Reaction Kinetics Reaction Kinetics The study of the


- Slides: 9

Reaction Kinetics

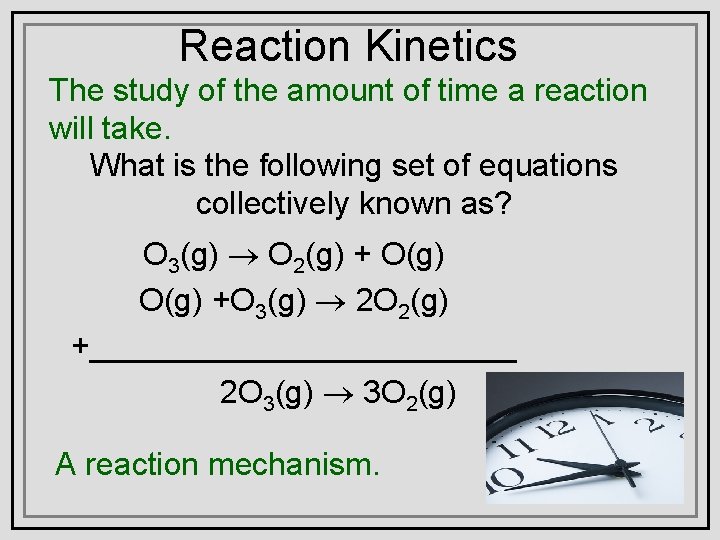
Reaction Kinetics The study of the amount of time a reaction will take. What is the following set of equations collectively known as? O 3(g) ® O 2(g) + O(g) +O 3(g) ® 2 O 2(g) +____________ 2 O 3(g) ® 3 O 2(g) A reaction mechanism.

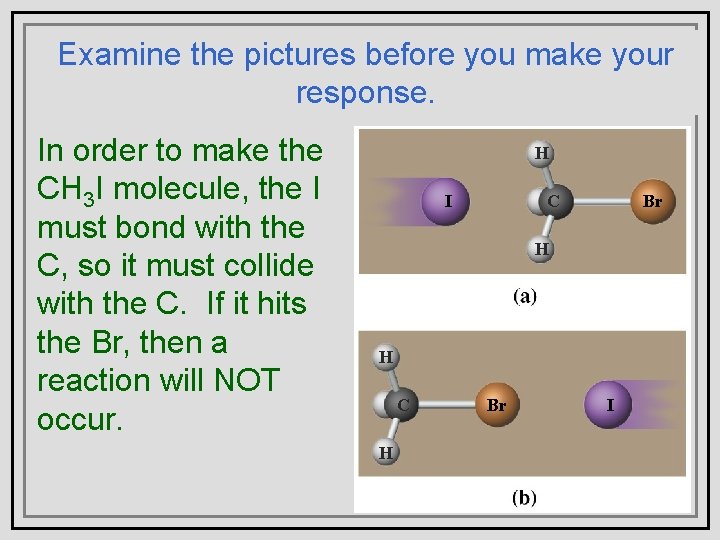
Examine the pictures before you make your Collision Theory response. • In. Picture shows the order (a) to make correct orientation CH I molecule, the I 3 whilebond (b) does must withnot. the • C, Inso order to make a it must collide molecule of CH 3 I from with the C. If it hits an atom of I and a the Br, then a Br, molecule of CH 3 reaction willorientation NOT why is the occur. of the collision important? H I C Br H H C H Br I

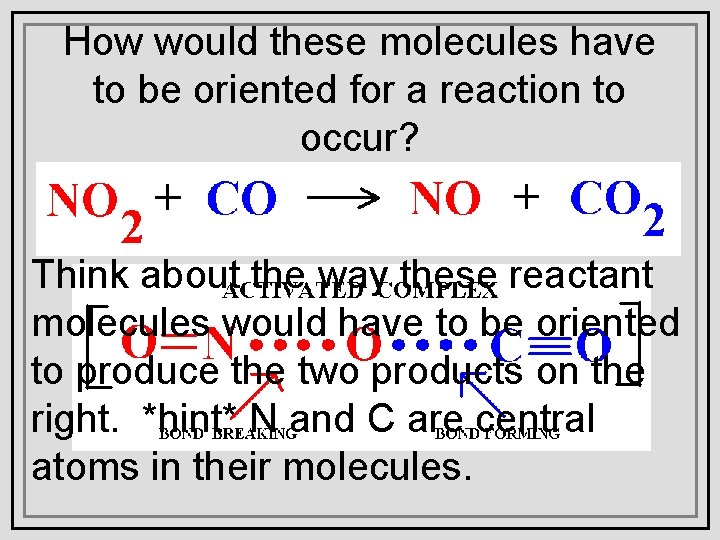
How would these molecules have to be oriented for a reaction to occur? Think about the way these reactant molecules would have to be oriented to produce the two products on the right. *hint* N and C are central atoms in their molecules.

Rate Influencing Factors Collision Theory • orientation • . surface area • temperature • concentration / pressure of reactants • presence of a catalyst

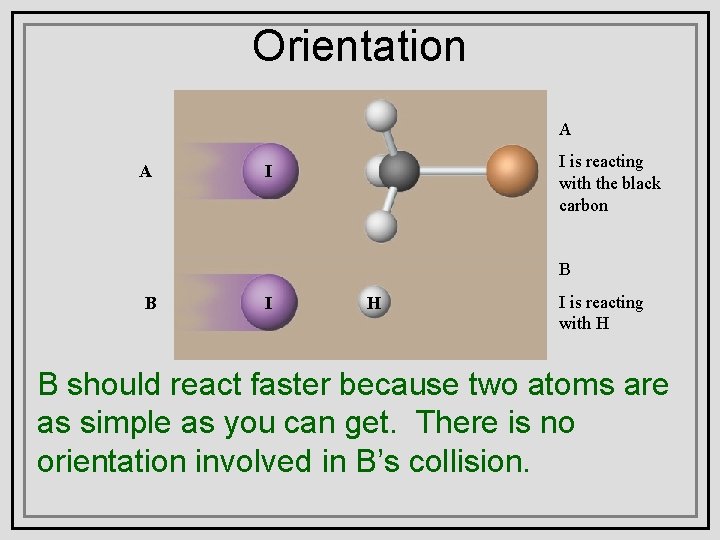
Orientation A A I is reacting with the black carbon I B B I H I is reacting with H BWhich should faster because A two are setreact of two substances, or atoms B, do you asthink simple you faster? can get. Why? There is no willasreact orientation involved in B’s collision.

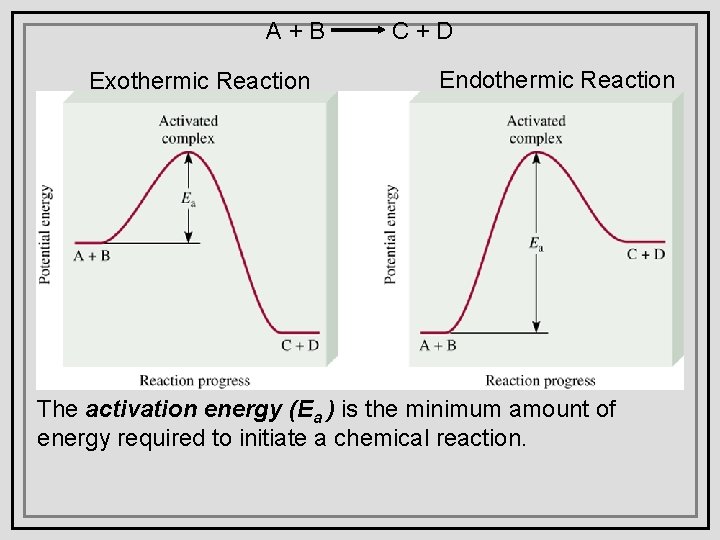
A+B Exothermic Reaction C+D Endothermic Reaction The activation energy (Ea ) is the minimum amount of energy required to initiate a chemical reaction.

Activation Energy. . • . . is energy needed so there is enough energy to break reactant bonds. • . . is the energy needed to get molecules in the correct orientation. • . . is the energy needed to reach the transition state or activated complex Activation energy is always a positive number

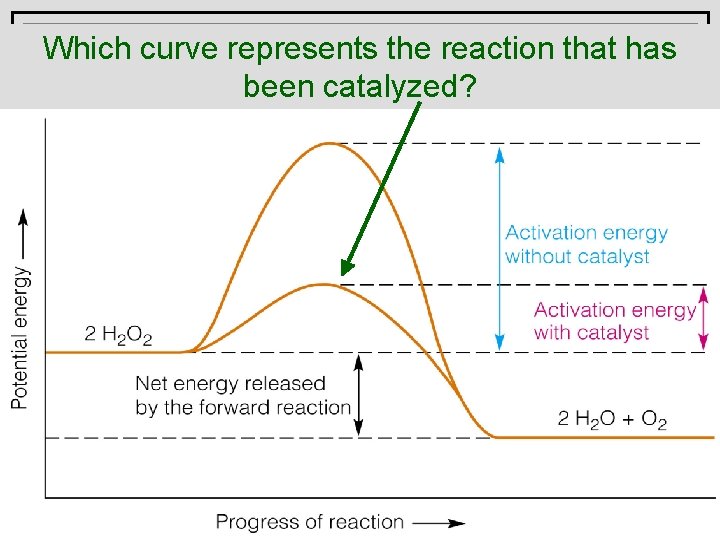
A catalyst Which curve represents the reaction that has beenactivation catalyzed? energy. lowers the
 Kinetics reaction
Kinetics reaction Unit of rate of reaction for first order reaction
Unit of rate of reaction for first order reaction Addition reaction and substitution reaction
Addition reaction and substitution reaction Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Half life formula
Half life formula Molecularity of reaction
Molecularity of reaction Kinetics and equilibrium
Kinetics and equilibrium First order drug elimination
First order drug elimination Planar kinetics of a rigid body
Planar kinetics of a rigid body Difference between 1st order and zero order kinetics
Difference between 1st order and zero order kinetics