Chemical Kinetics Chapter 16 Kinetics Reaction Rates Factors












![Rate Laws k = m, n = rate constant order rate = k[NO 2]n Rate Laws k = m, n = rate constant order rate = k[NO 2]n](https://slidetodoc.com/presentation_image_h/e280e39e9024ff90347689401918cc80/image-24.jpg)




![Half-life, first order reactions Integrated law: Half-life: Half of initial reacted [A]t = ½[A]0 Half-life, first order reactions Integrated law: Half-life: Half of initial reacted [A]t = ½[A]0](https://slidetodoc.com/presentation_image_h/e280e39e9024ff90347689401918cc80/image-36.jpg)

![Half-life, second order reactions Integrated law: Half-life: Half of initial reacted [A]t = ½[A]0 Half-life, second order reactions Integrated law: Half-life: Half of initial reacted [A]t = ½[A]0](https://slidetodoc.com/presentation_image_h/e280e39e9024ff90347689401918cc80/image-40.jpg)

![First order ln[A]0 slope = -k ln[A] Plot: ln[A] vs. time First order ln[A]0 slope = -k ln[A] Plot: ln[A] vs. time](https://slidetodoc.com/presentation_image_h/e280e39e9024ff90347689401918cc80/image-44.jpg)
![Second order slope = k 1 [A] Plot: 1 vs. time [A] 1 [A]o Second order slope = k 1 [A] Plot: 1 vs. time [A] 1 [A]o](https://slidetodoc.com/presentation_image_h/e280e39e9024ff90347689401918cc80/image-45.jpg)
![Zero order [A]0 slope = -k [A] Plot: [A] vs. time Zero order [A]0 slope = -k [A] Plot: [A] vs. time](https://slidetodoc.com/presentation_image_h/e280e39e9024ff90347689401918cc80/image-46.jpg)




























- Slides: 86

Chemical Kinetics Chapter 16

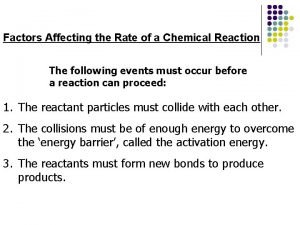
Kinetics Reaction Rates Factors affecting rate Quantitative rate expressions Determination Factors Models for Rates Reaction Mechanisms Effects of catalysts

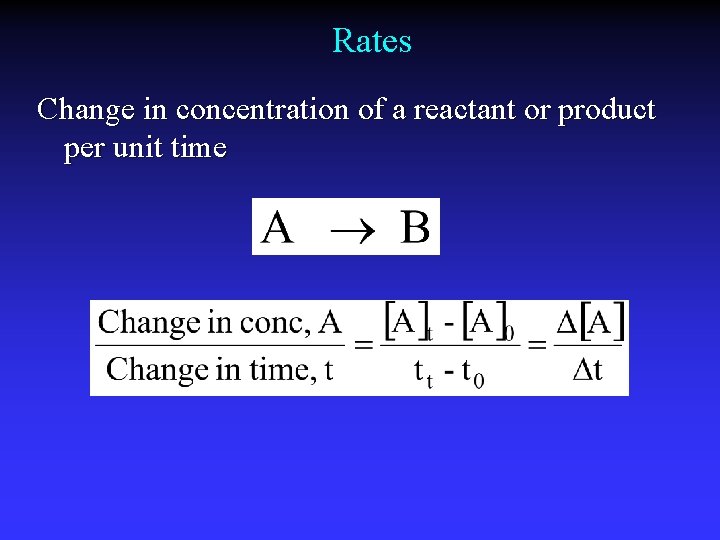
Rates Change in concentration of a reactant or product per unit time



Factors affecting rates Nature of the reactants State of subdivision/surface area Concentration Temperature Catalysts

Reactants Complexity Bond strengths Etc.








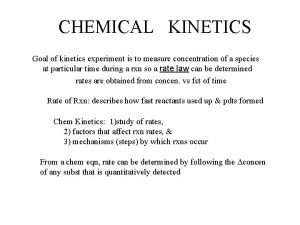
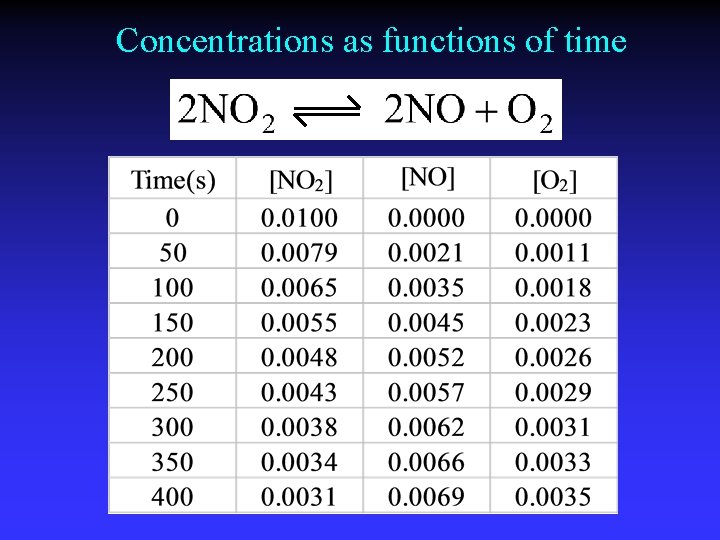
Concentrations as functions of time

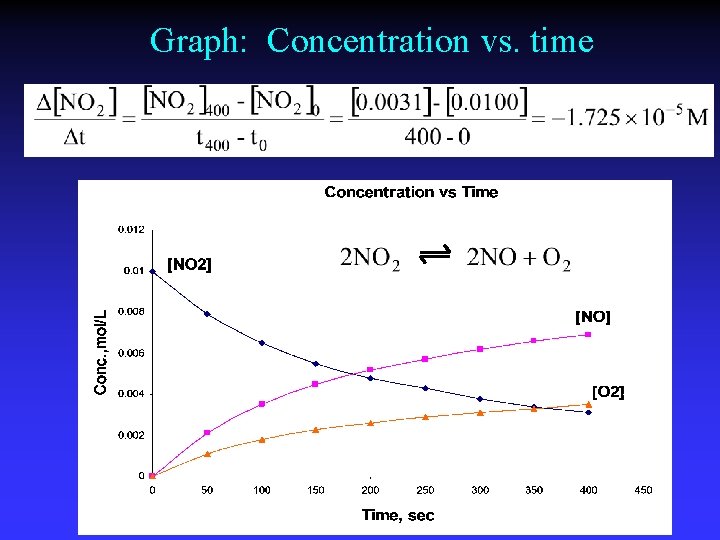
Graph: Concentration vs. time

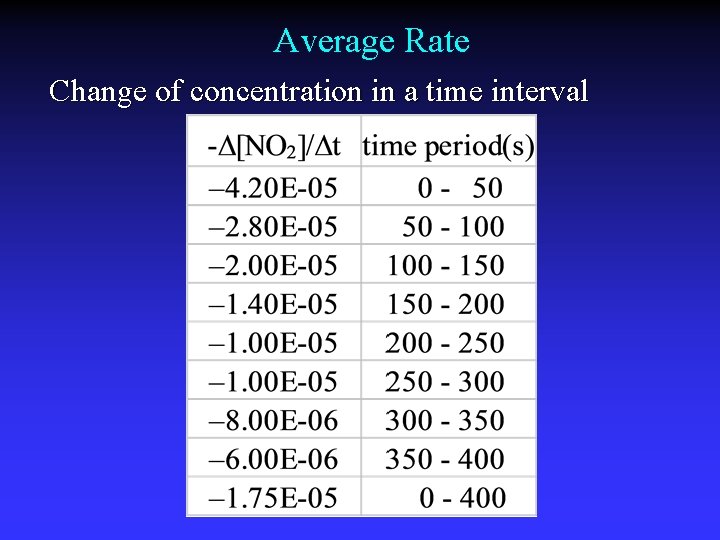
Average Rate Change of concentration in a time interval

Average Rate Slope of line between two points on the graph

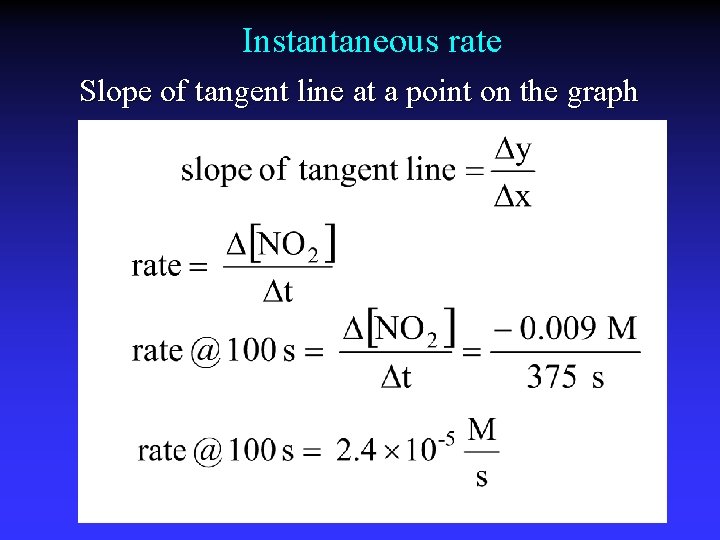
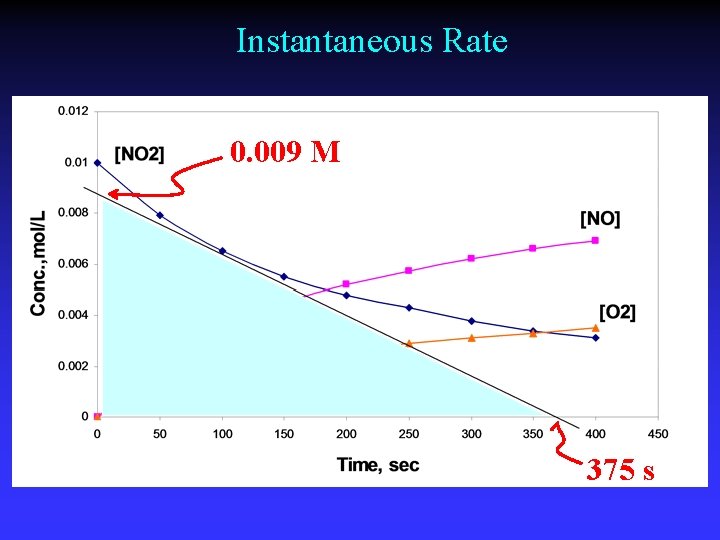
Instantaneous rate Slope of tangent line at a point on the graph

Instantaneous Rate 0. 009 M 375 s


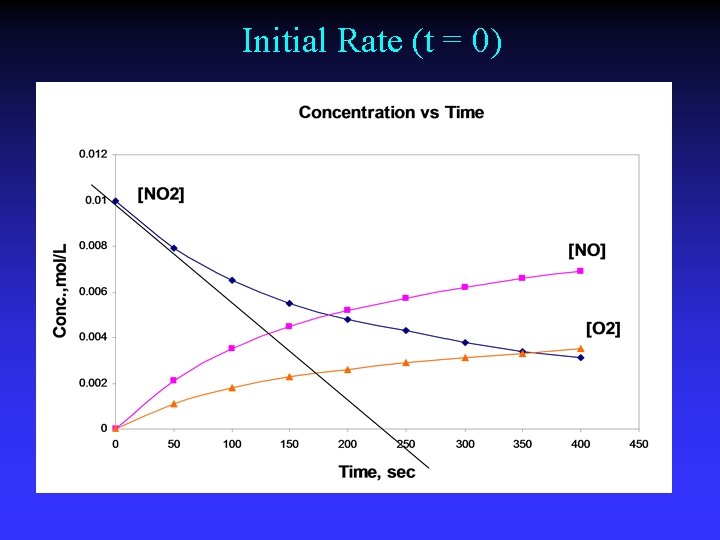
Initial Rate (t = 0)

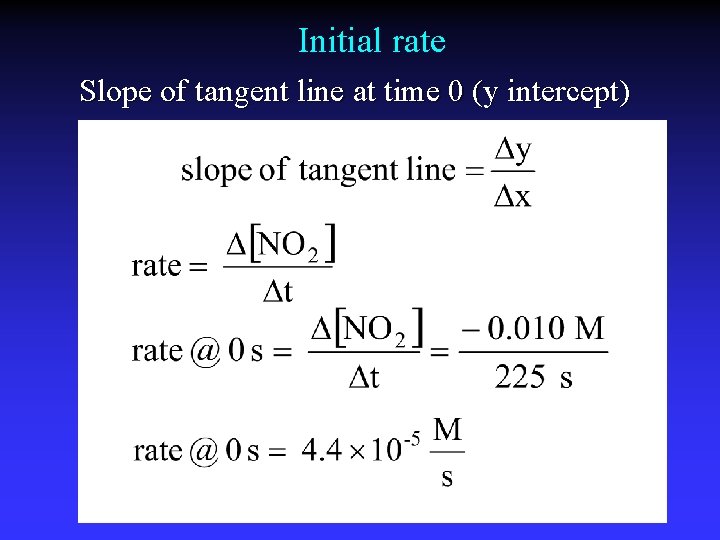
Initial rate Slope of tangent line at time 0 (y intercept)
![Rate Laws k m n rate constant order rate kNO 2n Rate Laws k = m, n = rate constant order rate = k[NO 2]n](https://slidetodoc.com/presentation_image_h/e280e39e9024ff90347689401918cc80/image-24.jpg)
Rate Laws k = m, n = rate constant order rate = k[NO 2]n

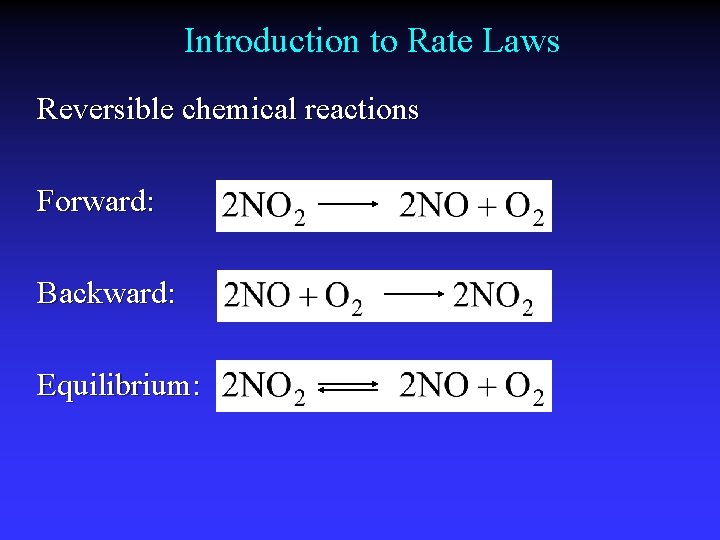
Introduction to Rate Laws Reversible chemical reactions Forward: Backward: Equilibrium:

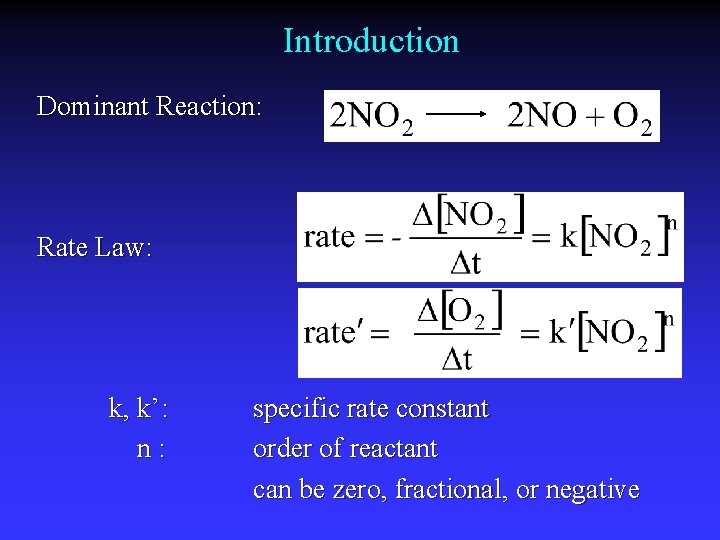
Introduction Dominant Reaction: Rate Law: k, k’: n: specific rate constant order of reactant can be zero, fractional, or negative

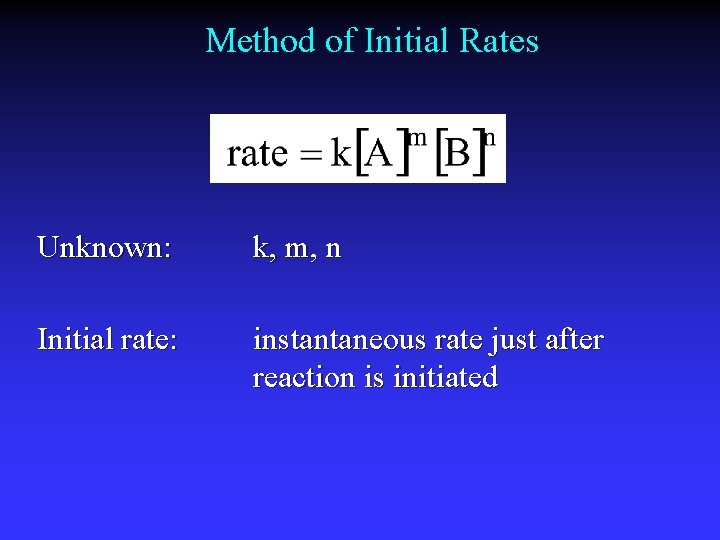
Method of Initial Rates Unknown: k, m, n Initial rate: instantaneous rate just after reaction is initiated

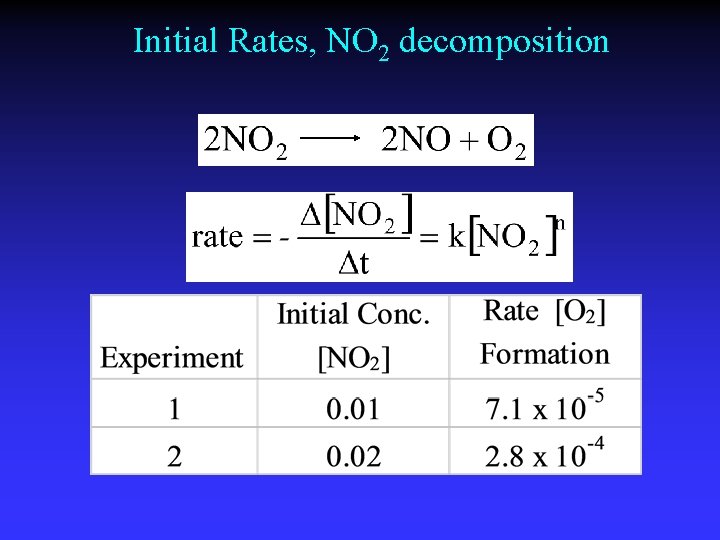
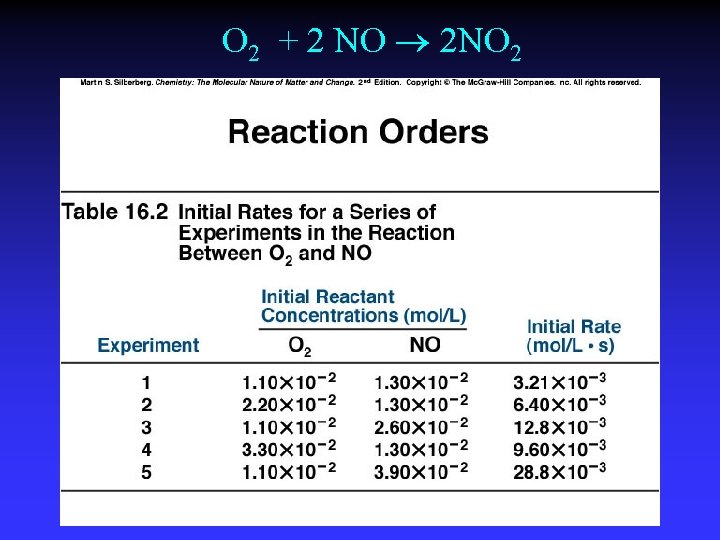
Initial Rates, NO 2 decomposition

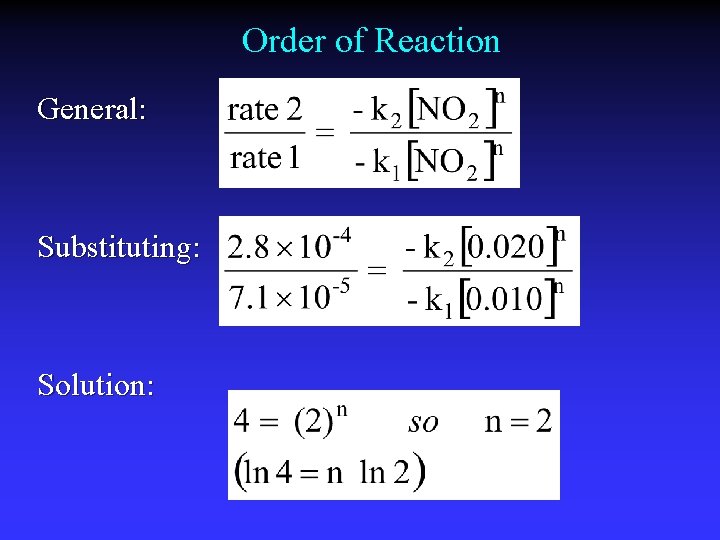
Order of Reaction General: Substituting: Solution:

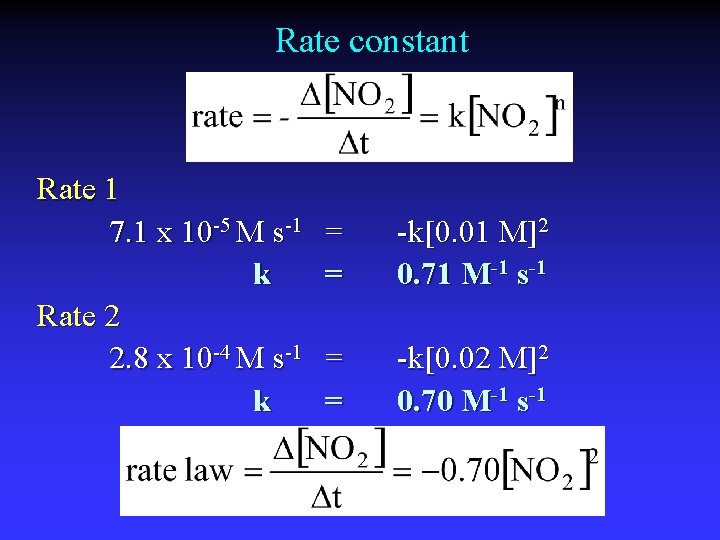
Rate constant Rate 1 7. 1 x 10 -5 M s-1 k Rate 2 2. 8 x 10 -4 M s-1 k = = -k[0. 01 M]2 0. 71 M-1 s-1 = = -k[0. 02 M]2 0. 70 M-1 s-1

You try

O 2 + 2 NO 2 NO 2

Overall Order Sum: 1 = + 6 2 Overall order of reaction: + 6 3

Types Differential: Rate dependence on concentration Integrated: Concentration dependence on time

First Order Reactions For a. A products Differential: Integrated:
![Halflife first order reactions Integrated law Halflife Half of initial reacted At ½A0 Half-life, first order reactions Integrated law: Half-life: Half of initial reacted [A]t = ½[A]0](https://slidetodoc.com/presentation_image_h/e280e39e9024ff90347689401918cc80/image-36.jpg)
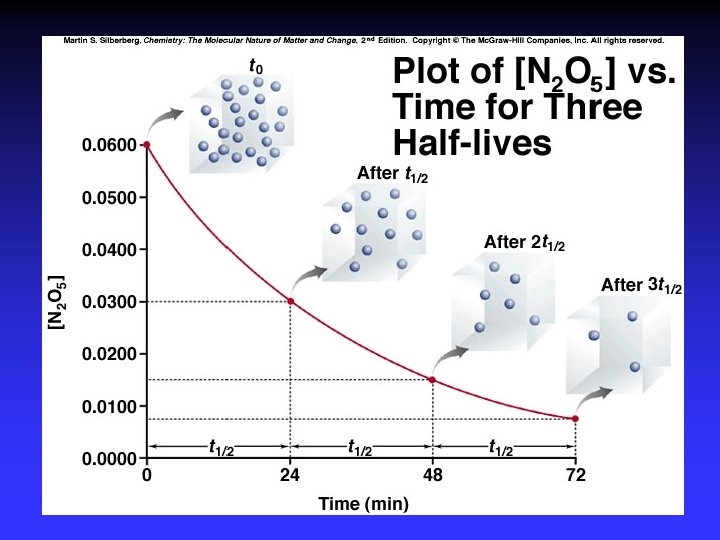
Half-life, first order reactions Integrated law: Half-life: Half of initial reacted [A]t = ½[A]0 Independent of [A]0



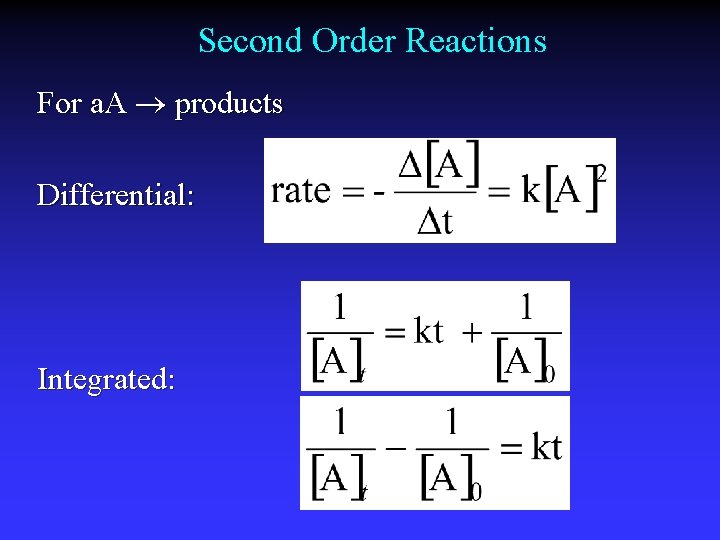
Second Order Reactions For a. A products Differential: Integrated:
![Halflife second order reactions Integrated law Halflife Half of initial reacted At ½A0 Half-life, second order reactions Integrated law: Half-life: Half of initial reacted [A]t = ½[A]0](https://slidetodoc.com/presentation_image_h/e280e39e9024ff90347689401918cc80/image-40.jpg)
Half-life, second order reactions Integrated law: Half-life: Half of initial reacted [A]t = ½[A]0 Inversely proportional to [A]0

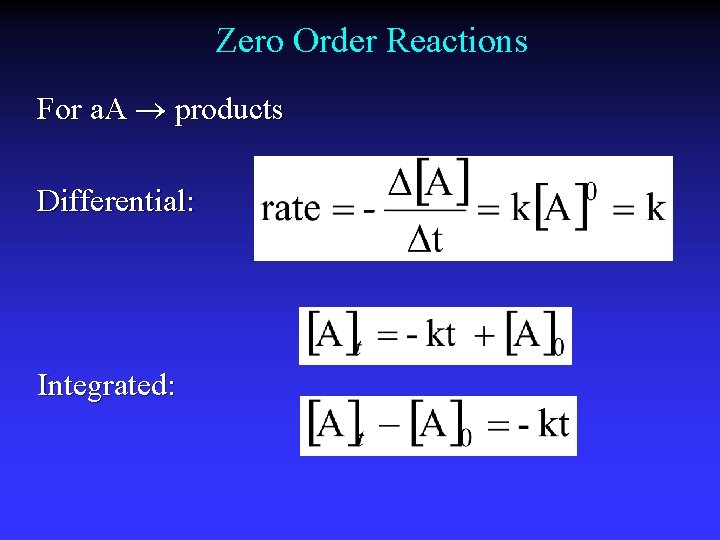
Zero Order Reactions For a. A products Differential: Integrated:


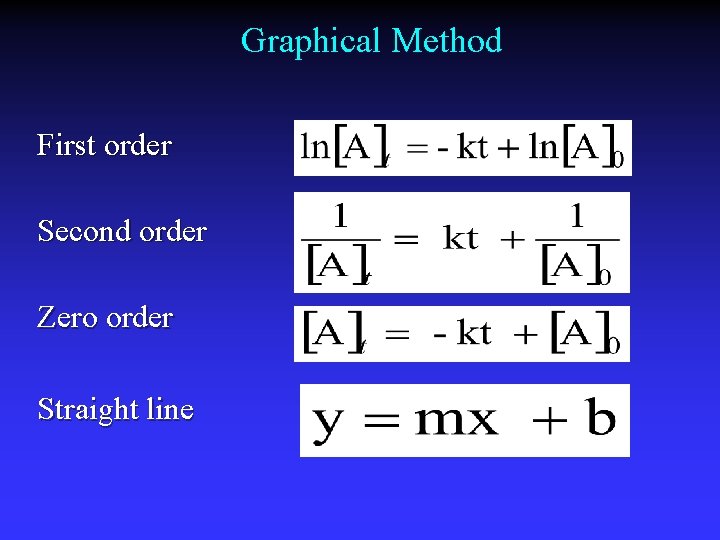
Graphical Method First order Second order Zero order Straight line
![First order lnA0 slope k lnA Plot lnA vs time First order ln[A]0 slope = -k ln[A] Plot: ln[A] vs. time](https://slidetodoc.com/presentation_image_h/e280e39e9024ff90347689401918cc80/image-44.jpg)
First order ln[A]0 slope = -k ln[A] Plot: ln[A] vs. time
![Second order slope k 1 A Plot 1 vs time A 1 Ao Second order slope = k 1 [A] Plot: 1 vs. time [A] 1 [A]o](https://slidetodoc.com/presentation_image_h/e280e39e9024ff90347689401918cc80/image-45.jpg)
Second order slope = k 1 [A] Plot: 1 vs. time [A] 1 [A]o time
![Zero order A0 slope k A Plot A vs time Zero order [A]0 slope = -k [A] Plot: [A] vs. time](https://slidetodoc.com/presentation_image_h/e280e39e9024ff90347689401918cc80/image-46.jpg)
Zero order [A]0 slope = -k [A] Plot: [A] vs. time



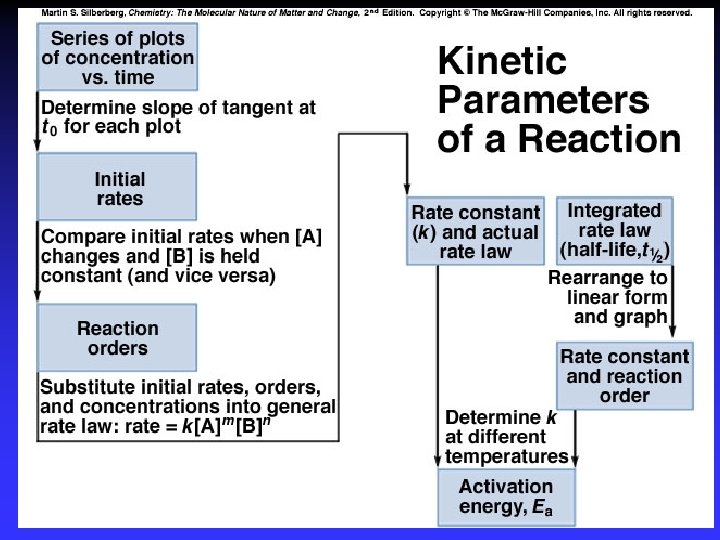
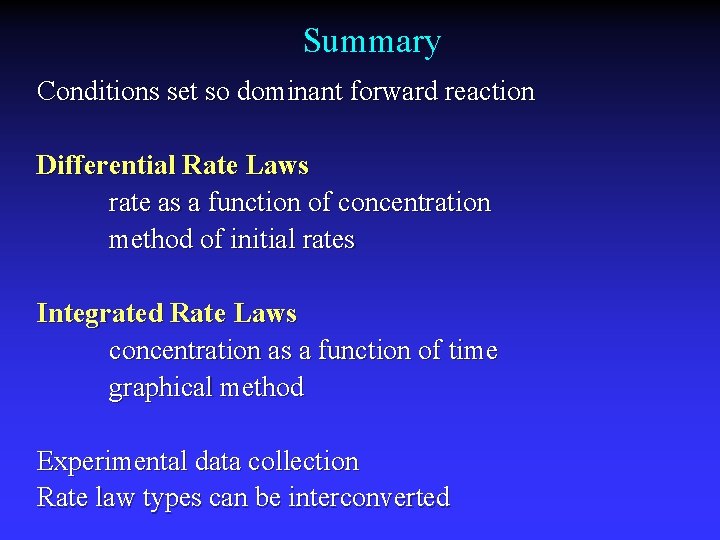
Summary Conditions set so dominant forward reaction Differential Rate Laws rate as a function of concentration method of initial rates Integrated Rate Laws concentration as a function of time graphical method Experimental data collection Rate law types can be interconverted

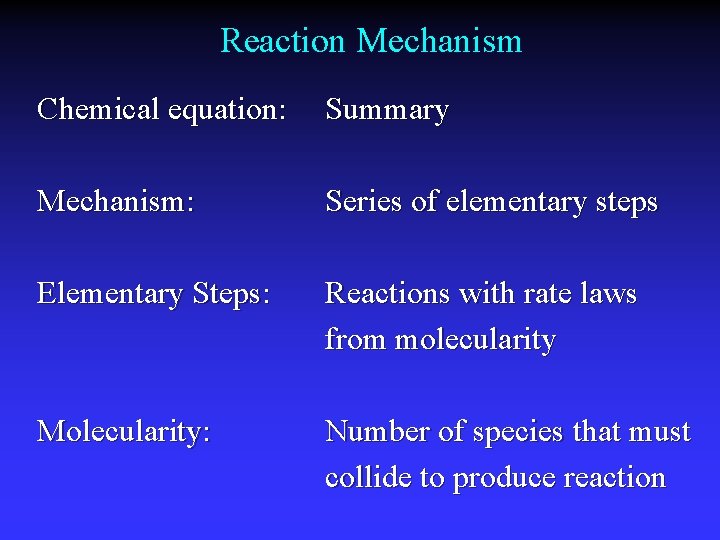
Reaction Mechanism Chemical equation: Summary Mechanism: Series of elementary steps Elementary Steps: Reactions with rate laws from molecularity Molecularity: Number of species that must collide to produce reaction


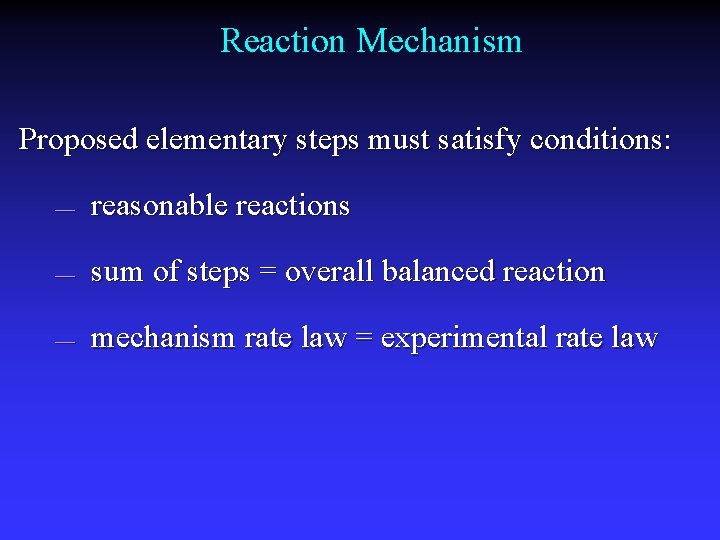
Reaction Mechanism Proposed elementary steps must satisfy conditions: — reasonable reactions — sum of steps = overall balanced reaction — mechanism rate law = experimental rate law

Intermediates appear in steps — produced in one step — used in subsequent — not in overall equation —

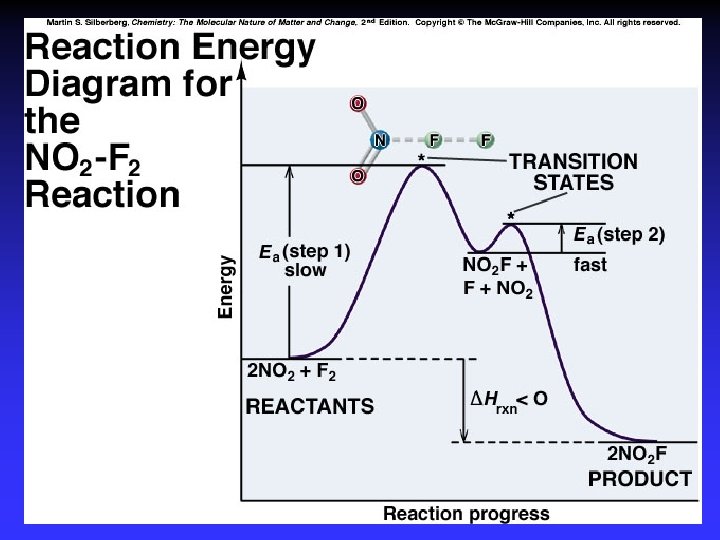
Rate-determining step In a multi-step process: SLOWEST step Determines overall reaction rate “Bottleneck”

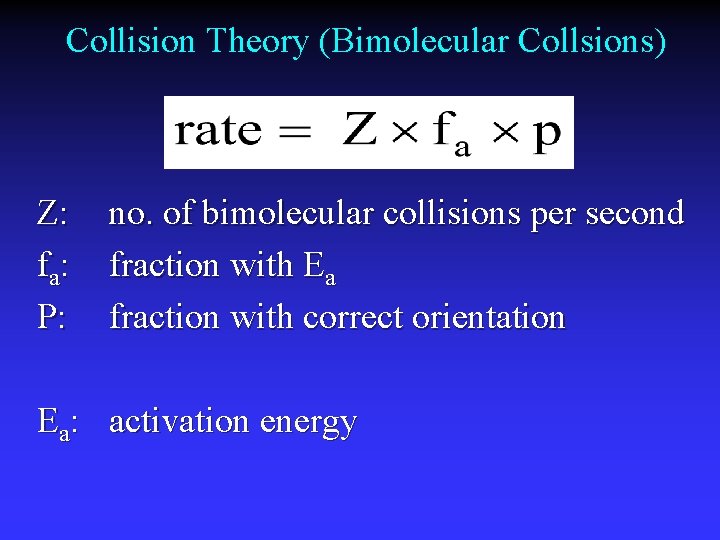
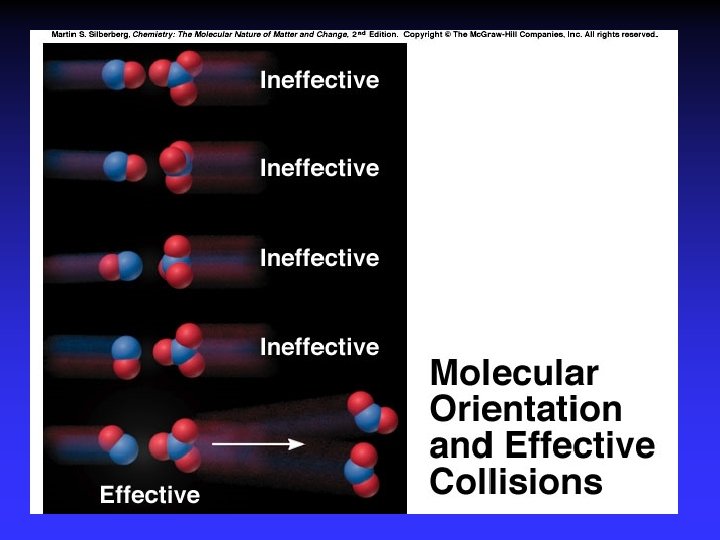
Model for Kinetics Collision Theory rate determined by particle collisions collision frequency and energy Transition State Theory how reactants convert to products

Collision Theory (Bimolecular Collsions) Z: fa : P: no. of bimolecular collisions per second fraction with Ea fraction with correct orientation Ea: activation energy







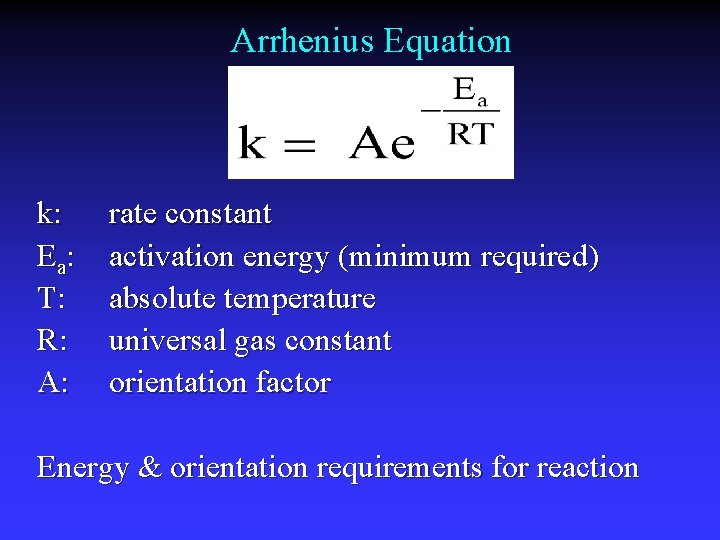
Arrhenius Equation k: E a: T: R: A: rate constant activation energy (minimum required) absolute temperature universal gas constant orientation factor Energy & orientation requirements for reaction

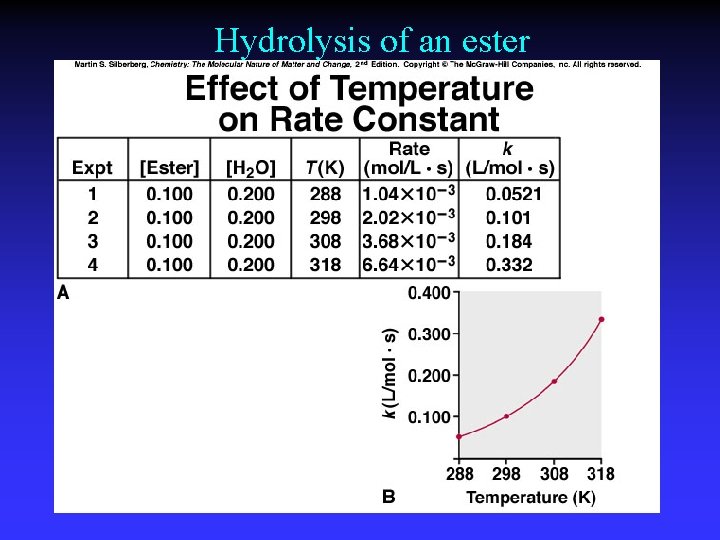
Hydrolysis of an ester




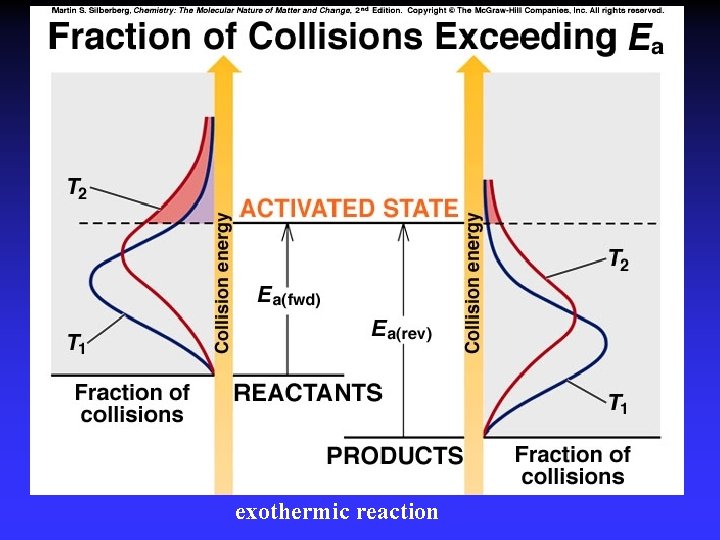
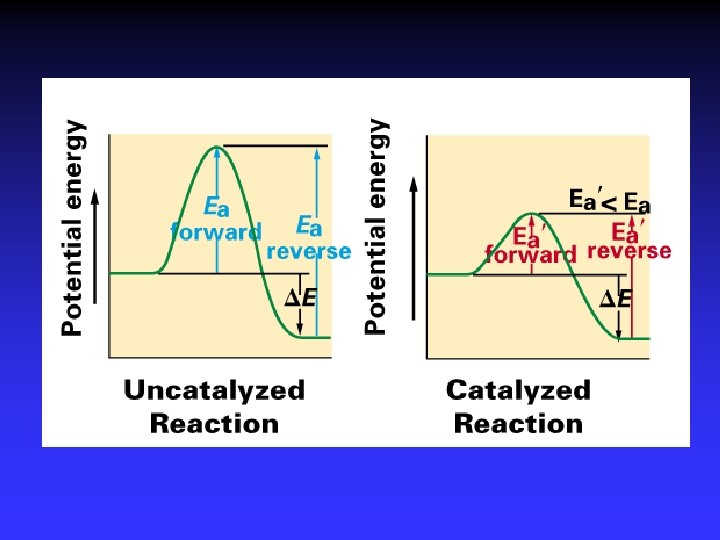
Transition State Theory Ea and internal energy: Bonds breaking and forming Atoms rearranging “Transition State” Unstable intermediate At point of highest energy

forward reaction reverse reaction

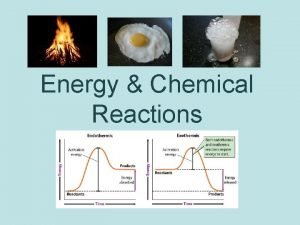
exothermic reaction


I- + CH 3 Cl Cl- + CH 3 I






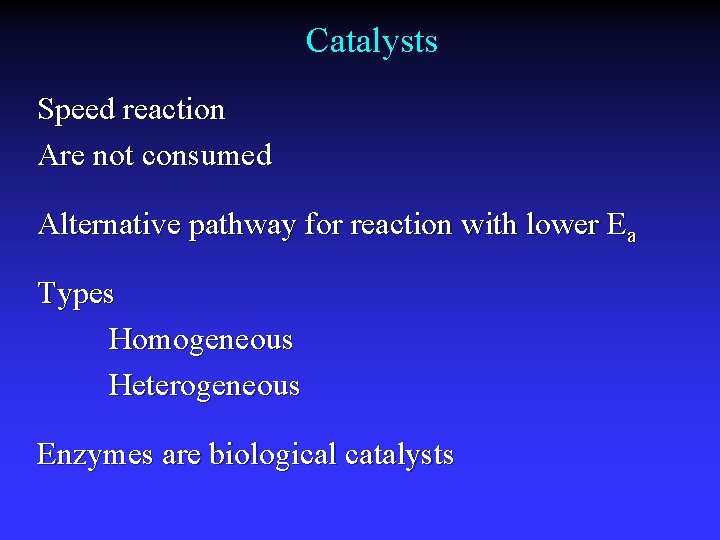
Catalysts Speed reaction Are not consumed Alternative pathway for reaction with lower Ea Types Homogeneous Heterogeneous Enzymes are biological catalysts




Adsorption, activation, reaction, desorption




 Half time equation
Half time equation Is a ratio a rate
Is a ratio a rate Ratios rates and unit rates guided notes
Ratios rates and unit rates guided notes Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates Reaction rates and equilibrium worksheet answers chapter 19
Reaction rates and equilibrium worksheet answers chapter 19 Chapter 18 reaction rates and equilibrium answer key
Chapter 18 reaction rates and equilibrium answer key Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium What factors influence the rate of a chemical reaction
What factors influence the rate of a chemical reaction Kinetics reaction
Kinetics reaction Molecularity of reaction
Molecularity of reaction Chemistry unit 4 grade 11
Chemistry unit 4 grade 11 Kinetics half life
Kinetics half life Chemical kinetics definition
Chemical kinetics definition Chemical kinetics experiment
Chemical kinetics experiment Applications of chemical kinetics
Applications of chemical kinetics Steady state kinetics definition
Steady state kinetics definition Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Section 4 reaction rates and equilibrium
Section 4 reaction rates and equilibrium Expressing reaction rates
Expressing reaction rates Rate of reaction quiz
Rate of reaction quiz Expressing reaction rates
Expressing reaction rates Did a chemical reaction occur
Did a chemical reaction occur Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds Chemical formulas and chemical compounds chapter 7
Chemical formulas and chemical compounds chapter 7 Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Reaction rate equation
Reaction rate equation Addition reaction and substitution reaction
Addition reaction and substitution reaction Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Acid rain chemical reaction
Acid rain chemical reaction Word equation examples
Word equation examples Chemical reaction types
Chemical reaction types Breathalyzer redox reaction
Breathalyzer redox reaction Stoichiometry refers to
Stoichiometry refers to Rancimat principle
Rancimat principle How to calculate excess reactant
How to calculate excess reactant Which type of reaction
Which type of reaction Percent yeild
Percent yeild Reactions of pyrrole
Reactions of pyrrole Rancidity chemical reaction
Rancidity chemical reaction Phosphorus reaction with oxygen equation
Phosphorus reaction with oxygen equation Combustion reaction of methane
Combustion reaction of methane A chemist shorthand way of representing chemical reaction.
A chemist shorthand way of representing chemical reaction. Fatty acids definition
Fatty acids definition Rules of chemical reaction
Rules of chemical reaction 5 general types of chemical reactions
5 general types of chemical reactions In a chemical reaction... atoms aren't rearranged
In a chemical reaction... atoms aren't rearranged Balanced equations
Balanced equations A chemist shorthand way of representing chemical reaction.
A chemist shorthand way of representing chemical reaction. Chemist shorthand way of representing chemical reaction
Chemist shorthand way of representing chemical reaction Rainoutcomes
Rainoutcomes What are 5 indicators of a chemical change
What are 5 indicators of a chemical change 5 indicators of chemical reaction
5 indicators of chemical reaction What are 2 examples of endothermic reactions
What are 2 examples of endothermic reactions Yeast chemical reaction
Yeast chemical reaction Is the halloween clock reaction a chemical change
Is the halloween clock reaction a chemical change Double replacement example
Double replacement example The starting substances in a chemical reaction.
The starting substances in a chemical reaction. Is photosynthesis endothermic or exothermic
Is photosynthesis endothermic or exothermic Chemical reaction engineering
Chemical reaction engineering Chemical reaction engineering
Chemical reaction engineering Chemical reaction engineering
Chemical reaction engineering Describing chemical reactions
Describing chemical reactions Chemical reaction engineering
Chemical reaction engineering Series reaction example
Series reaction example Smog chemical reaction
Smog chemical reaction Indications of chemical reactions
Indications of chemical reactions Toothpaste chemical equation
Toothpaste chemical equation Concentration in chemical reaction
Concentration in chemical reaction Elephant toothpaste chemical reaction
Elephant toothpaste chemical reaction Product of reaction
Product of reaction Odor change chemical reaction example
Odor change chemical reaction example 50 unbalanced chemical equations
50 unbalanced chemical equations Concentration in chemical reaction
Concentration in chemical reaction Mole
Mole Combination chemical reaction examples
Combination chemical reaction examples What are the five general types of chemical reactions
What are the five general types of chemical reactions Evidence of chemical reaction
Evidence of chemical reaction Answer key
Answer key Rust chemical reaction
Rust chemical reaction Metabolism chemical reaction
Metabolism chemical reaction Chemical reaction engineering
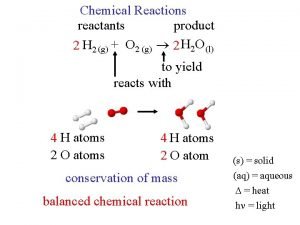
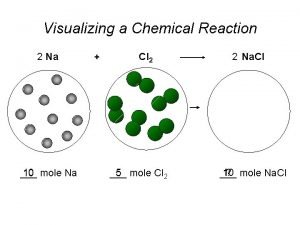
Chemical reaction engineering State the law of conservation of mass
State the law of conservation of mass Welding chemical reaction
Welding chemical reaction Chopping wood is a physical or chemical change
Chopping wood is a physical or chemical change Larry made this picture to represent a chemical reaction
Larry made this picture to represent a chemical reaction