Kinetics Kinetics Kinetics rates of chemical reactions and










- Slides: 25

Kinetics

Kinetics • Kinetics - rates of chemical reactions and the mechanisms by which they occur • Rate of a chemical reaction - change in the concentration of products and reactants in a given time

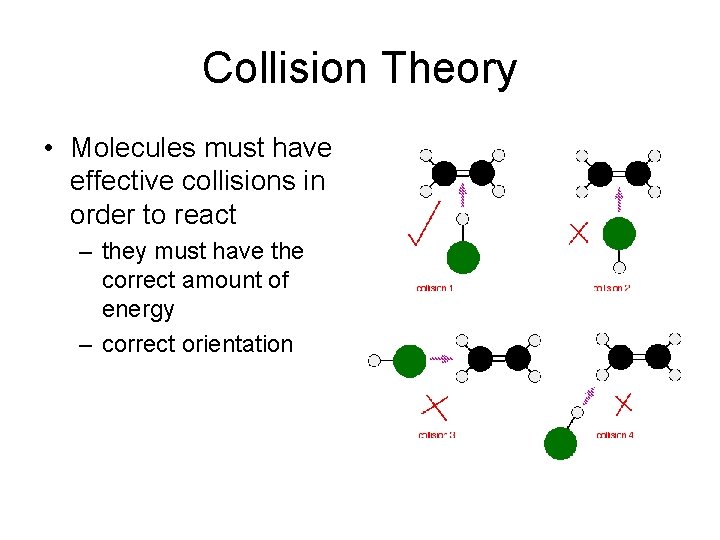
Collision Theory • Molecules must have effective collisions in order to react – they must have the correct amount of energy – correct orientation

Collision Theory • Not all collisions are successful • How to increase the rate of a reaction? – Increase the number of collisions – Increase the effectiveness of the collisions

Factors that Affect Reaction Rates • Nature of the Reactants • Anything that increases the number of collisions will increase the reaction rate – Concentration – Temperature – Catalyst – Surface Area – Pressure (only for gases)

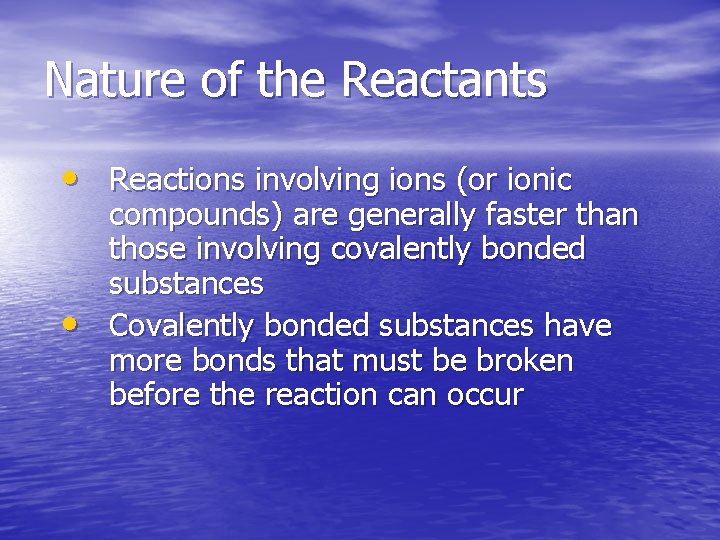
Nature of the Reactants • Reactions involving ions (or ionic • compounds) are generally faster than those involving covalently bonded substances Covalently bonded substances have more bonds that must be broken before the reaction can occur

Nature of the Reactants § Energy required to break bonds is proportional to the stability of the bond – More stable bonds (stronger bond) require more energy to break, slower reaction, less reactive – Weaker bonds are broken with less energy, faster reaction, more reactive

Concentration • When the concentration of one or more of the reactants is increased, the reaction proceeds faster – As concentration increases, there are more particles, more likely to collide

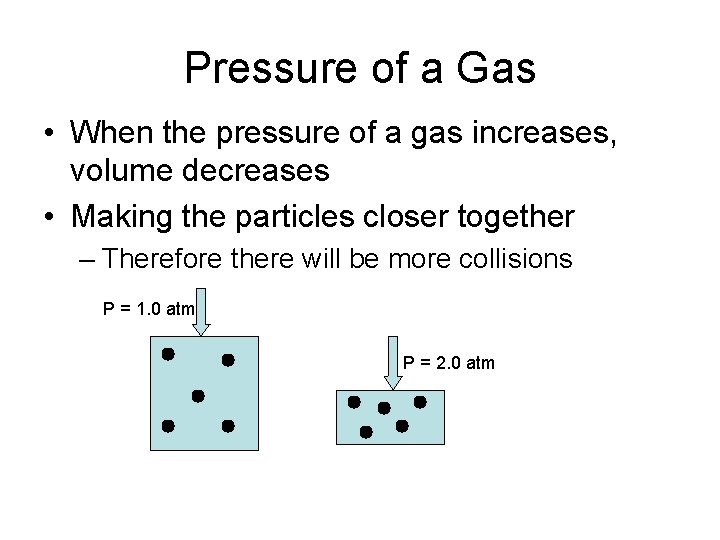
Pressure of a Gas • When the pressure of a gas increases, volume decreases • Making the particles closer together – Therefore there will be more collisions P = 1. 0 atm P = 2. 0 atm

Temperature • An increase in temperature increases the rate of a reaction – Higher temperatures cause particles to move faster and have more kinetic energy – Therefore, more collisions and more effective collisions, due to the increased kinetic energy

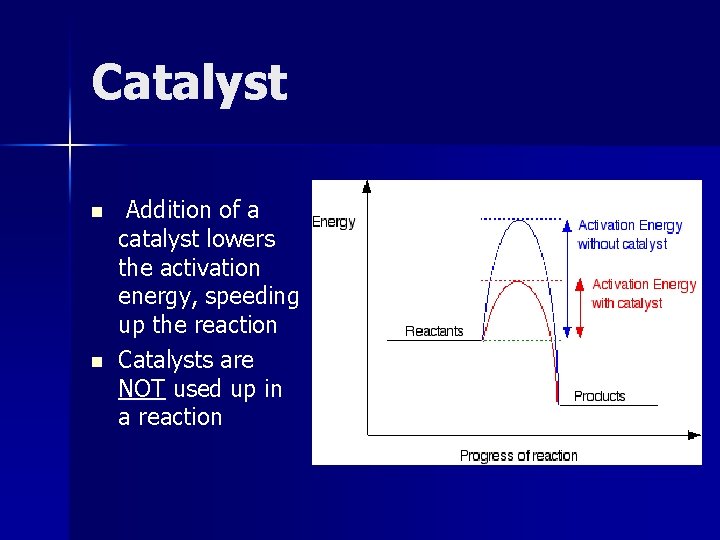
Catalyst n n Addition of a catalyst lowers the activation energy, speeding up the reaction Catalysts are NOT used up in a reaction

Surface Area n More surface area, faster reaction – With more area exposed, there will be more collisions Example: Both samples represent 2. 5 g of Mg, which would react faster with 25 m. L of 1. 0 M HCl? Sample A Sample B

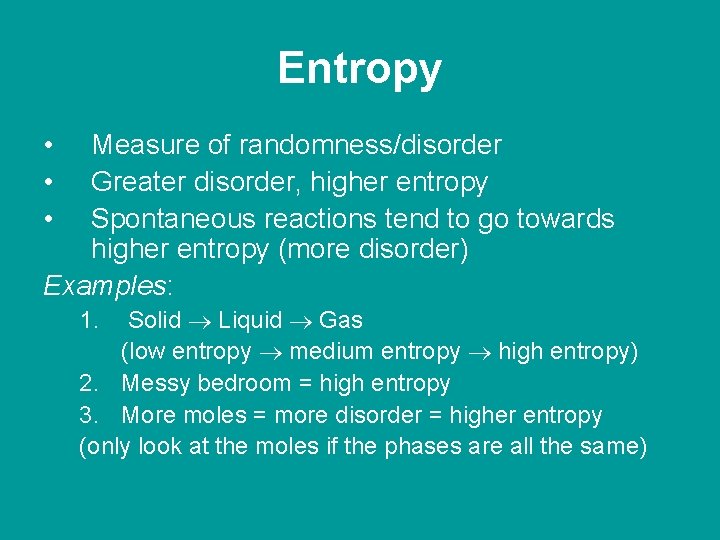
Entropy • • • Measure of randomness/disorder Greater disorder, higher entropy Spontaneous reactions tend to go towards higher entropy (more disorder) Examples: Solid Liquid Gas (low entropy medium entropy high entropy) 2. Messy bedroom = high entropy 3. More moles = more disorder = higher entropy (only look at the moles if the phases are all the same) 1.

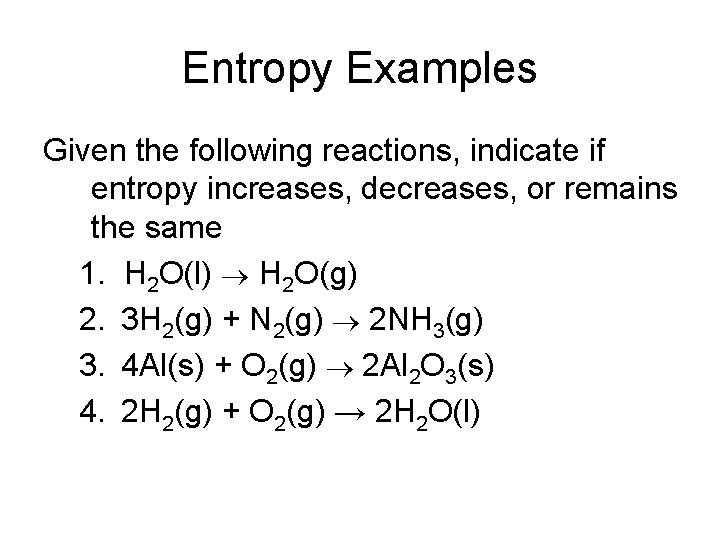
Entropy Examples Given the following reactions, indicate if entropy increases, decreases, or remains the same 1. H 2 O(l) H 2 O(g) 2. 3 H 2(g) + N 2(g) 2 NH 3(g) 3. 4 Al(s) + O 2(g) 2 Al 2 O 3(s) 4. 2 H 2(g) + O 2(g) → 2 H 2 O(l)

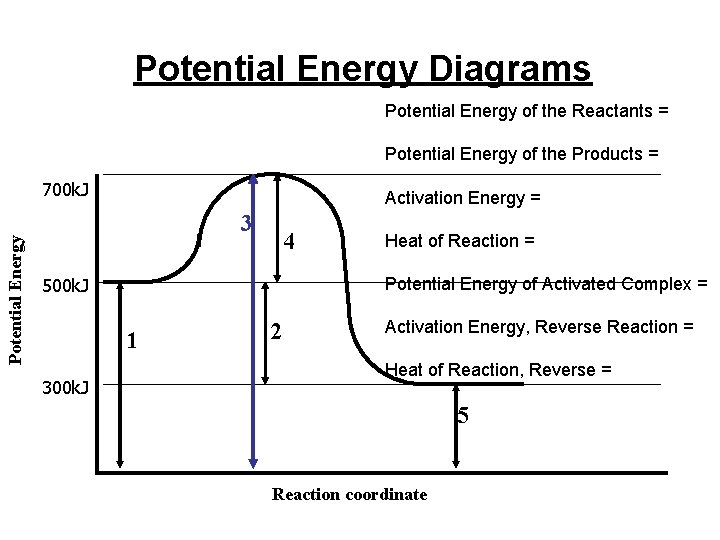
Potential Energy Diagrams Potential Energy of the Reactants = Potential Energy of the Products = Potential Energy 700 k. J Activation Energy = 3 4 500 k. J Potential Energy of Activated Complex = 1 300 k. J Heat of Reaction = 2 Activation Energy, Reverse Reaction = Heat of Reaction, Reverse = 5 Reaction coordinate

Activation Energy Minimum energy required to initiate a chemical reaction (energy to break bonds) Equal to the difference between the Potential Energy of activated complex and potential energy of the reactants n difference from the starting point to the top The Larger the Activation Energy, the slower the reaction

Activated Complex Highest point on Potential Energy curve It represents a transition state between the products and reactants

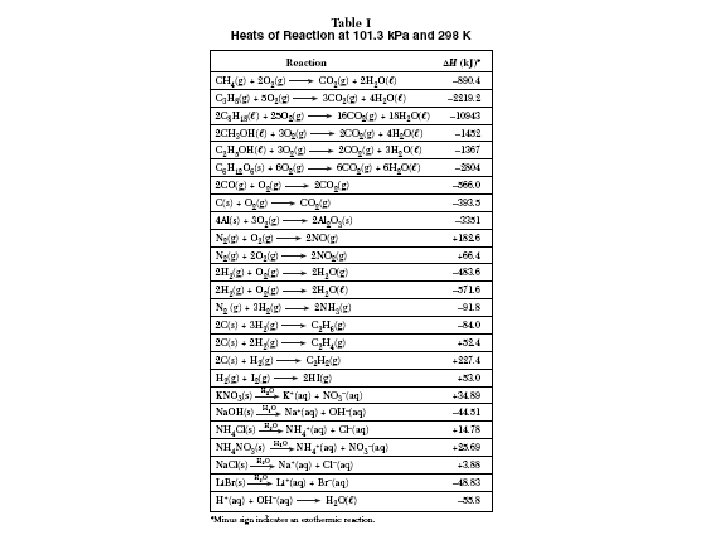
Heat of Reaction (Enthalpy, H) Difference between the potential energy of the products and the potential energy of the reactants Energy given off or absorbed by the reaction Found on Reference Table I H = Hp - Hr

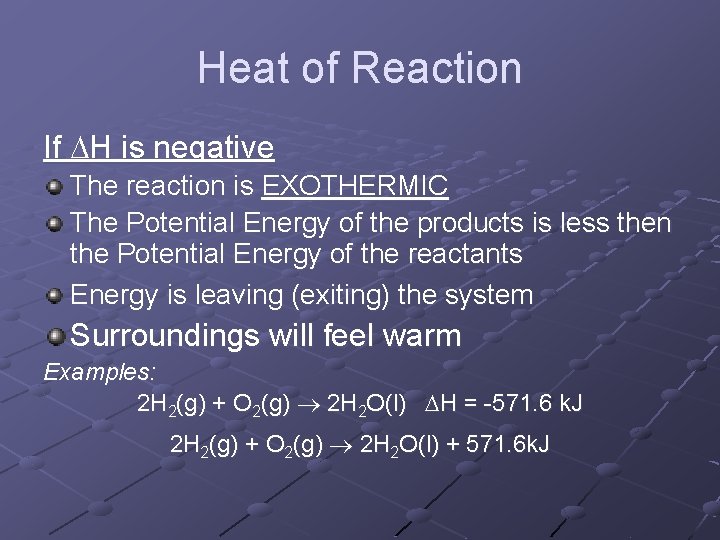
Heat of Reaction If H is negative The reaction is EXOTHERMIC The Potential Energy of the products is less then the Potential Energy of the reactants Energy is leaving (exiting) the system Surroundings will feel warm Examples: 2 H 2(g) + O 2(g) 2 H 2 O(l) H = -571. 6 k. J 2 H 2(g) + O 2(g) 2 H 2 O(l) + 571. 6 k. J

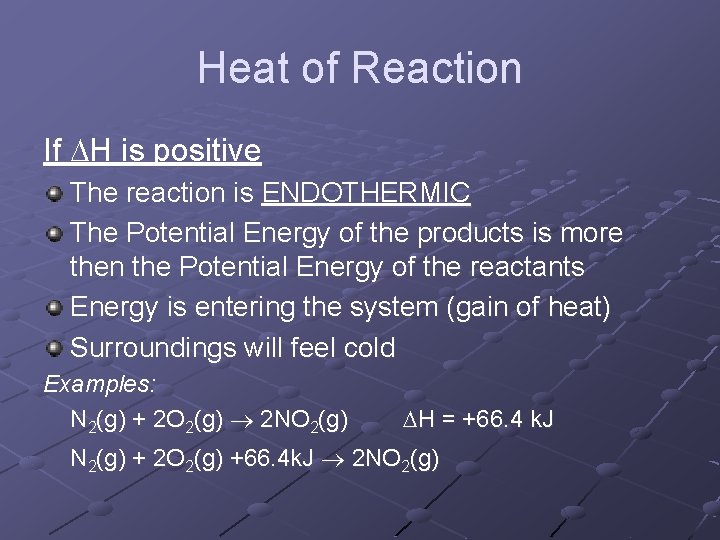
Heat of Reaction If H is positive The reaction is ENDOTHERMIC The Potential Energy of the products is more then the Potential Energy of the reactants Energy is entering the system (gain of heat) Surroundings will feel cold Examples: N 2(g) + 2 O 2(g) 2 NO 2(g) H = +66. 4 k. J N 2(g) + 2 O 2(g) +66. 4 k. J 2 NO 2(g)


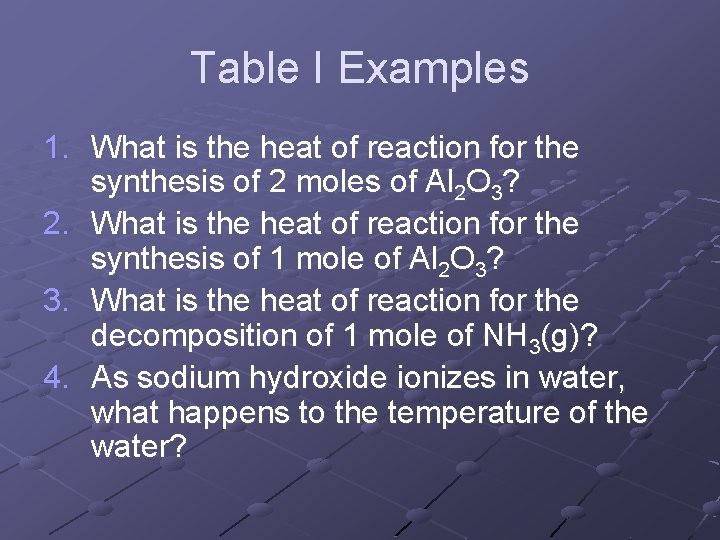
Table I Examples 1. What is the heat of reaction for the synthesis of 2 moles of Al 2 O 3? 2. What is the heat of reaction for the synthesis of 1 mole of Al 2 O 3? 3. What is the heat of reaction for the decomposition of 1 mole of NH 3(g)? 4. As sodium hydroxide ionizes in water, what happens to the temperature of the water?

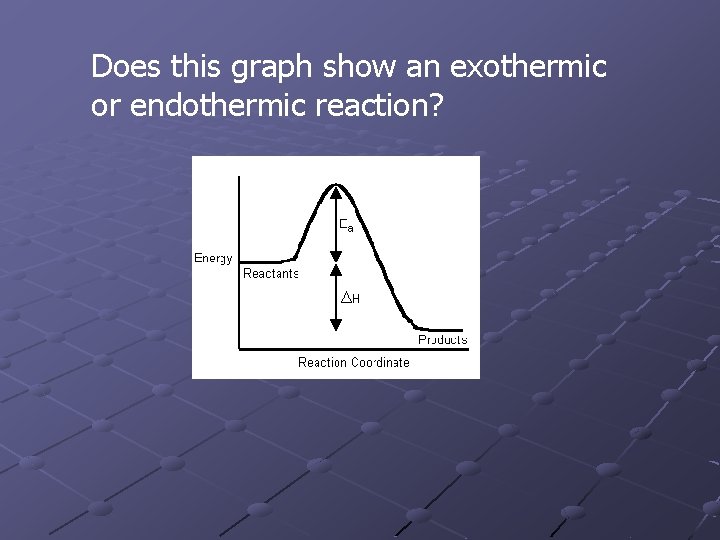
Does this graph show an exothermic or endothermic reaction?

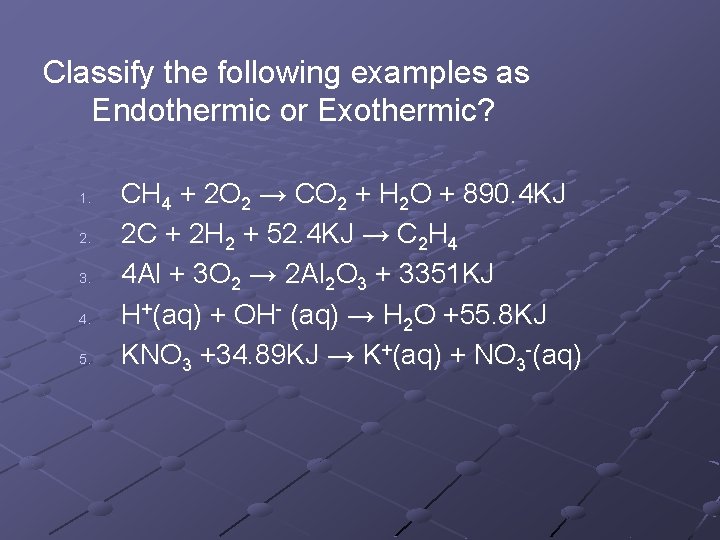
Classify the following examples as Endothermic or Exothermic? 1. 2. 3. 4. 5. CH 4 + 2 O 2 → CO 2 + H 2 O + 890. 4 KJ 2 C + 2 H 2 + 52. 4 KJ → C 2 H 4 4 Al + 3 O 2 → 2 Al 2 O 3 + 3351 KJ H+(aq) + OH- (aq) → H 2 O +55. 8 KJ KNO 3 +34. 89 KJ → K+(aq) + NO 3 -(aq)

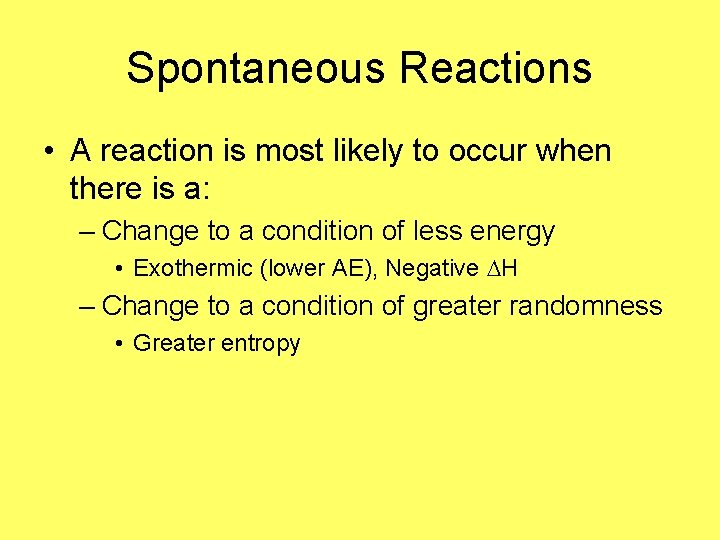
Spontaneous Reactions • A reaction is most likely to occur when there is a: – Change to a condition of less energy • Exothermic (lower AE), Negative H – Change to a condition of greater randomness • Greater entropy