Chemical Kinetics Kinetics Reaction Rates The speed of



![Reaction Rates ¨ Consider reaction A B Rate = -D[A]/ Dt = D[B]/ Dt Reaction Rates ¨ Consider reaction A B Rate = -D[A]/ Dt = D[B]/ Dt](https://slidetodoc.com/presentation_image_h/c3773afdbcfdf52f99a37e2a4004aac6/image-6.jpg)
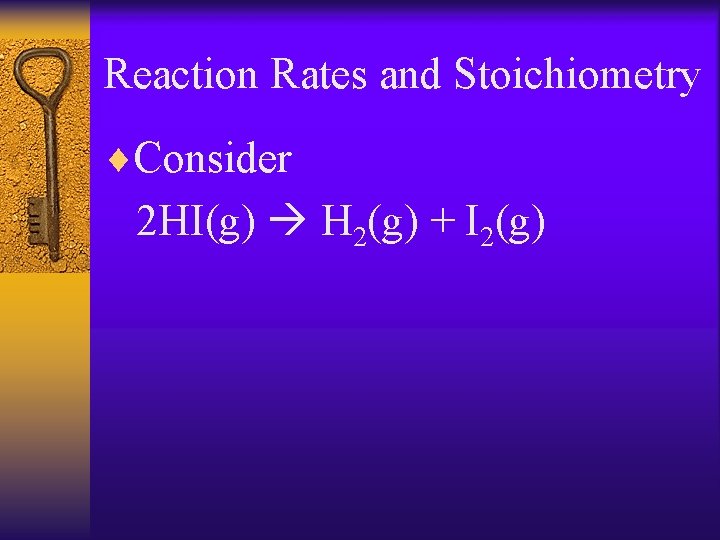

![Rate Laws ¨a. A + b. B c. C + d. D ¨Rate= k[A]m[B]n Rate Laws ¨a. A + b. B c. C + d. D ¨Rate= k[A]m[B]n](https://slidetodoc.com/presentation_image_h/c3773afdbcfdf52f99a37e2a4004aac6/image-11.jpg)





![Rate Laws ¨ 1 st order ¨Rate = k [A] ¨Units of k ( Rate Laws ¨ 1 st order ¨Rate = k [A] ¨Units of k (](https://slidetodoc.com/presentation_image_h/c3773afdbcfdf52f99a37e2a4004aac6/image-20.jpg)
![Rate Laws ¨ 2 nd order ¨Rate=k[A]2 or Rate = k[A][B] ¨Units of k Rate Laws ¨ 2 nd order ¨Rate=k[A]2 or Rate = k[A][B] ¨Units of k](https://slidetodoc.com/presentation_image_h/c3773afdbcfdf52f99a37e2a4004aac6/image-21.jpg)
![Rate Laws ¨ 3 rd order ¨Rate = k[A]2[B] ¨Units of k ( ) Rate Laws ¨ 3 rd order ¨Rate = k[A]2[B] ¨Units of k ( )](https://slidetodoc.com/presentation_image_h/c3773afdbcfdf52f99a37e2a4004aac6/image-22.jpg)

![Zero order rxns ¨ ¨ ¨ Rate = -D[A]/ Dt =k Integrated Rate Law Zero order rxns ¨ ¨ ¨ Rate = -D[A]/ Dt =k Integrated Rate Law](https://slidetodoc.com/presentation_image_h/c3773afdbcfdf52f99a37e2a4004aac6/image-27.jpg)
![First order rxns ¨ ¨ ¨ Rate = -D[A]/ Dt =k[A] Integrate Rate Law First order rxns ¨ ¨ ¨ Rate = -D[A]/ Dt =k[A] Integrate Rate Law](https://slidetodoc.com/presentation_image_h/c3773afdbcfdf52f99a37e2a4004aac6/image-28.jpg)
![Second order rxns ¨ Rate = -D[A]/ Dt =k[A]2 ¨ 1/[A]t = kt +1/[A]0 Second order rxns ¨ Rate = -D[A]/ Dt =k[A]2 ¨ 1/[A]t = kt +1/[A]0](https://slidetodoc.com/presentation_image_h/c3773afdbcfdf52f99a37e2a4004aac6/image-29.jpg)




















- Slides: 61

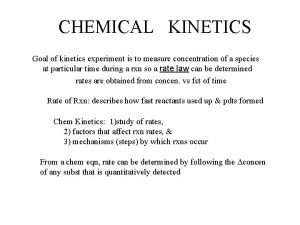
Chemical Kinetics

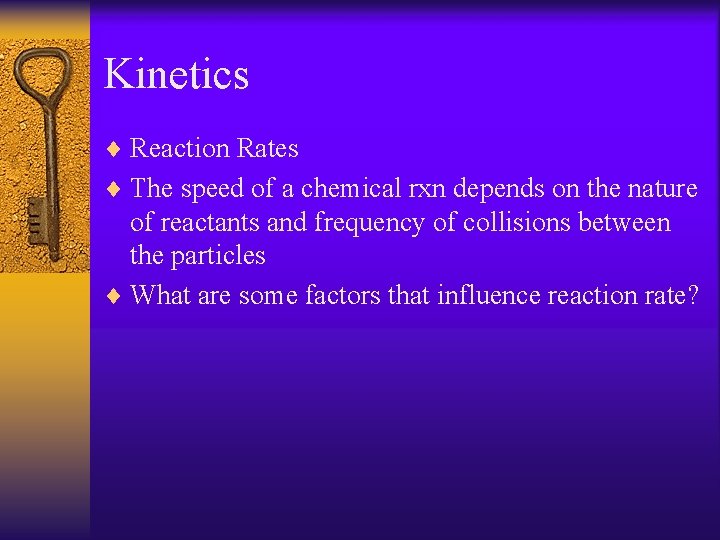
Kinetics ¨ Reaction Rates ¨ The speed of a chemical rxn depends on the nature of reactants and frequency of collisions between the particles ¨ What are some factors that influence reaction rate?

Factors that affect reaction rates ¨ 1. The physical state of the reactants ¨ 2. The concentration of reactants ¨ 3. The temperature at which the reaction occurs ¨ 4. The presence of a catalyst

Reaction Rates ¨ A rxn rate is the change in concentration of reactants or products per unit time (M/s) ¨ Can be based on rate of disappearance of the reactant or the rate of appearance of a product

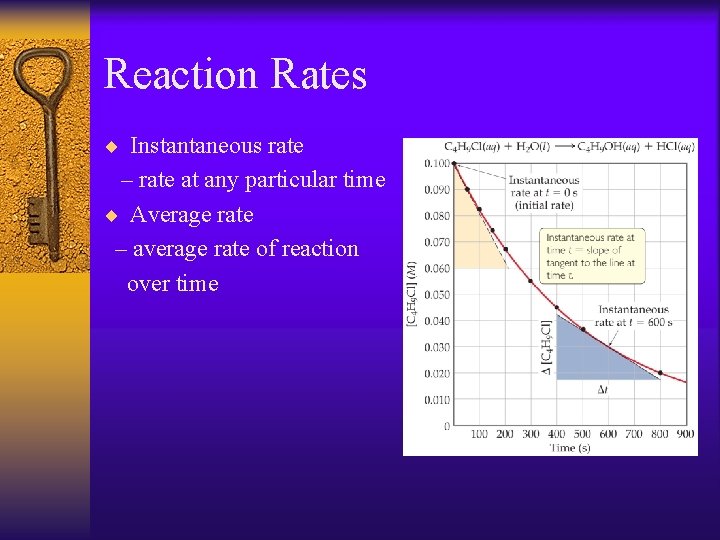
Reaction Rates ¨ Instantaneous rate – rate at any particular time ¨ Average rate – average rate of reaction over time
![Reaction Rates Consider reaction A B Rate DA Dt DB Dt Reaction Rates ¨ Consider reaction A B Rate = -D[A]/ Dt = D[B]/ Dt](https://slidetodoc.com/presentation_image_h/c3773afdbcfdf52f99a37e2a4004aac6/image-6.jpg)
Reaction Rates ¨ Consider reaction A B Rate = -D[A]/ Dt = D[B]/ Dt

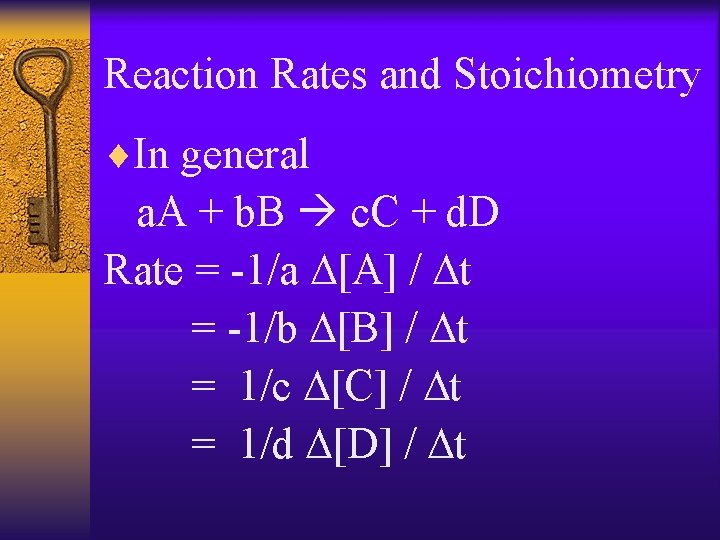
Reaction Rates and Stoichiometry ¨In general a. A + b. B c. C + d. D Rate = -1/a D[A] / Dt = -1/b D[B] / Dt = 1/c D[C] / Dt = 1/d D[D] / Dt
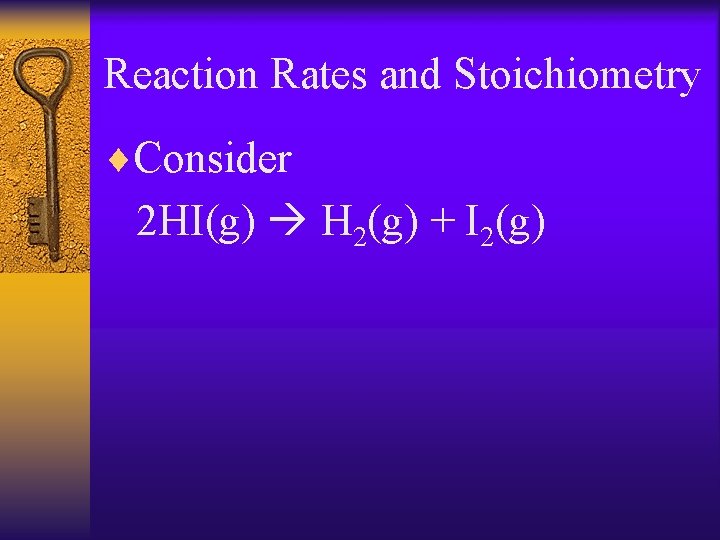
Reaction Rates and Stoichiometry ¨Consider 2 HI(g) H 2(g) + I 2(g)

Sample Exercise 14. 3 Relating Rates at Which Products Appear and Reactants Disappear ¨ (a) How is the rate at which ozone disappears related to the rate at which oxygen appears in the reaction 2 O 3(g) → 3 O 2(g)? (b) If the rate at which O 2 appears, Δ[O 2]/ Δt, is 6. 0 × 10– 5 M/s at a particular instant, at what rate is O 3 disappearing at this same time, –Δ[O 3]/Δ t?

Rate Laws ¨A rate law tells how the rate depends on the reactants
![Rate Laws a A b B c C d D Rate kAmBn Rate Laws ¨a. A + b. B c. C + d. D ¨Rate= k[A]m[B]n](https://slidetodoc.com/presentation_image_h/c3773afdbcfdf52f99a37e2a4004aac6/image-11.jpg)
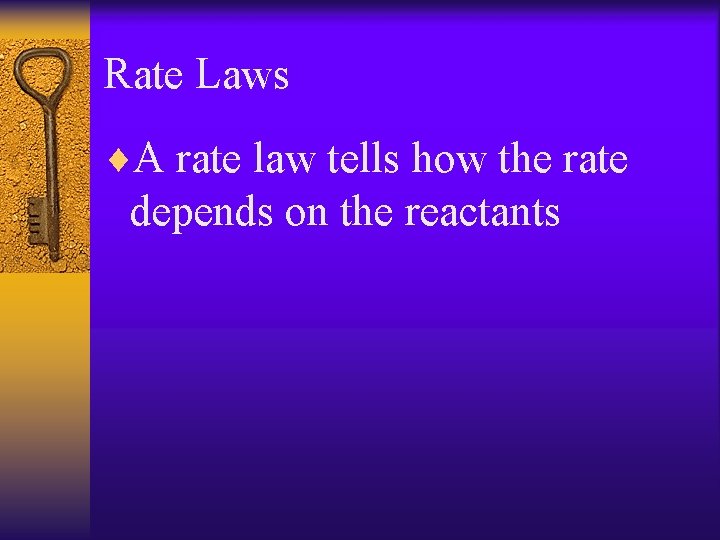
Rate Laws ¨a. A + b. B c. C + d. D ¨Rate= k[A]m[B]n ¨k is the rate constant affected by T ¨m and n are the rxn orders

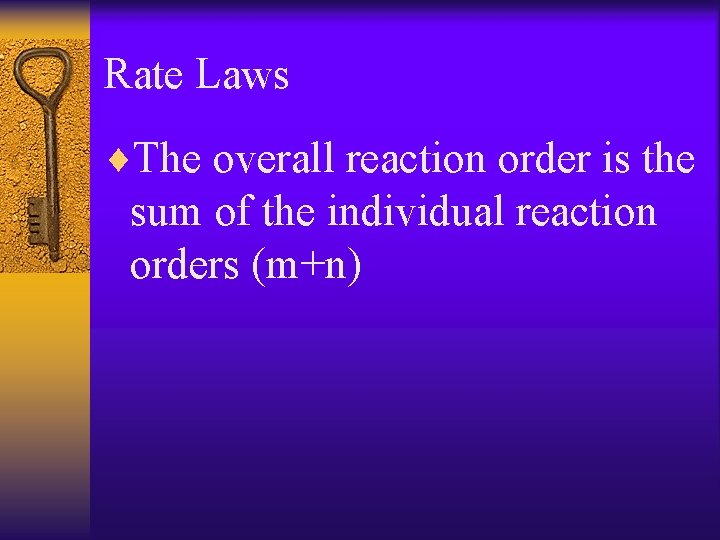
Rate Laws ¨The overall reaction order is the sum of the individual reaction orders (m+n)

Rate Laws ¨The exponents in the rate law indicate how the rate is affected by the concentration of each reactant

Rate Laws ¨A rxn order of 0 means that if the conc. doubles, the rate stays the same (no change to rate)

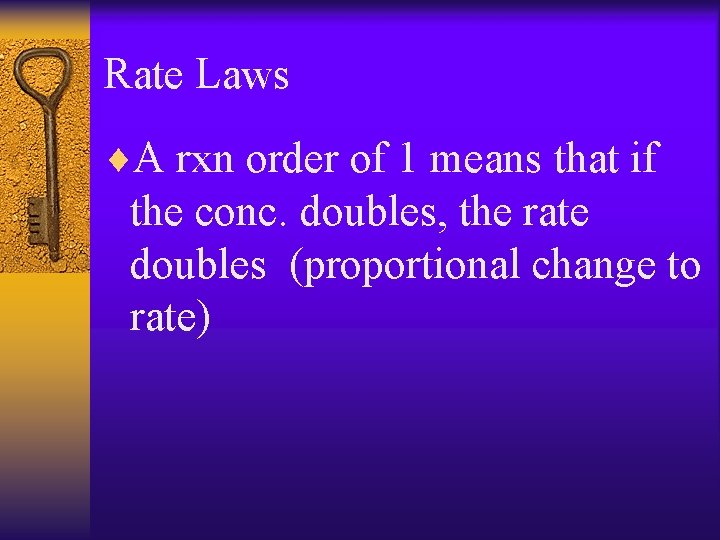
Rate Laws ¨A rxn order of 1 means that if the conc. doubles, the rate doubles (proportional change to rate)

Rate Laws ¨A rxn order of 2 means that if the conc. doubles, the rate quadruples (the increase factor is squared and multiplied by rate)

Rate Laws ¨ The values of the exponents must be determined experimentally or based on information from the rxn mechanism ¨ The overall balanced equation does not provide the rate law unless the rxn occurs in one step

Rate Laws ¨The units of rate constants depend on the overall reaction order.

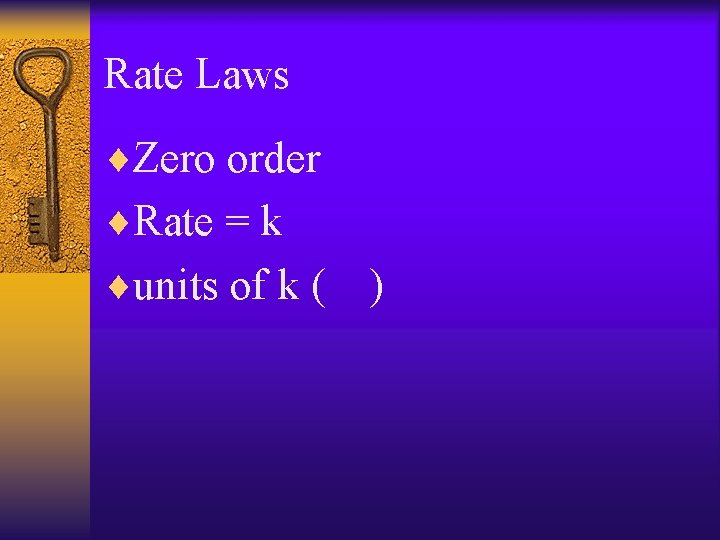
Rate Laws ¨Zero order ¨Rate = k ¨units of k ( )
![Rate Laws 1 st order Rate k A Units of k Rate Laws ¨ 1 st order ¨Rate = k [A] ¨Units of k (](https://slidetodoc.com/presentation_image_h/c3773afdbcfdf52f99a37e2a4004aac6/image-20.jpg)
Rate Laws ¨ 1 st order ¨Rate = k [A] ¨Units of k ( )
![Rate Laws 2 nd order RatekA2 or Rate kAB Units of k Rate Laws ¨ 2 nd order ¨Rate=k[A]2 or Rate = k[A][B] ¨Units of k](https://slidetodoc.com/presentation_image_h/c3773afdbcfdf52f99a37e2a4004aac6/image-21.jpg)
Rate Laws ¨ 2 nd order ¨Rate=k[A]2 or Rate = k[A][B] ¨Units of k ( )
![Rate Laws 3 rd order Rate kA2B Units of k Rate Laws ¨ 3 rd order ¨Rate = k[A]2[B] ¨Units of k ( )](https://slidetodoc.com/presentation_image_h/c3773afdbcfdf52f99a37e2a4004aac6/image-22.jpg)
Rate Laws ¨ 3 rd order ¨Rate = k[A]2[B] ¨Units of k ( )

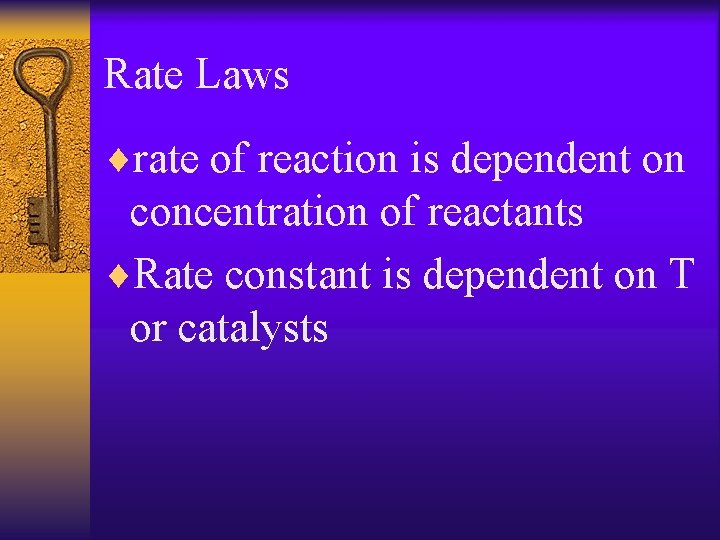
Rate Laws ¨rate of reaction is dependent on concentration of reactants ¨Rate constant is dependent on T or catalysts

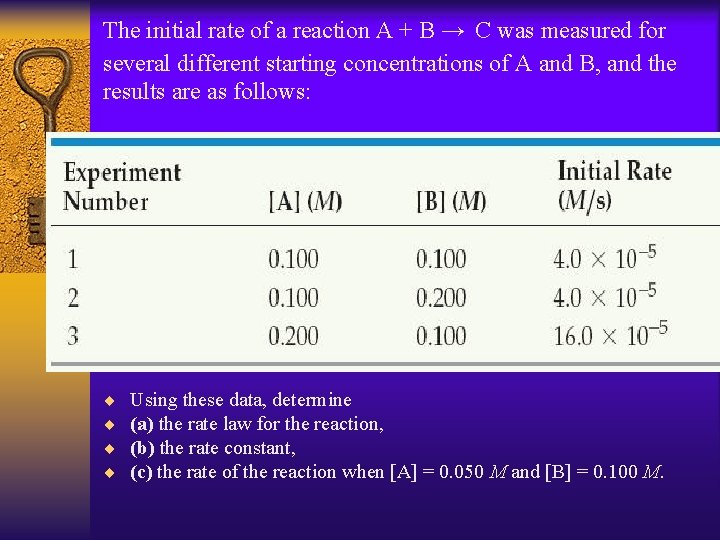
The initial rate of a reaction A + B → C was measured for several different starting concentrations of A and B, and the results are as follows: ¨ ¨ Using these data, determine (a) the rate law for the reaction, (b) the rate constant, (c) the rate of the reaction when [A] = 0. 050 M and [B] = 0. 100 M.

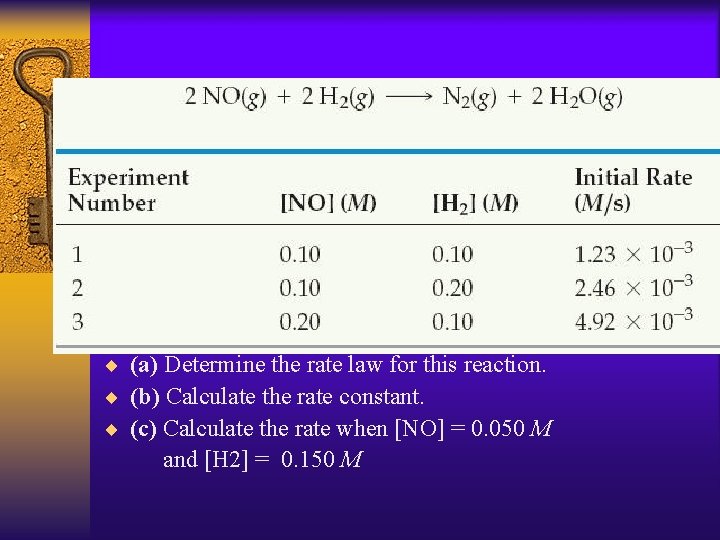
¨ (a) Determine the rate law for this reaction. ¨ (b) Calculate the rate constant. ¨ (c) Calculate the rate when [NO] = 0. 050 M and [H 2] = 0. 150 M

The change of concentration with time ¨A rate law tells how the rate changes at a particular T as the reactant conc. changes st ¨To distinguish between zero, 1 , and 2 nd order rxns, graphs can be plotted
![Zero order rxns Rate DA Dt k Integrated Rate Law Zero order rxns ¨ ¨ ¨ Rate = -D[A]/ Dt =k Integrated Rate Law](https://slidetodoc.com/presentation_image_h/c3773afdbcfdf52f99a37e2a4004aac6/image-27.jpg)
Zero order rxns ¨ ¨ ¨ Rate = -D[A]/ Dt =k Integrated Rate Law [A]t = -kt + [A]0 y = mx + b A graph of concentration versus time yields a straight line with slope -k
![First order rxns Rate DA Dt kA Integrate Rate Law First order rxns ¨ ¨ ¨ Rate = -D[A]/ Dt =k[A] Integrate Rate Law](https://slidetodoc.com/presentation_image_h/c3773afdbcfdf52f99a37e2a4004aac6/image-28.jpg)
First order rxns ¨ ¨ ¨ Rate = -D[A]/ Dt =k[A] Integrate Rate Law ln [A]t = -kt + ln[A]0 y = mx + b A graph of ln[A]t vs. time will yield a straight line with slope –k and y-intercept ln[A]0
![Second order rxns Rate DA Dt kA2 1At kt 1A0 Second order rxns ¨ Rate = -D[A]/ Dt =k[A]2 ¨ 1/[A]t = kt +1/[A]0](https://slidetodoc.com/presentation_image_h/c3773afdbcfdf52f99a37e2a4004aac6/image-29.jpg)
Second order rxns ¨ Rate = -D[A]/ Dt =k[A]2 ¨ 1/[A]t = kt +1/[A]0 ¨ y = mx + b ¨ A graph of 1/[A]t vs. time will yield a straight line with slope k and y-intercept 1/[A]0

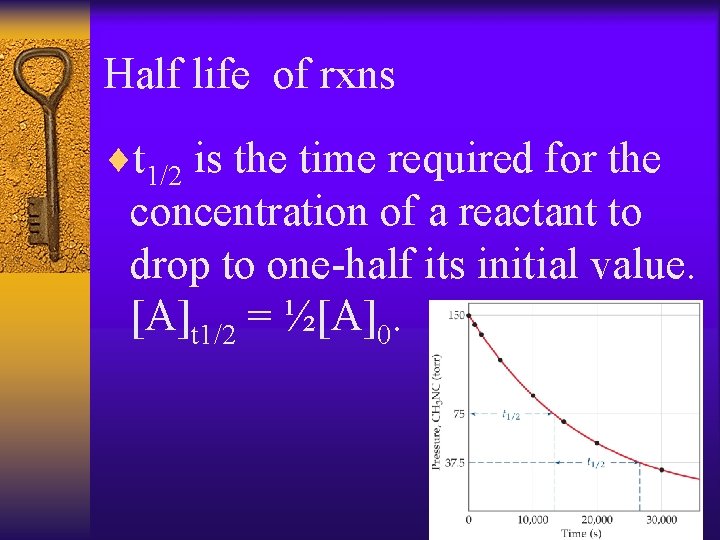
Half life of rxns ¨t 1/2 is the time required for the concentration of a reactant to drop to one-half its initial value. [A]t 1/2 = ½[A]0.

Half life of rxns ¨For 1 st order rxns ¨t 1/2 = 0. 693/k ¨For 2 nd order rxns ¨ t 1/2= 1/k[A]0

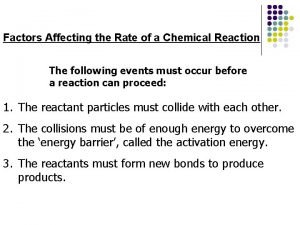
Temperature and Rate ¨ The Collision Theory ¨ the rate of rxn depends on the number of collisions between molecules or ions, the average energy of the collisions, and their effectiveness – Temperature, concentration, orientation, and activation energy affect collisions and rate

Temperature and Rate ¨ The Collision Theory ¨ Does the general effect of concentration and T on reaction rate support the collision theory? Explain.


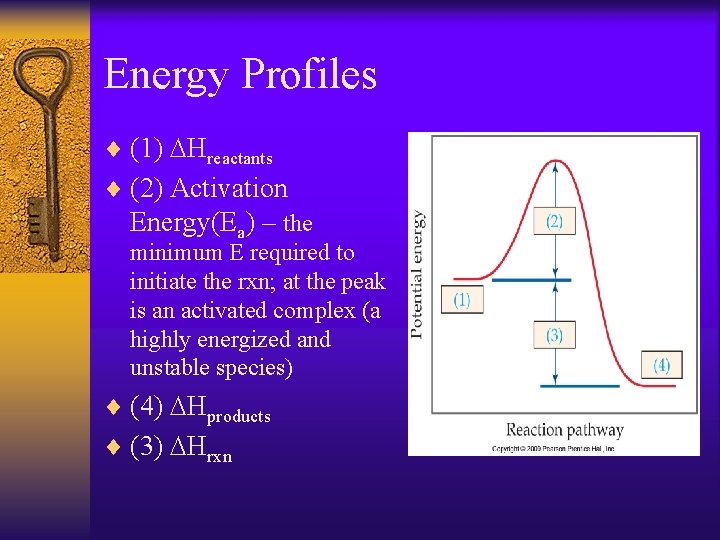
Energy Profiles ¨ (1) DHreactants ¨ (2) Activation Energy(Ea) – the minimum E required to initiate the rxn; at the peak is an activated complex (a highly energized and unstable species) ¨ (4) DHproducts ¨ (3) DHrxn

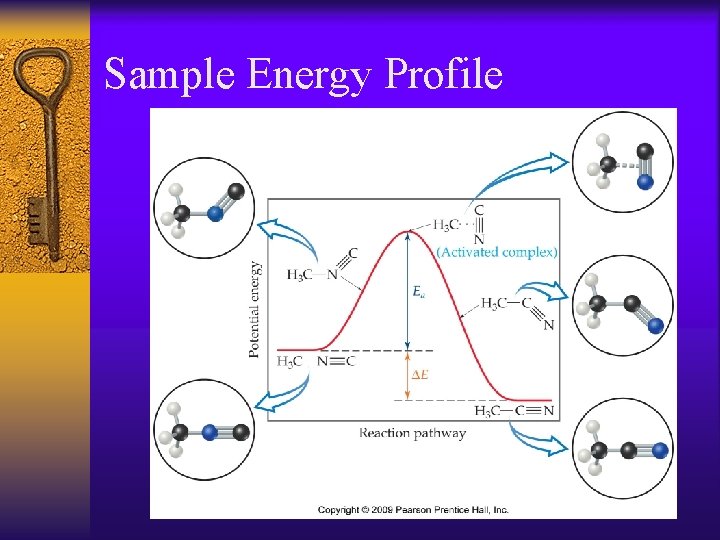
Sample Energy Profile

The Arrhenius Equation ¨k = Ae-Ea/RT ¨k = rate constant ¨Ea ¨R = 8. 314 J/mol-K ¨T ¨A is frequency factor

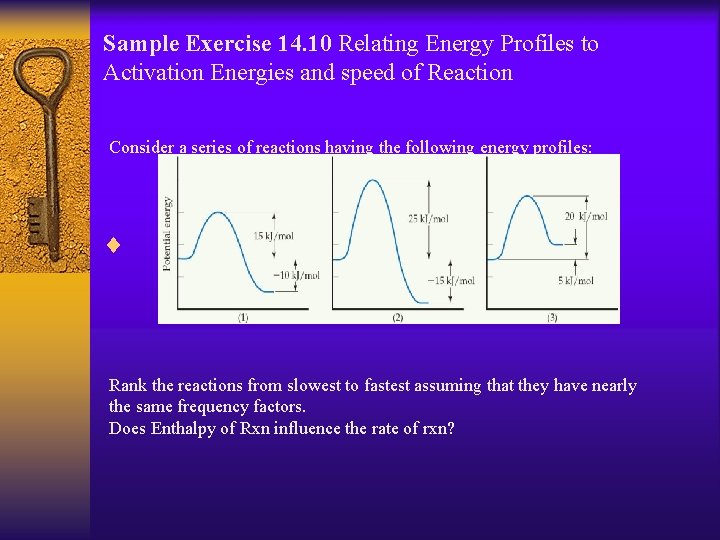
Sample Exercise 14. 10 Relating Energy Profiles to Activation Energies and speed of Reaction Consider a series of reactions having the following energy profiles: ¨ Rank the reactions from slowest to fastest assuming that they have nearly the same frequency factors. Does Enthalpy of Rxn influence the rate of rxn?

Reaction Mechanisms ¨The process by which a rxn occurs is a rxn mechanism which will describe the order that the bonds are broken and formed and changes positions of atoms

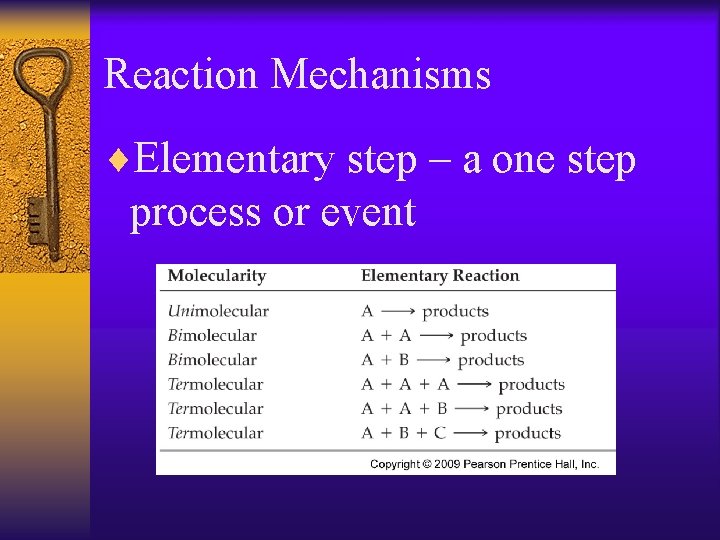
Reaction Mechanisms ¨Elementary step – a one step process or event

Reaction Mechanisms ¨Multistep Mechanism – A net change represented by a balanced chemical equation consisting of a sequence of elementary steps

Reaction Mechanisms ¨The elementary steps in a multistep mechanism must always add to give the chemical equation of the overall process

Reaction Mechanisms ¨An intermediate is a substance that is neither a product or reactant, it is formed in one elementary step and consumed in another

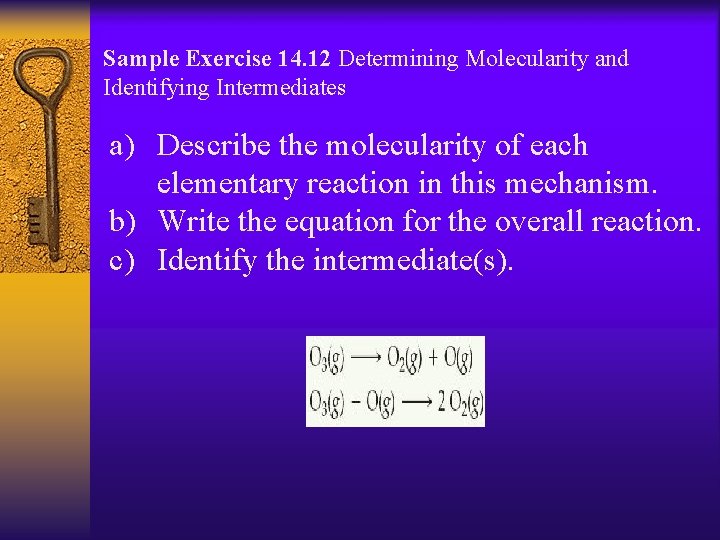
Sample Exercise 14. 12 Determining Molecularity and Identifying Intermediates a) Describe the molecularity of each elementary reaction in this mechanism. b) Write the equation for the overall reaction. c) Identify the intermediate(s).

Sample Exercise 14. 12 Determining Molecularity and Identifying Intermediates Practice Exercise For the reaction the proposed mechanism is (a) Is the proposed mechanism consistent with the equation for the overall reaction? (b) What is the molecularity of each step of the mechanism? (c) Identify the intermediate(s).

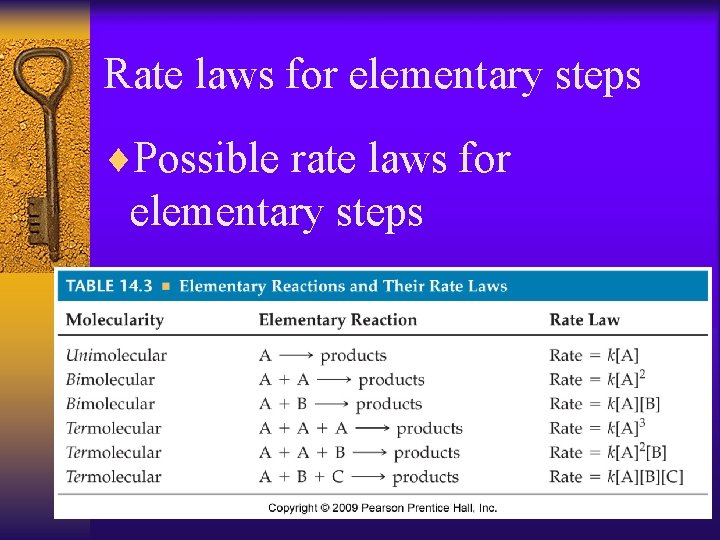
Rate laws for elementary steps ¨If we know a reaction is an elementary step then the rate law is based on molecularity

Rate laws for elementary steps ¨Possible rate laws for elementary steps

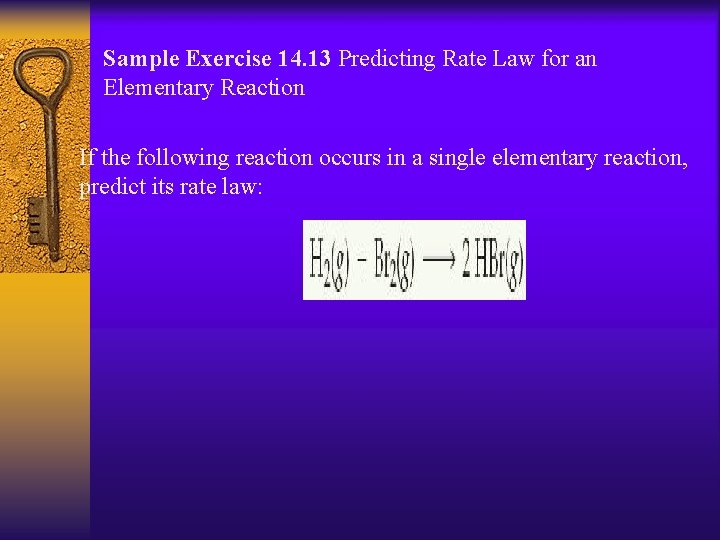
Sample Exercise 14. 13 Predicting Rate Law for an Elementary Reaction If the following reaction occurs in a single elementary reaction, predict its rate law:

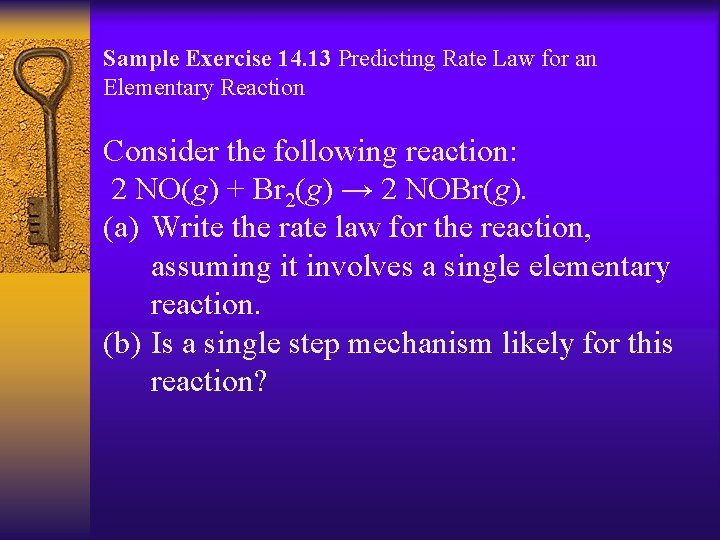
Sample Exercise 14. 13 Predicting Rate Law for an Elementary Reaction Consider the following reaction: 2 NO(g) + Br 2(g) → 2 NOBr(g). (a) Write the rate law for the reaction, assuming it involves a single elementary reaction. (b) Is a single step mechanism likely for this reaction?

Rate laws for multistep mechanism ¨the overall rxn rate can not exceed the rate of the slowest elementary step of its mechanism

Rate laws for multistep mechanism ¨rate-determining step = slow step

Rate laws for multistep mechanism ¨If the rate determining step is the first step, then the faster step has no effect on the overall rate

Rate laws for multistep mechanism ¨If the slow step is preceded by fast steps, intermediates are formed and accumulate before being used in the slow step

Sample Exercise 14. 14 Determining the Rate Law for a Multistep Mechanism The decomposition of nitrous oxide, N 2 O, is believed to occur by a two-step mechanism: (a) Write the equation for the overall reaction. (b) Write the rate law for the overall reaction.

Sample Exercise 14. 14 Determining the Rate Law for a Multistep Mechanism Ozone reacts with nitrogen dioxide to produce dinitrogen pentoxide and oxygen: The reaction is believed to occur in two steps: The experimental rate law is rate = k[O 3][NO 2]. What can you say about the relative rates of the two steps of the mechanism?

Sample Exercise 14. 15 Deriving the Rate Law for a Mechanism with a Fast Initial Step Prove the rate law is R=k[NO]2[Br 2]

Sample Exercise 14. 15 Deriving the Rate Law for a Mechanism with a Fast Initial Step The first step of a mechanism involving the reaction of bromine is What is the expression relating the concentration of Br(g) to that of Br 2(g)?

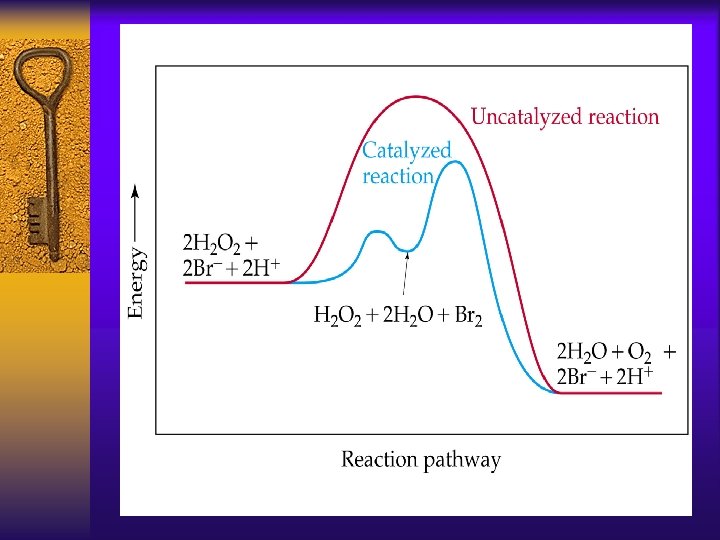
Catalysis ¨a catalyst is a substance that can increase or decrease the speed of a chemical rxn without a change in chemical composition by altering Ea or A and providing a different mechanism

Catalysis ¨It is not consumed in a chemical reaction and can be recovered at the end


 Difference between nuclear reaction and chemical reaction
Difference between nuclear reaction and chemical reaction Is a ratio a rate
Is a ratio a rate Ratio guided notes
Ratio guided notes Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates Kinetics reaction
Kinetics reaction Molecularity of reaction
Molecularity of reaction Chemistry grade 11 unit 4
Chemistry grade 11 unit 4 Order kinetics
Order kinetics Chemical kinetics definition
Chemical kinetics definition Chemical kinetics experiment
Chemical kinetics experiment Applications of chemical kinetics
Applications of chemical kinetics Steady state approximation examples
Steady state approximation examples Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Chapter 18 review chemical equilibrium section 3 answer key
Chapter 18 review chemical equilibrium section 3 answer key Section 4 reaction rates and equilibrium
Section 4 reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Expressing reaction rates
Expressing reaction rates Rate of reaction quiz
Rate of reaction quiz Expressing reaction rates
Expressing reaction rates Reaction rates
Reaction rates How to find speed
How to find speed Narrow field of vision of 140 degrees or less
Narrow field of vision of 140 degrees or less Speed detection of moving vehicle using speed cameras ppt
Speed detection of moving vehicle using speed cameras ppt Enzymes affect the reaction in living cells by changing the
Enzymes affect the reaction in living cells by changing the Reaction rate formula
Reaction rate formula Ictahedron
Ictahedron Leukoerythroblastic reaction vs leukemoid reaction
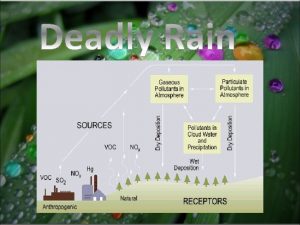
Leukoerythroblastic reaction vs leukemoid reaction Chemical reaction of acid rain
Chemical reaction of acid rain Chemical reaction examples
Chemical reaction examples Types of reaction
Types of reaction Cathode and anode
Cathode and anode Stoichiometry refers to
Stoichiometry refers to Rancimat principle
Rancimat principle Rate of reaction formula
Rate of reaction formula Which type of reaction
Which type of reaction Percent yield
Percent yield Why is pyrrole aromatic
Why is pyrrole aromatic Rancidity chemical reaction
Rancidity chemical reaction Factors affecting rate of chemical reaction
Factors affecting rate of chemical reaction Phosphorus reaction with oxygen equation
Phosphorus reaction with oxygen equation Incomplete combustion of hexane
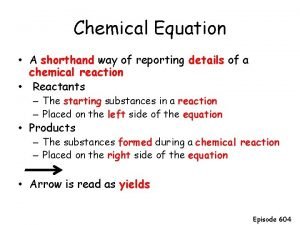
Incomplete combustion of hexane Chemist shorthand way of representing chemical reaction
Chemist shorthand way of representing chemical reaction Lipids definition
Lipids definition Rules of chemical reaction
Rules of chemical reaction Five chemical
Five chemical Can we rearrange atoms
Can we rearrange atoms Change in temperature chemical reaction example
Change in temperature chemical reaction example A chemists shorthand way of representing chemical reaction
A chemists shorthand way of representing chemical reaction Chemist shorthand way of representing chemical reaction
Chemist shorthand way of representing chemical reaction Acid rain chemical reaction
Acid rain chemical reaction 5 indicators of a chemical change
5 indicators of a chemical change What are 5 indicators of a chemical change
What are 5 indicators of a chemical change Chemical reaction examples
Chemical reaction examples Chemical reaction in bread
Chemical reaction in bread Is the halloween clock reaction a chemical change
Is the halloween clock reaction a chemical change Double replacement example
Double replacement example The starting substances in a chemical reaction.
The starting substances in a chemical reaction. Is photosynthesis endothermic
Is photosynthesis endothermic Chemical reaction engineering
Chemical reaction engineering