Chemical Equilibrium Chemical Kinetics n Chemical kinetics is

Chemical Equilibrium

Chemical Kinetics n Chemical kinetics is a branch of chemistry which deals strictly with the speed of chemical reactions n otherwise known as the rate of reaction

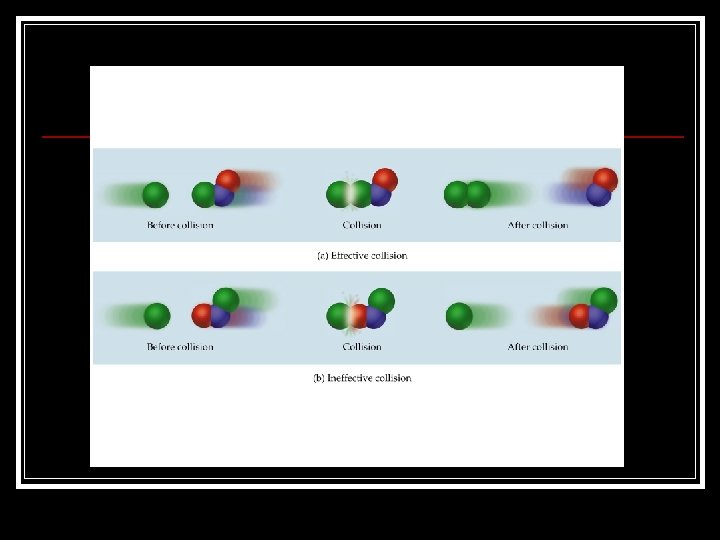
The Collision Theory Reactions occur when atoms or molecules collide in an effective collision. An effective collision is one which results in a chemical reaction. In order to have an effective collision the particles must collide with: n u A proper alignment. Enough force to affect electrons and bonds.
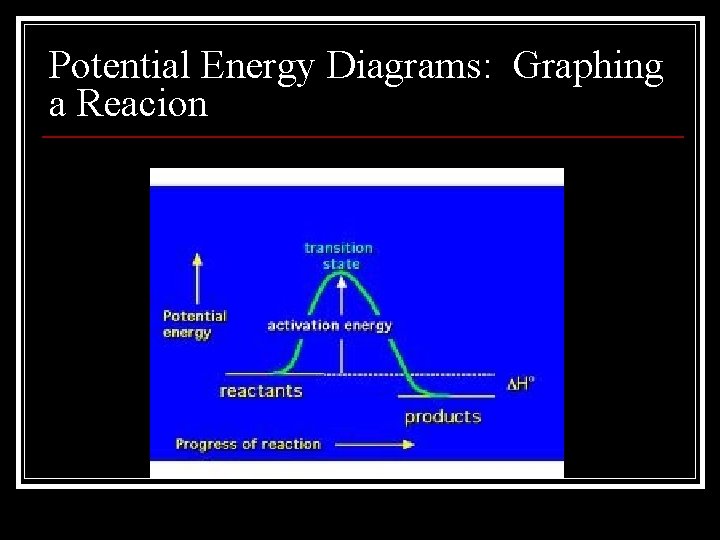
Potential Energy Diagrams: Graphing a Reacion

Activation Energy n Activation energy is the energy required to initiate a chemical reaction n An activated complex is a molecule in an intermediate state between the reactant and the product
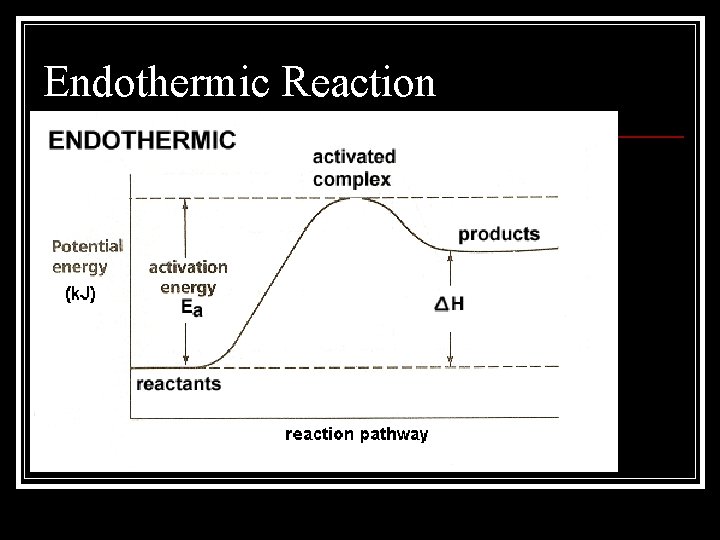
Endothermic Reaction
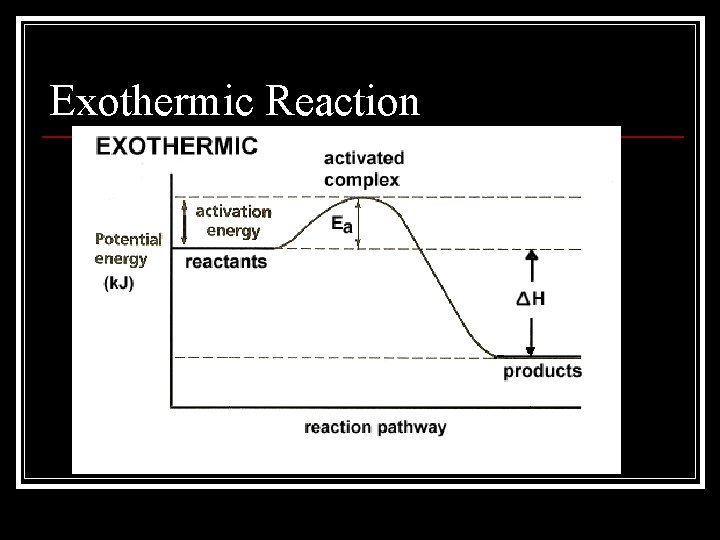
Exothermic Reaction

Intermediates n Molecules that are created in the first stage of a chemical reaction that is in the middle between the initial reactant and the final product n n The intermediate is completely consumed in a reaction The intermediate is the species that is canceled out when applying Hess’s Law

Reaction Mechanism n A reaction mechanism is the way in which a chemical reaction takes place and is expressed in a series of chemical equations

Rate-Limiting Step n The limiting reactant controls the amount of product that is formed n The Rate-Limiting Step is the slowest step of a multi-step reaction that determines the overall speed or rate
• Which car is the fastest? • Why is the red car slower than the yellow car?

What factors affect rate? n To determine which factors affect rate, it is important to determine what happens during a chemical reaction first of all n n The particles of each of the reactants are colliding with one another and transferring energy The speed of the reaction depends on the number of collisions and how effective those collisions are

And those factors are … Nature of the Reactants n Concentration of the Reactants n Temperature n Surface Area n Catalysts n

Nature of Reactants n Bond Types n n Remember those IM forces! Metallic, Hydrogen, Ionic, Polar Covalent and Non-polar Covalent The stronger the bond, the slower the reaction HOWEVER, remember that an Ionic compound in an aqueous solution is broken down into it’s cations and it’s anions which react quickly
Concentration of Reactants As the concentration of the reactants increases, the frequency of collisions increases as does the rate n In a gas system, the greater the concentration, the greater the pressure; therefore, increase in pressure leads to an increase in reaction rate n

Temperature n As the average kinetic energy increases, that would be the temperature, the number of collisions increases and therefore the rate of reaction increases

Surface Area n The greater the surface area, the greater the probability of surface collisions; therefore, the reaction rate increases

Catalyst n The presence of a catalyst lowers the activation energy required to initiate a reaction by providing an alternative pathway. This alternative pathway allows for a faster reaction rate
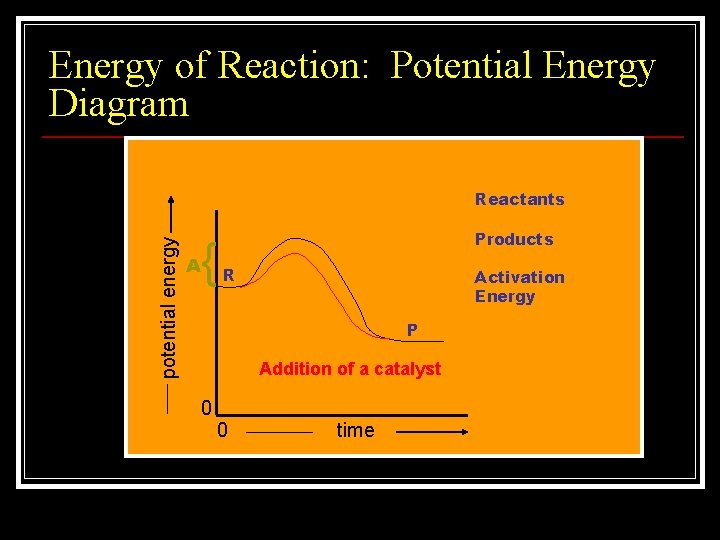
Energy of Reaction: Potential Energy Diagram potential energy Reactants { A Products R Activation Energy P Addition of a catalyst 0 0 time

Reversible Reactions & Dynamic Equilibrium

Define these terms. Reactant n Product n Dynamic Equilibrium n Reversible Reaction n Completion Reaction n

Reversible Reaction n A chemical reaction that proceeds in both directions at the same time. n n As the product decomposes back into reactants as it is being produced Ex. Ca 2+(aq) + SO 42 -(aq) Ca. SO 4(s)

Equilibrium When two opposing changes occur at the same rate Physical: evaporation condensation Chemical: H 2 + I 2 2 HI HC 2 H 3 O 2 + H 2 O H 3 O+ + C 2 H 3 O 2 -

Chemical Equilibrium n The point in a chemical reaction when dynamic equilibrium has been achieved and the concentration of the reactants and products remains constant

Dynamic Equilibrium The rate of the forward reaction equals the rate of the reverse reaction; AND, n the concentration of the products and reactants remain the same. n
Rates can be graphed n Reaction rates are graphed by plotting the concentration in moles per liter by the time in seconds
Comparison of Rates n Let’s look at the reversible reaction of Hydrogen and Iodine to form Hydrogen iodide H 2 + I 2 2 HI
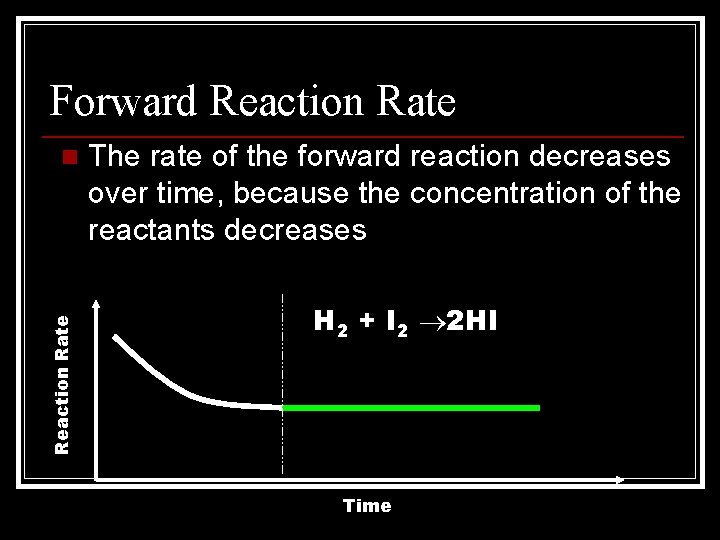
Forward Reaction Rate n The rate of the forward reaction decreases over time, because the concentration of the reactants decreases H 2 + I 2 2 HI Time
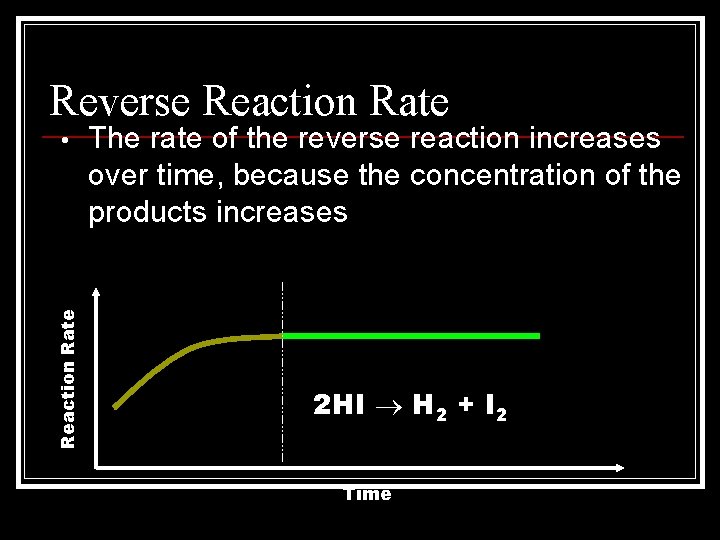
Reverse Reaction Rate • The rate of the reverse reaction increases over time, because the concentration of the products increases 2 HI H 2 + I 2 Time
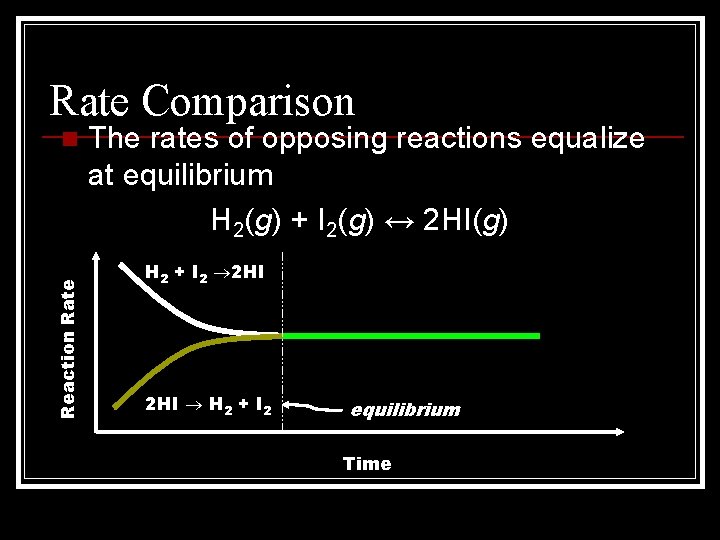
Rate Comparison Reaction Rate n The rates of opposing reactions equalize at equilibrium H 2(g) + I 2(g) ↔ 2 HI(g) H 2 + I 2 2 HI H 2 + I 2 equilibrium Time

n What evidence might lead you to believe that a chemical reaction was NOT at equilibrium?

The Equilibrium Constant n The equilibrium constant (Keq) is a number that represents the concentrations of reactants and products of a reversible reaction at a given temperature Keq = [products] [reactants]

What does it mean? n If the equilibrium constant is a high number, the reaction favors the products; the concentration of the products is greater than that of the reactants

Calculating Keq Write and balance the reaction equation including states of matter. 2. Set up the equation: [product 1]coefficient 1 ÷ [reactant 1]coefficient 1 *the concentration of any solid or pure liquid is left out because the concentration remains constant 1.

Decomposition of water to form Hydrogen gas and Oxygen gas. 1. 2. 2 H 2 O(l) 2 H 2(g) + O 2(g) Keq= [O 2] x [H 2]2

Solubility Constant n The solubility product constant (Ksp) is a number that represents the concentrations of a solid and its aqueous ions at a given temperature Keq = [ion]

What it means n If the solubility product constant is high, the ions are favored over the solid. The higher the Ksp, the greater the extent of dissolution.
n When would Ksp not apply?

Le Châtelier’s Principle n How will a system at equilibrium respond to additional stress? n “When a system at equilibrium is subjected to a stress, the system shifts in order to relieve the effects of the stress and restore the equilibrium conditions as closely as possible. ”

What do you do when your foot falls asleep? n Adjust your legs so that the circulation will be restored to your feet.

What do you do when your stomache growls from hunger? n Feed it!

What do you do when your checking account is at a zero balance? n Have your mom deposit more money, of course! n All of these are examples of how a system at equilibrium responds to a stress in order to regain the state of equilibrium

Huh? n Chemical reactions respond to similar stresses to the system n Note: when a system returns to a state of equilibrium, there is a new equilibrium point because the original conditions have been changed.

Effects of Le Châtelier’s n Stresses due to change in concentration, temperature and pressure are subject to Le Châtelier’s Principle

Chemical Shift n A chemical shift is when either the forward or reverse reaction is favored by the introduction of a stress. Equilibrium

So. . . n A forward shift is to the right of the reaction in response to a stress n A reverse shift is to the left of the reaction in response to a stress

For Example Let’s look at the Haber Process

Haber Process n The Haber Process is a process that is used to produce ammonia N 2(g) + 3 H 2(g) 2 NH 3(g) n Notice that it is a reversible reaction.

Haber Equilibrium n At equilibrium, the rate of ammonia production equals the rate at which ammonia is decomposed into its elements. n The concentrations (molarity) of ammonia, nitrogen and hydrogen are constant.
![Effect of Concentration n If the [N 2] is increased, in other words, we Effect of Concentration n If the [N 2] is increased, in other words, we](http://slidetodoc.com/presentation_image_h2/097f355e7b378791e320c23087127d17/image-51.jpg)
Effect of Concentration n If the [N 2] is increased, in other words, we add more reactant, n Then, the reaction will shift to the right, forward shift, in order to remove any additional nitrogen
![Forward Shift n As a result, the [NH 3] increases and the [H 2] Forward Shift n As a result, the [NH 3] increases and the [H 2]](http://slidetodoc.com/presentation_image_h2/097f355e7b378791e320c23087127d17/image-52.jpg)
Forward Shift n As a result, the [NH 3] increases and the [H 2] decreases N 2 (g )+ 3 H 2 (g) 2 NH 3 (g)
![Reverse Shift Suppose instead of nitrogen, the [NH 3] is increased. n As a Reverse Shift Suppose instead of nitrogen, the [NH 3] is increased. n As a](http://slidetodoc.com/presentation_image_h2/097f355e7b378791e320c23087127d17/image-53.jpg)
Reverse Shift Suppose instead of nitrogen, the [NH 3] is increased. n As a result of the [NH 3] increasing, the [N 2] and the [H 2] increases n )+ g ( N 2 ) g ( 3 H 2 2 ) g ( NH 3

Completion n A chemical reaction in which one of the products is continuously removed will never achieve equilibrium. n This reaction is said to go to completion

Completion Reactions n A chemical reaction that continues to completion n All (or nearly all) of the reactants are used up in the chemical reaction. Products do not re-form any of the reactants. Also known as an irreversible reaction.

Common Ion Effect n In a saturated solution of an ionic compound, the ions are in equilibrium with it’s solid form Ag. Cl(s) n Ag+(aq) + Cl-(aq) If you additional Cl- from a different ionic parent, more Ag. Cl will be produced

Reduction of Solubility n The common ion effect reduces the solubility of slightly soluble compounds

Effect of Volume Change n What happens when you reduce the volume of a system? n n The pressure increases, and the particles are closer together The stress can be relieved by producing a smaller number of particles

Example n Let’s look again at the Haber process N 2(g) + 3 H 2(g) 2 NH 3(g) n There are 4 moles of reactants and 2 moles of product n Reducing the volume would shift the reaction to the right where there are fewer particles

Another Example n Let’s look at the reaction of Hydrogen and Chlorine to form Hydrochloric Acid H 2(g) + Cl 2(g) 2 HCl(g) n There are 2 moles of reactant AND product so an increase or a decrease in volume would not cause the reaction to shift

Pressure Changes n Pressure changes have almost no effect on equilibrium reactions in solution n Pressure effects the equilibrium of gaseous species

Inert Gas n The pressure of an equilibrium system can be changed by adding an inert gas n An inert gas does not react with any of the reactants or products and so therefore does not affect the equilibrium of the system
Changing the Temperature n We can raise the temperature of a system by adding energy in the form of heat n n Adding heat to a system is endothermic Removing heat from a system is exothermic

Lower the Temperature n Because an exothermic reaction releases heat, it will favor a decrease in the temperature n Lower the temperature in a system and the reaction will shift to the exothermic side in order to replace some of the lost heat
Raise the Temperature n Because an endothermic reaction absorbs heat, it will favor an increase in the temperature n Raise the temperature in a system and the reaction will shift to the endothermic side in order to absorb the excess

Again with the Haber Process n Let’s add the change in enthalpy as part of our reaction and see what happens N 2(g) + 3 H 2(g) 2 NH 3(g) + 91. 8 k. J n What happens when we lower the temperature?

Lower the Temperature in the Haber Process n When we lower the temperature, the reaction shifts toward the exothermic side N (g) + 3 H (g) 2 NH 3(g) + 91. 8 k. J 2 2 n The [NH 3] is increased and the [N 2] and [H 2] is decreased

Effects of a Catalyst n At equilibrium, a catalyst increases the forward and reverse reactions equally n However, if a system is NOT at equilibrium, a catalyst will shorten the time needed to achieve equilibrium

Summary of Effects

Problems, Yahoo! n Consider the following reaction 904 k. J + 6 H 2 O(g) + 4 NO(g) n What is the effect of. . . n decreasing the temperature n n a shift to the left decreasing the volume n shift right 4 NH 3(g) + 5 O 2(g)

Problems, continued n 904 k. J + 6 H 2 O(g) + 4 NO(g) n What is the effect of. . . n increasing [NO] n n shift right decreasing [H 2 O] n shift left 4 NH 3(g) + 5 O 2(g)

The Equilibrium Constant (Keq) n The Equilibrium Constant, known by Keq or K, tells us how much reactant used or product produced

What does that tell you? n The larger the value for the equilibrium constant the more the reaction goes to completion. Irreversible reactions can be thought to have an infinite equilibrium constant so there are no reactants left.

That means. . . The value for K is large when products dominate the mixture. n The value for K is small when the reactants dominate the mixture. n
![Homogenous System n For a homogenous system at equilibrium, you divide the [product] by Homogenous System n For a homogenous system at equilibrium, you divide the [product] by](http://slidetodoc.com/presentation_image_h2/097f355e7b378791e320c23087127d17/image-75.jpg)
Homogenous System n For a homogenous system at equilibrium, you divide the [product] by the [reactant] A(g) B(g) Keq = [B] / [A]
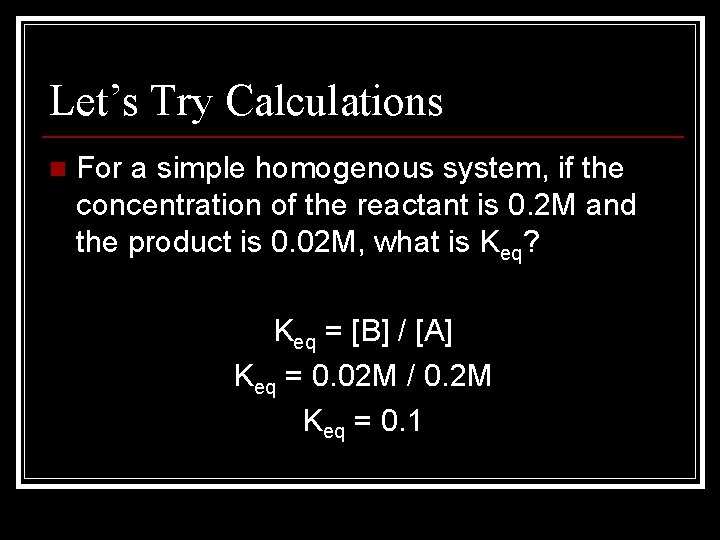
Let’s Try Calculations n For a simple homogenous system, if the concentration of the reactant is 0. 2 M and the product is 0. 02 M, what is Keq? Keq = [B] / [A] Keq = 0. 02 M / 0. 2 M Keq = 0. 1
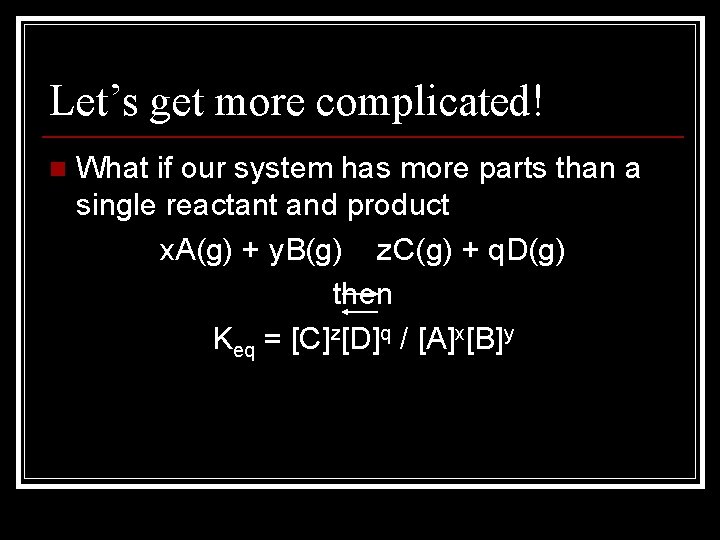
Let’s get more complicated! n What if our system has more parts than a single reactant and product x. A(g) + y. B(g) z. C(g) + q. D(g) then Keq = [C]z[D]q / [A]x[B]y

Solve it! n Write a Keq equation for the following reaction and evaluate Keq if [NH 3]=0. 2 M; [N 2]=0. 04 M, and [H 2]=0. 01 M. N 2(g) + 3 H 2(g) 2 NH 3(g) Keq = [NH 3]2 / [N 2][H 2]3 Keq = (0. 2 M)2/(0. 04 M)(0. 01 M)3 Keq = 1 x 106 ignore the units!!!

Solubility Product Constant (Ksp) n The Solubility Product Constant (Ksp) is a special type of equilibrium that measures the concentration of ionic compounds in water

Ionic Compounds Dissociate in Water n An ionic compound dissolves in water AB(s) A+(aq) + B-(aq) Keq = [A+][B-] / [AB] so. . . Keq. [AB] = [A+][B-] = Ksp

Let’s write some Ksp n Write the Ksp expression for Ba. SO 4 Ba 2+ + SO 42 Ksp Ba. SO 4 = [Ba 2+]. [SO 42 -] n Now, try to write the Ksp for Pb. I 2 Pb 2+ + 2 IKsp = [Pb 2+]. [ I-]2

Problems with Keq n Problem: In an experiment, hydrogen gas and iodine gas were placed in a container which was sealed and allowed to come to equilibrium with Hydrogen iodide gas. At the start of the experiment, [H 2]=0. 001 M and [I 2]=0. 002; and at the end, the [HI]=0. 00186 M. n n a. calculate [ ] for H 2 and I 2 in M b. calculate Keq

Step 1: n Write out the equation for the reaction H 2(g) + I 2(g) 2 HI(g)

Step 2: n Start solving the problem by first constructing a table for the information

Step 3: n Use the coefficients as simple whole number ratios in order to calculate the proportions of products to reactants n n It takes 1 mole of H 2 and 1 mole of I 2 to produce 2 moles of HI Therefore, if there were 2 moles of HI produced, it required 1 mole of H 2 and 1 mole of I 2

Step 3: continued n Calculate the proportions and add the information into the table

Step 4: n If you can calculate the change in concentration, you can calculate the equilibrium concentration by taking the initial concentration and adding or subtracting the change
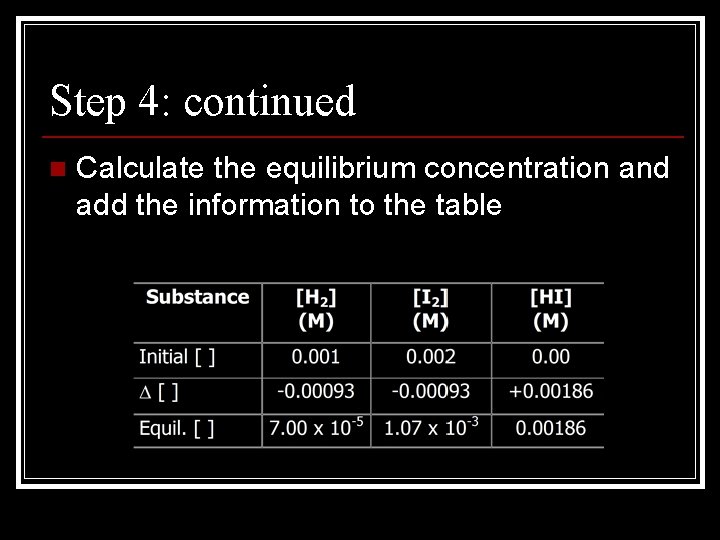
Step 4: continued n Calculate the equilibrium concentration and add the information to the table
- Slides: 88