Unit 8 Chemical Kinetics Thermodynamics Chemical Kinetics Chemical



![Determining the Order of a Reaction [A] (M) x 2 0. 10 Rate (M/s) Determining the Order of a Reaction [A] (M) x 2 0. 10 Rate (M/s)](https://slidetodoc.com/presentation_image_h2/9ccc2a277d7a4dcef9a6e6646723d007/image-19.jpg)
![Rate Order of a Reaction Sample Problem x 2 [NO 2] (M) 0. 10 Rate Order of a Reaction Sample Problem x 2 [NO 2] (M) 0. 10](https://slidetodoc.com/presentation_image_h2/9ccc2a277d7a4dcef9a6e6646723d007/image-20.jpg)
![Finding the Rate Constant Sample Problem [NO 2] (M) [CO] (M) Rate (M/s) 0. Finding the Rate Constant Sample Problem [NO 2] (M) [CO] (M) Rate (M/s) 0.](https://slidetodoc.com/presentation_image_h2/9ccc2a277d7a4dcef9a6e6646723d007/image-21.jpg)


- Slides: 41

Unit 8 Chemical Kinetics & Thermodynamics

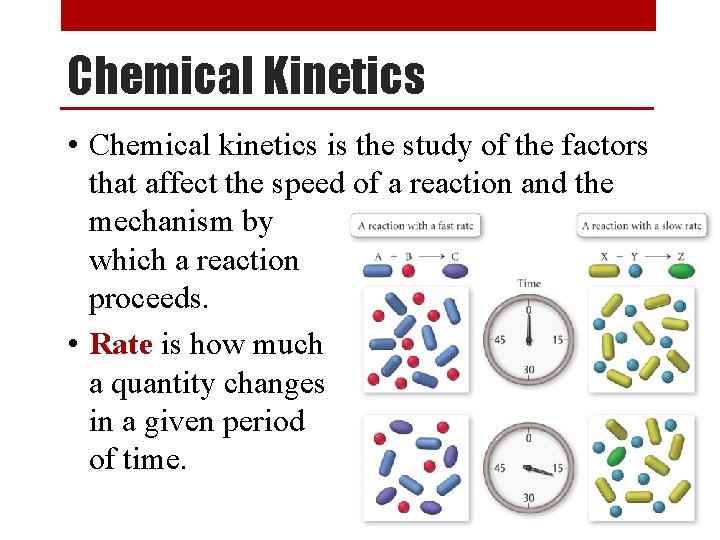
Chemical Kinetics • Chemical kinetics is the study of the factors that affect the speed of a reaction and the mechanism by which a reaction proceeds. • Rate is how much a quantity changes in a given period of time.

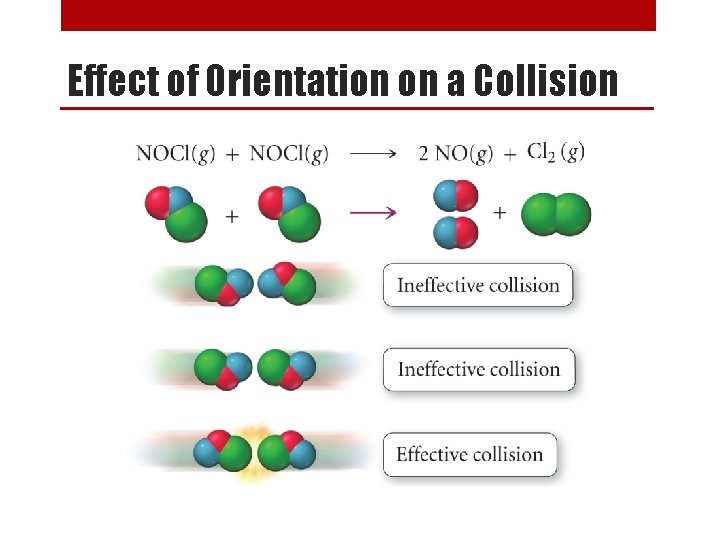
Collision Theory • Collision Theory says that in order for reactions to occur between substances, particles must collide. • For a collision to be effective, particles must: 1. Collide with sufficient energy. 2. Have a favorable orientation.

Effect of Orientation on a Collision

Rate-Influencing Factors • The rate of a chemical reaction depends on the number of collisions between particles. • Rate-influencing factors are: • Nature of Reactants • Surface Area Smaller pieces = larger surface area • Temperature • Concentration • Presence of Catalysts Visual Concept

Effect of Concentration • The rate of a reaction often depends on the concentration of one or more of the reactants. • Greater concentration of reactant particles = more collisions = increase in reaction rate.

Rate Law Expressions • Rate law equations are based on the Molarity concentrations of the reactant(s). (Indicate Molarity concentrations with brackets. ) • The Rate (R) involves the product of the concentrations of the individual reactants. • Experiments have proven that coefficients of reactants have an exponential effect on R. Ex: For the equation R = k [A][B]2 A+2 B→C (k = rate law constant)

Rate Law Expressions Sample Problem For the following single-step chemical reaction: 2 SO 2 + O 2 → 2 SO 3 1. Write the rate law expression. R = k [SO 2]2 [O 2] 2. What happens to the Rate if the [SO 2] is cut by ½? Rate is 4 x slower 3. What happens to the Rate if the [O 2] is tripled? Rate is 3 x greater

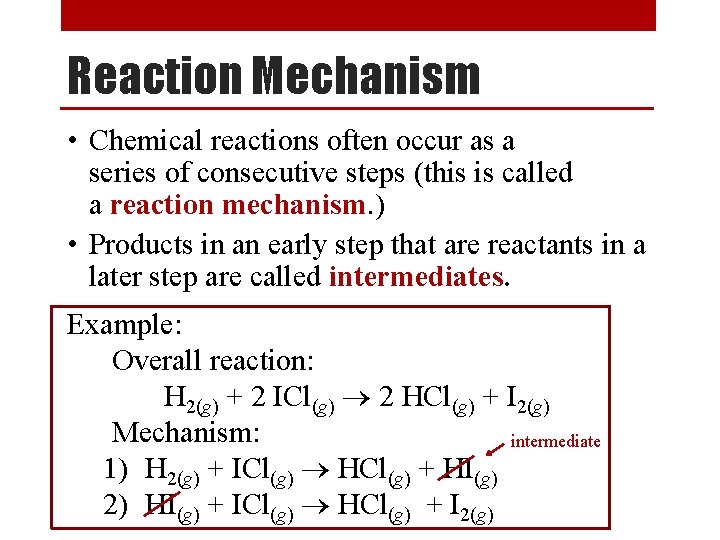
Reaction Mechanism • Chemical reactions often occur as a series of consecutive steps (this is called a reaction mechanism. ) • Products in an early step that are reactants in a later step are called intermediates. Example: Overall reaction: H 2(g) + 2 ICl(g) 2 HCl(g) + I 2(g) Mechanism: intermediate 1) H 2(g) + ICl(g) HCl(g) + HI(g) 2) HI(g) + ICl(g) HCl(g) + I 2(g)

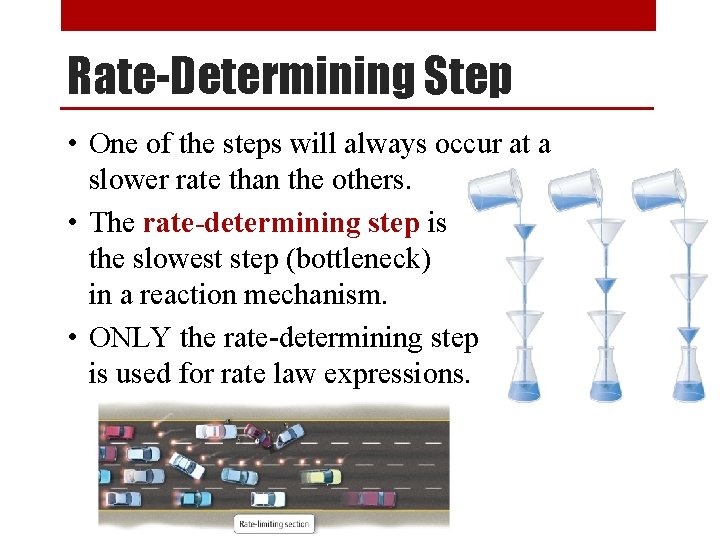
Rate-Determining Step • One of the steps will always occur at a slower rate than the others. • The rate-determining step is the slowest step (bottleneck) in a reaction mechanism. • ONLY the rate-determining step is used for rate law expressions.

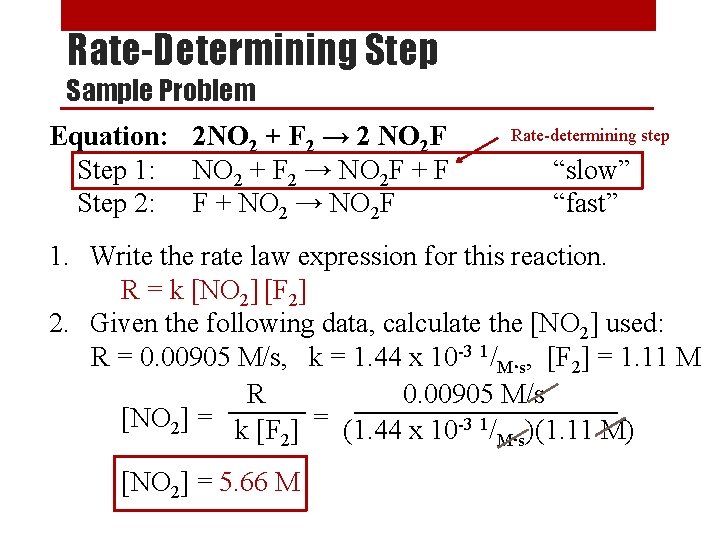
Rate-Determining Step Sample Problem Equation: 2 NO 2 + F 2 → 2 NO 2 F Step 1: NO 2 + F 2 → NO 2 F + F Step 2: F + NO 2 → NO 2 F Rate-determining step “slow” “fast” 1. Write the rate law expression for this reaction. R = k [NO 2] [F 2] 2. Given the following data, calculate the [NO 2] used: R = 0. 00905 M/s, k = 1. 44 x 10 -3 1/M s, [F 2] = 1. 11 M R 0. 00905 M/s [NO 2] = k [F ] = (1. 44 x 10 -3 1/ )(1. 11 M) 2 Ms ● ● [NO 2] = 5. 66 M

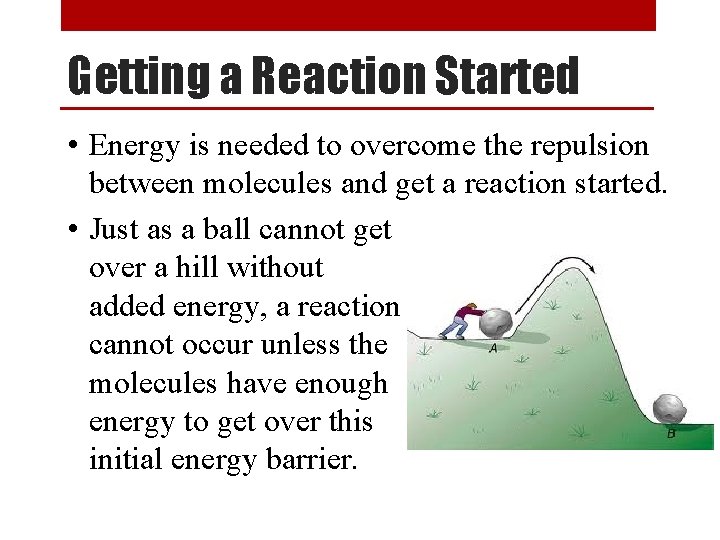
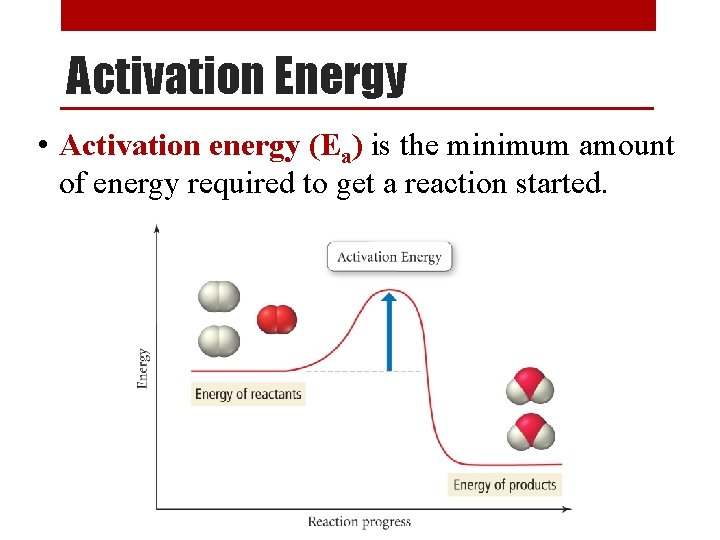
Getting a Reaction Started • Energy is needed to overcome the repulsion between molecules and get a reaction started. • Just as a ball cannot get over a hill without added energy, a reaction cannot occur unless the molecules have enough energy to get over this initial energy barrier.

Activation Energy • Activation energy (Ea) is the minimum amount of energy required to get a reaction started.

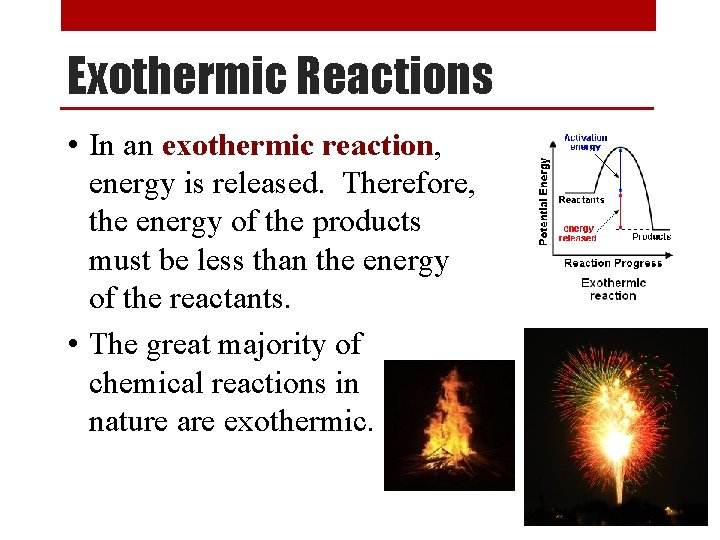
Exothermic Reactions • In an exothermic reaction, energy is released. Therefore, the energy of the products must be less than the energy of the reactants. • The great majority of chemical reactions in nature are exothermic.

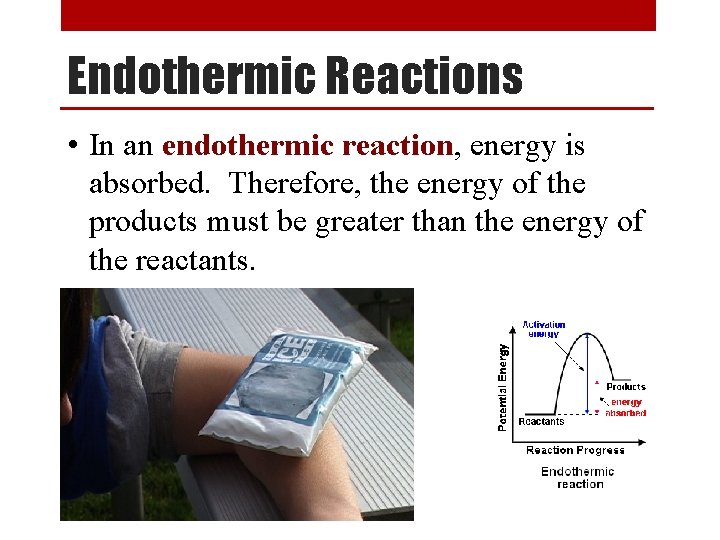
Endothermic Reactions • In an endothermic reaction, energy is absorbed. Therefore, the energy of the products must be greater than the energy of the reactants.

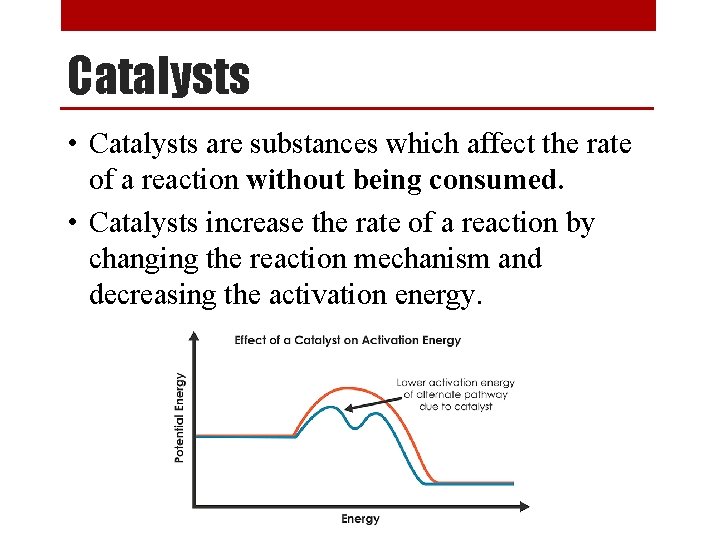
Catalysts • Catalysts are substances which affect the rate of a reaction without being consumed. • Catalysts increase the rate of a reaction by changing the reaction mechanism and decreasing the activation energy.

Enzymes • Due to the complexity of organic molecules, most biological reactions need a catalyst to proceed at a reasonable rate. • Protein molecules that catalyze biological reactions are called enzymes. • Enzymes pull the reactants onto an active site that orients them for the reaction.

Rate Order • Experiments have shown that there are 3 ways that a reactant’s concentration can affect the overall rate of the reaction: • Rate Order 0 ([X]0) - The concentration of a reactant has no effect on reaction rate. • Rate Order 1 ([X]1) - The concentration of a reactant has a direct effect on reaction rate. • Rate Order 2 ([X]2) - The concentration of a reactant has an exponential effect on reaction rate. • The order of a reaction can be determined only by experiment.
![Determining the Order of a Reaction A M x 2 0 10 Rate Ms Determining the Order of a Reaction [A] (M) x 2 0. 10 Rate (M/s)](https://slidetodoc.com/presentation_image_h2/9ccc2a277d7a4dcef9a6e6646723d007/image-19.jpg)
Determining the Order of a Reaction [A] (M) x 2 0. 10 Rate (M/s) [A] (M) Rate (M/s) 0. 015 x 2 0. 10 0. 015 x 1 x 2 0. 10 0. 015 x 4 0. 20 0. 030 0. 20 0. 015 0. 20 0. 060 0. 40 0. 015 0. 40 0. 240 When [A] doubles, the rate doubles When [A] doubles, rate doesn’t change When [A] doubles, the rate quadruples First Order Zero Order Second Order Rate = k[A]1 Rate = k[A]0 = k Rate = k[A]2
![Rate Order of a Reaction Sample Problem x 2 NO 2 M 0 10 Rate Order of a Reaction Sample Problem x 2 [NO 2] (M) 0. 10](https://slidetodoc.com/presentation_image_h2/9ccc2a277d7a4dcef9a6e6646723d007/image-20.jpg)
Rate Order of a Reaction Sample Problem x 2 [NO 2] (M) 0. 10 [CO] (M) Rate (M/s) 0. 10 constant 0. 0021 0. 20 0. 10 0. 20 constant 0. 20 0. 40 0. 10 x 4 0. 0082 x 2 0. 0083 x 1 0. 033 From the data above, determine the following: 1. Rate order of each reactant. second order in [NO 2] and zero order in [CO] 2. Overall rate order of the reaction. 2+0=2 second order reaction 3. The rate law for the reaction. Rate = k [NO 2]2
![Finding the Rate Constant Sample Problem NO 2 M CO M Rate Ms 0 Finding the Rate Constant Sample Problem [NO 2] (M) [CO] (M) Rate (M/s) 0.](https://slidetodoc.com/presentation_image_h2/9ccc2a277d7a4dcef9a6e6646723d007/image-21.jpg)
Finding the Rate Constant Sample Problem [NO 2] (M) [CO] (M) Rate (M/s) 0. 10 0. 0021 0. 20 0. 10 0. 0082 0. 20 0. 0083 0. 40 0. 10 0. 033 Use data from any one line of chart to find k Using the data above, and the rate law we just found, (R = k [NO 2]2), determine the following: 0. 0021 M/s 1. Value of k. k = R -1 s-1 = = 0. 21 M [NO 2]2 (0. 10 M)2 2. Reaction rate when the concentration of [NO 2] is 0. 50 M and [CO] is 1. 00 M. R = k [NO 2]2 = (0. 21 M-1 s-1) (0. 50 M)2 = 0. 053 M/s

Thermodynamics • Thermodynamics is the study of energy transfer in reactions.

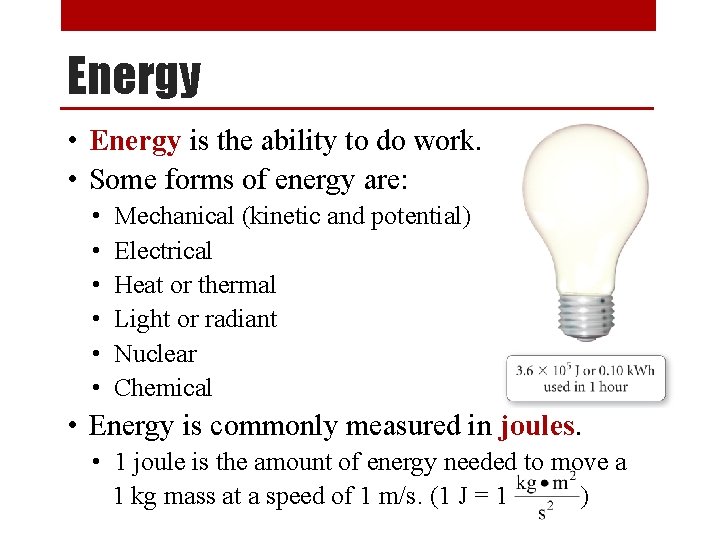
Energy • Energy is the ability to do work. • Some forms of energy are: • • • Mechanical (kinetic and potential) Electrical Heat or thermal Light or radiant Nuclear Chemical • Energy is commonly measured in joules. • 1 joule is the amount of energy needed to move a 1 kg mass at a speed of 1 m/s. (1 J = 1 )

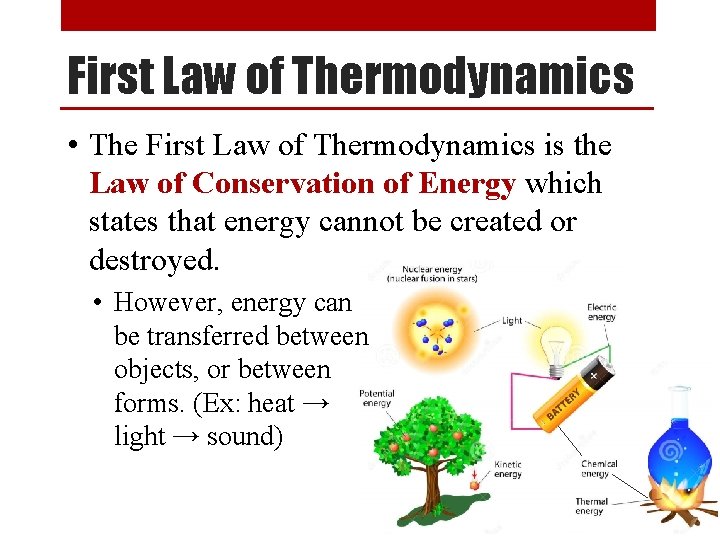
First Law of Thermodynamics • The First Law of Thermodynamics is the Law of Conservation of Energy which states that energy cannot be created or destroyed. • However, energy can be transferred between objects, or between forms. (Ex: heat → light → sound)

Energy Exchange • Conservation of Energy requires that the total energy change in a system and its surroundings must be zero. • Energy is exchanged between system and surroundings through either heat exchange or work being done. • Heat is the exchange of thermal energy between a system and its surroundings.

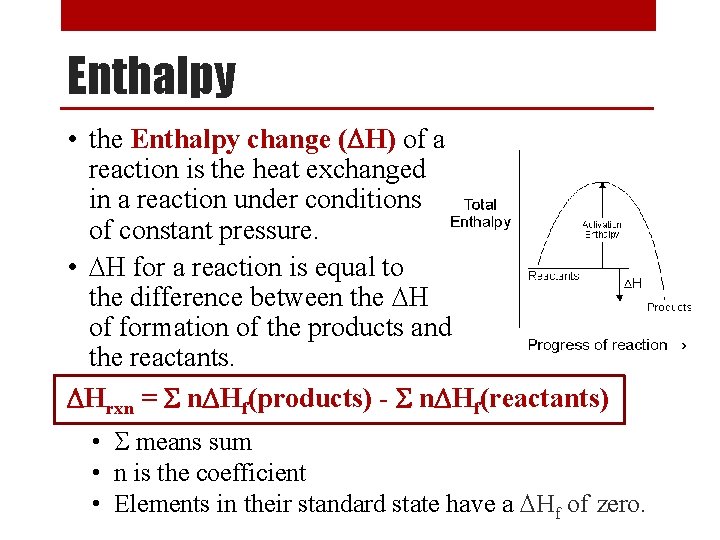
Enthalpy • the Enthalpy change (DH) of a reaction is the heat exchanged in a reaction under conditions of constant pressure. • DH for a reaction is equal to the difference between the DH of formation of the products and the reactants. DHrxn = S n. DHf(products) - S n. DHf(reactants) • S means sum • n is the coefficient • Elements in their standard state have a DHf of zero.

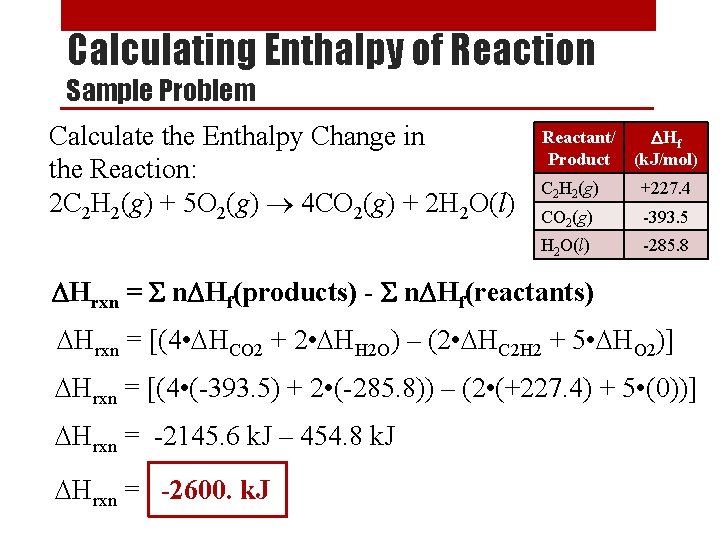
Calculating Enthalpy of Reaction Sample Problem Calculate the Enthalpy Change in the Reaction: 2 C 2 H 2(g) + 5 O 2(g) 4 CO 2(g) + 2 H 2 O(l) Reactant/ DHf Product (k. J/mol) C 2 H 2(g) +227. 4 CO 2(g) -393. 5 H 2 O(l) -285. 8 DHrxn = S n. DHf(products) - S n. DHf(reactants) DHrxn = [(4 • DHCO 2 + 2 • DHH 2 O) – (2 • DHC 2 H 2 + 5 • DHO 2)] DHrxn = [(4 • (-393. 5) + 2 • (-285. 8)) – (2 • (+227. 4) + 5 • (0))] DHrxn = -2145. 6 k. J – 454. 8 k. J DHrxn = -2600. k. J

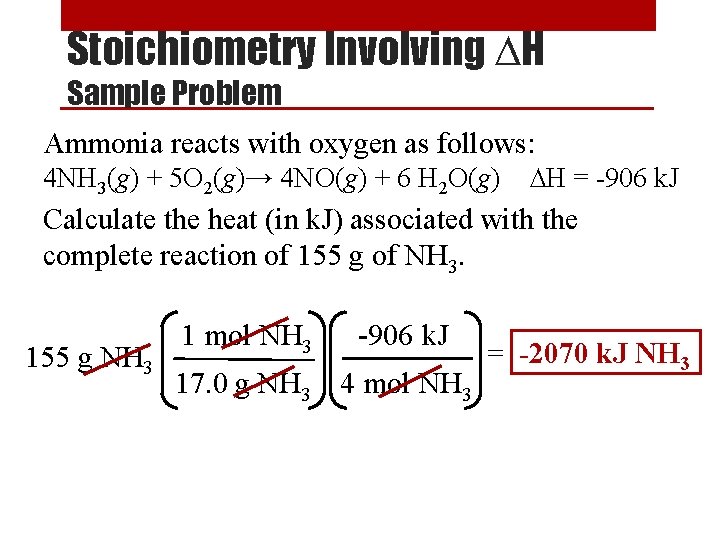
Stoichiometry Involving ∆H • The amount of heat generated or absorbed during a reaction depends on the amount of the substances reacting. Example: C 3 H 3(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O(g) ∆H = -2044 k. J Means 2044 k. J of heat is given off when 1 mole of C 3 H 3 reacts, or when 3 moles of CO 2 is produced. • These relationships can be used as ratios in stoichiometric conversions.

Stoichiometry Involving ∆H Sample Problem Ammonia reacts with oxygen as follows: 4 NH 3(g) + 5 O 2(g)→ 4 NO(g) + 6 H 2 O(g) ∆H = -906 k. J Calculate the heat (in k. J) associated with the complete reaction of 155 g of NH 3. 155 g NH 3 1 mol NH 3 -906 k. J 17. 0 g NH 3 4 mol NH 3 = -2070 k. J NH 3

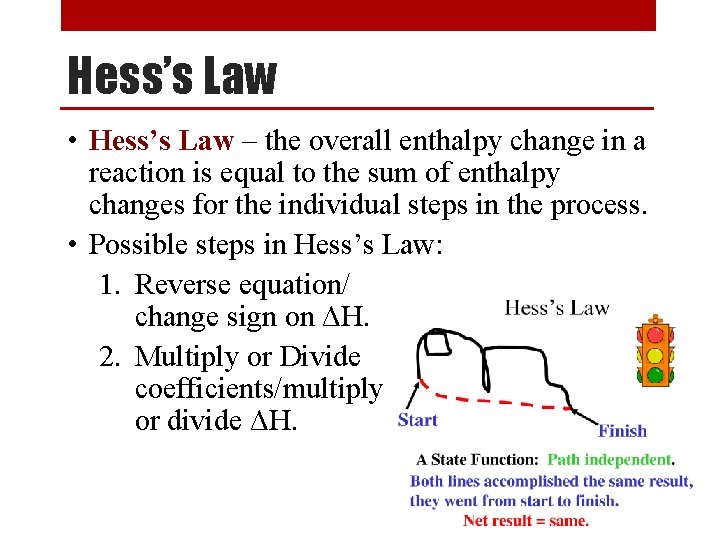
Hess’s Law • Hess’s Law – the overall enthalpy change in a reaction is equal to the sum of enthalpy changes for the individual steps in the process. • Possible steps in Hess’s Law: 1. Reverse equation/ change sign on ΔH. 2. Multiply or Divide coefficients/multiply or divide ΔH.

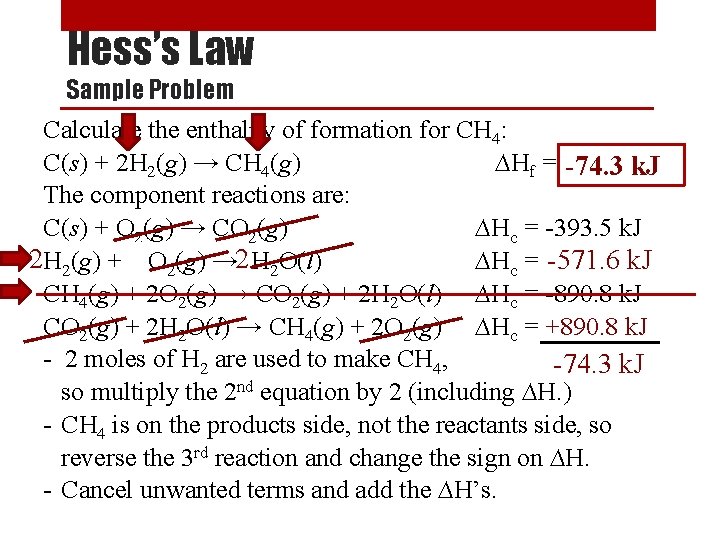
Hess’s Law Sample Problem Calculate the enthalpy of formation for CH 4: C(s) + 2 H 2(g) → CH 4(g) ∆Hf = ? -74. 3 k. J The component reactions are: C(s) + O 2(g) → CO 2(g) ∆Hc = -393. 5 k. J 2 H 2(g) + ½O 2(g) → 2 H 2 O(l) -571. 6 k. J ∆Hc = -285. 8 CH 4(g) + 2 O 2(g) → CO 2(g) + 2 H 2 O(l) ∆Hc = -890. 8 k. J CO 2(g) + 2 H 2 O(l) → CH 4(g) + 2 O 2(g) ∆Hc = +890. 8 k. J - 2 moles of H 2 are used to make CH 4, -74. 3 k. J so multiply the 2 nd equation by 2 (including ∆H. ) - CH 4 is on the products side, not the reactants side, so reverse the 3 rd reaction and change the sign on ∆H. - Cancel unwanted terms and add the ∆H’s.

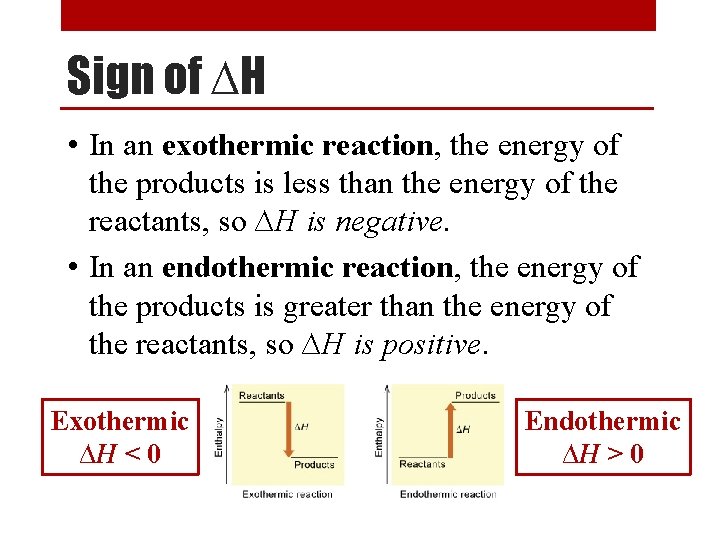
Sign of ∆H • In an exothermic reaction, the energy of the products is less than the energy of the reactants, so ∆H is negative. • In an endothermic reaction, the energy of the products is greater than the energy of the reactants, so ∆H is positive. Exothermic ∆H < 0 Endothermic ∆H > 0

Spontaneous Processes • A spontaneous process is one that occurs without ongoing outside intervention. • A nonspontaneous process is not impossible, but it requires energy input. • if a process is spontaneous in one direction, it must be nonspontaneous in the other.

Enthalpy and Reaction Tendency • Most spontaneous reactions are exothermic (∆H is negative). • Products have less energy than reactants. • Less energy = greater stability. • But not all spontaneous reactions are exothermic. • Ex: The melting of ice is endothermic and spontaneous (at >0 o. C). • Enthalpy must not be the sole criteria for spontaneity.

Entropy • Entropy (S) is a measure of the degree of disorder in a system. • Changes that increase entropy: • Physical state (phase) changes (solid < liquid < gas) • A larger number of moles of products than reactants • Increase in temperature • Solids dissociating into ions upon dissolving • Expansion (greater volume) of gases

Second Law of Thermodynamics • The Second Law of Thermodynamics states that for any spontaneous process, the entropy of the universe increases (DSuniv is positive). • The entropy of the system could decrease as long as the entropy of the surroundings increases by a greater amount. (DSuniv = DSsys + DSsurr)

Temperature Dependence of DS • The increase in DSsurr often comes from the heat released in an exothermic reaction. • the amount the entropy of the surroundings changes depends on the temperature it is at originally. • the higher the original temperature, the less effect addition or removal of heat has.

Gibbs Free Energy • The total amount of energy available in the system to do work on the surroundings is called the Gibbs Free Energy (DG). • DG can be calculated using the following equation (T is the Kelvin temperature): DG = DH – TDS • Systems tend toward lower Gibbs free energy (lower chemical potential. ) • A negative DG = a spontaneous reaction. • A positive DG = a nonspontaneous reaction.

Gibbs Free Energy Sample Problem Given: CCl 4(g) C(s, graphite) + 2 Cl 2(g) DH = +95. 7 k. J and DS = +142. 2 J/K at 25°C. 1. Calculate DG and determine if it is spontaneous: DG = DH – TDS DG = 95. 7 x 103 J – (298 K) (142. 2 J/K) DG = +5. 33 x 104 J Nonspontaneous 2. Determine at what temperature (if any) the reaction becomes spontaneous: DG = DH – TDS < 0 Spontaneous at: 3 95. 7 x 10 J – T (142. 2 J/K) < 0 T > 673 K 95. 7 x 103 J < T (142. 2 J/K)

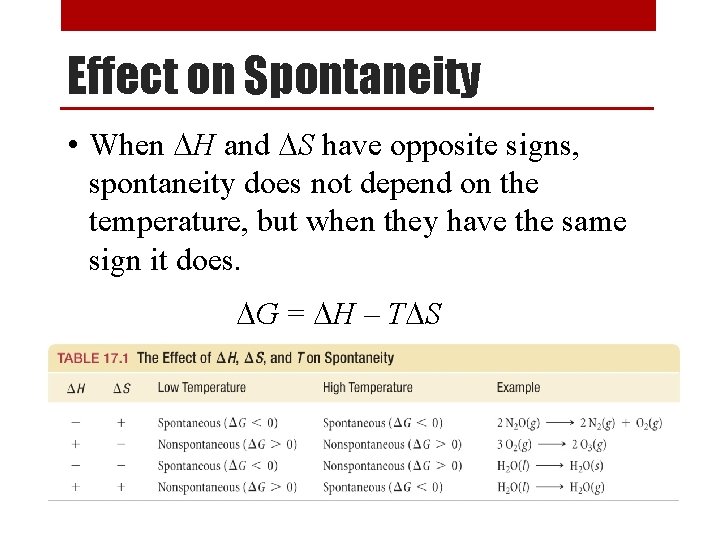
Effect on Spontaneity • When DH and DS have opposite signs, spontaneity does not depend on the temperature, but when they have the same sign it does. DG = DH – TDS

Third Law of Thermodynamics • The Third Law of Thermodynamics states that for a perfect crystal at absolute zero, absolute entropy (S) = 0. • So every substance that is not a perfect crystal at absolute zero has some energy from entropy. • Perfect crystals never occur in practice; imperfections just get "frozen in" at low temperatures. • Scientists have achieved temperatures extremely close to absolute zero.
 Chemical reactions grade 11
Chemical reactions grade 11 Definition of chemical kinetics in chemistry
Definition of chemical kinetics in chemistry Chemical kinetics half life
Chemical kinetics half life Chemical kinetics definition
Chemical kinetics definition Chemical kinetics experiment
Chemical kinetics experiment Applications of chemical kinetics
Applications of chemical kinetics Define steady state approximation
Define steady state approximation Reschual
Reschual Chemical engineering thermodynamics 8th solution chapter 3
Chemical engineering thermodynamics 8th solution chapter 3 Thermodynamics for chemical engineering
Thermodynamics for chemical engineering Thermodynamics
Thermodynamics Chapter 6
Chapter 6 Introduction to chemical engineering thermodynamics
Introduction to chemical engineering thermodynamics Chemical engineering thermodynamics 8th solution chapter 10
Chemical engineering thermodynamics 8th solution chapter 10 Chemical engineering thermodynamics 8th solution chapter 10
Chemical engineering thermodynamics 8th solution chapter 10 Endothermic enthalpy
Endothermic enthalpy Lesson 10 thermodynamics unit review
Lesson 10 thermodynamics unit review Unit 6 review questions
Unit 6 review questions Kinetics and equilibrium
Kinetics and equilibrium First order kinetics
First order kinetics Fbd and kd
Fbd and kd Antagonistic effect
Antagonistic effect Zero order kinetics
Zero order kinetics Collision theory of kinetics
Collision theory of kinetics Kinetics flotation chemicals
Kinetics flotation chemicals Planar kinetics of a rigid body force and acceleration
Planar kinetics of a rigid body force and acceleration Motion of rigid body in three dimensions
Motion of rigid body in three dimensions Kinetics of a particle force and acceleration
Kinetics of a particle force and acceleration Ap chem kinetics
Ap chem kinetics Kinetics of crystal violet fading
Kinetics of crystal violet fading Octet kinetics
Octet kinetics Km in enzyme kinetics
Km in enzyme kinetics Lineweaver-burk equation
Lineweaver-burk equation Kinetics of particles newton's second law
Kinetics of particles newton's second law Kinetics of a particle: impulse and momentum
Kinetics of a particle: impulse and momentum Dynafit software
Dynafit software Enzyme kinetics
Enzyme kinetics Abbas et al
Abbas et al Kinematics and kinetics of rigid bodies
Kinematics and kinetics of rigid bodies Data kinetics ltd
Data kinetics ltd Vmax equation
Vmax equation Planar kinetics of a rigid body work and energy
Planar kinetics of a rigid body work and energy