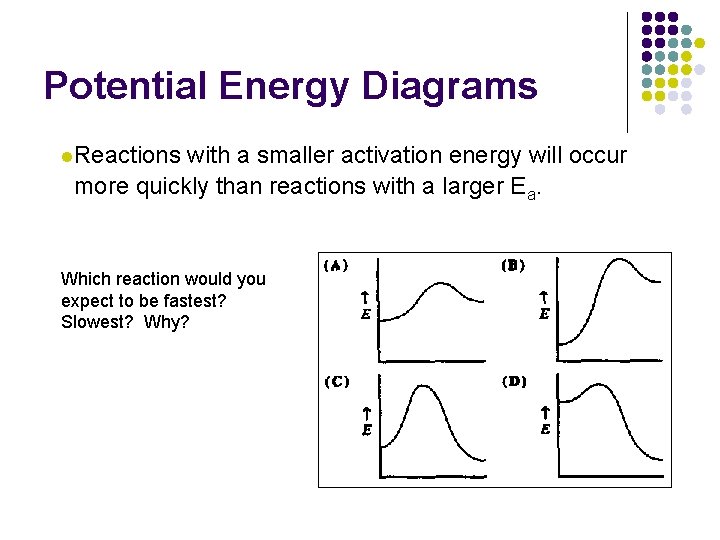
Potential Energy Diagrams l. Reactions with a smaller activation energy will occur more quickly than reactions with a larger Ea. Which reaction would you expect to be fastest? Slowest? Why?

Collision Theory l Basic premise: l l More collisions = faster reaction rate More collisions = greater likelihood for effective collisions

How can we speed up the rate of a reaction? l Increase temperature l l l Increase concentration l l Particles move more quickly, so more possible collisions More particles are likely to have enough energy to overcome activation energy barrier More particles, so more possible collisions Increase surface area l More particles are exposed, so more collisions are possible

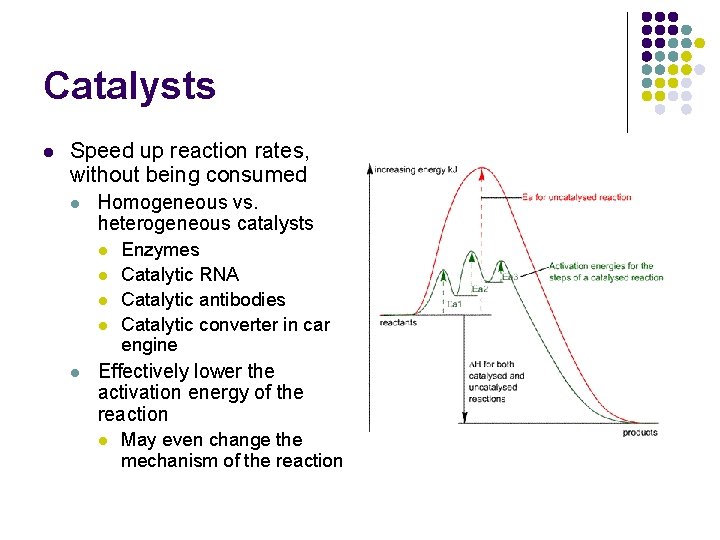
Catalysts l Speed up reaction rates, without being consumed l Homogeneous vs. heterogeneous catalysts l l l Enzymes Catalytic RNA Catalytic antibodies Catalytic converter in car engine Effectively lower the activation energy of the reaction l May even change the mechanism of the reaction
 Collision theory of kinetics
Collision theory of kinetics Use of arrhenius equation
Use of arrhenius equation Kinetics reaction
Kinetics reaction Unit rate
Unit rate Ratios and proportions guided notes
Ratios and proportions guided notes Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates Necessary for successful collisions to occur
Necessary for successful collisions to occur Collision theory states that
Collision theory states that Collision theory diagrams
Collision theory diagrams Collision theory class 12
Collision theory class 12 Collision theory
Collision theory Chemistry collision theory
Chemistry collision theory Systems in nature tend to undergo changes toward
Systems in nature tend to undergo changes toward Collision theory
Collision theory