How fast was that reaction Reaction Kinetics Reaction














![Data Analysis (cont’d) �The Rate Law will then be: Rate = k[H 2]2[Cl 2] Data Analysis (cont’d) �The Rate Law will then be: Rate = k[H 2]2[Cl 2]](https://slidetodoc.com/presentation_image_h2/43225cfd2fae1bab52d376f795085f4d/image-25.jpg)





- Slides: 44

How fast was that reaction? Reaction Kinetics, Reaction Rates

Introduction: �In your experiences in chemistry, you will have observed that every chemical reactions occurs instantly, or right away. �This, however, does not occur for all chemical changes. Some reactions occur very slowly, while others are fast, but observable. �Regardless of the type of chemical change, we can measure the speed at which a chemical reaction can occur. This, we call a reaction rate.

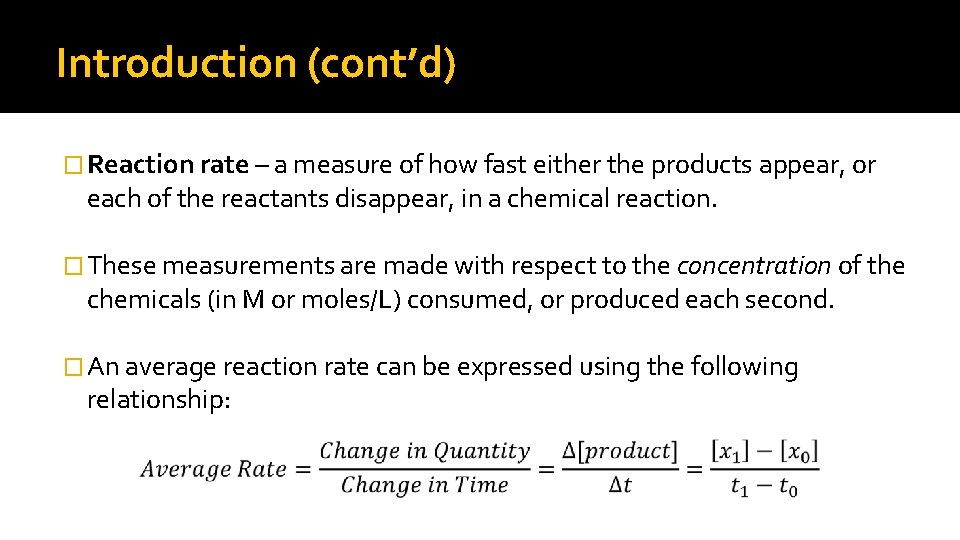
Introduction (cont’d) � Reaction rate – a measure of how fast either the products appear, or each of the reactants disappear, in a chemical reaction. � These measurements are made with respect to the concentration of the chemicals (in M or moles/L) consumed, or produced each second. � An average reaction rate can be expressed using the following relationship:

Reaction Rate: � How is the rate going to appear if we use the reactants instead of the products? � They will disappear, so we will have a negative number as a rate. We have defined that a reaction rate must always be positive, so to correct for this we must add a negative sign if we use the reactants. Average Rate = – change in Quantity = – ∆reactant = – [x]1 – [x]0 change in time ∆t t 1 – t 0

Collision Theory �Rates of a chemical reaction depend on one very important thing, the collision of the reactant molecules. �But is it just the collision of the molecules, you may ask? This is science, and it isn’t always that easy (big surprise). It also requires each of the reactant molecules to hit each other just the right way too. �This depends on what product is being formed. The right parts of both molecules must make contact in order to begin forming the bond between them.

Making Contact. . . the Right Way �When scientists studied chemical reactions carefully, they found that the number of successful reactions was much lower than the number of collisions that happened. Why? �Well, there are other factors that influence whether the reaction (change) occurs: 1. Orientation of colliding molecules For a bond to form between the right atoms, those atoms have to be the ones to make contact at the point of impact.

Activated Complex ▪ When this contact occurs, a short-lived intermediate called the “activated complex” is formed as bonds are forming and others bonds are breaking. A picture of this looks complicated, but essentially involves having the two molecules joined together. ▪ At this point, the activated complex, or transition state, may continue on to form the products, or go back to the reactants. 2. Activation Energy Even if the molecules do collide, they may not have enough energy to form the bonds needed (or break bonds) in order to form the product.

Activation Energy ▪ This energy is called the “activation energy” for the reaction or E a ▪ If Ea is high, fewer reactions will occur and the reaction rate will be lower. ▪ If Ea is low, more reactions will occur successfully and the reaction rate will be higher. Does Spontaneity Affect Reaction Rate? In a word, no. It does not affect rate. Remember that ∆G (free energy) only determines the natural tendency for a reaction to proceed.

Review the Idea �Answer These: What does reaction rate mean? How quickly product forms, or reactants disappear. How do we express reaction rate? (hint: unit) M/s or moles/L∙s What is Collision Theory and how does it relate to reaction rate? Successful collisions must occur, and the number of successful collisions determine the rate. According to Collision Theory, what has to occur in order for a Molecules in the correct orientation and with enough energy. reaction to happen? How is speed of a reaction related to the spontaneity of the reaction? Speed is not related to spontaneity.

Factors Affecting Reaction Rate �There are several factors that affect the rate of a chemical reaction: A. Nature of Reactants – reactants are different in how reactive they are. B. Concentration – when you increase the concentration of the reactants, you will increase the number of collisions possible, therefore you will increase reaction rate. C. Surface Area – increasing the surface area of a solid (like a wool or a powder, instead of a small block) will increase the reaction rate by allowing more contact surface for a reaction to occur.

Factors (cont’d) D. Temperature – increasing the temperature of the reaction will increase the reaction rate. It will cause the increase in both the collision frequency and the energy of the collision. E. Catalysts – a catalyst gets involved in a reaction but does not get used up (does not disappear). It aids a reaction by “reducing the activation energy” for a reaction. This is achieved by improving the number of collisions that produce the product. ▪ Another chemical, called an inhibitor does the opposite, reducing the reaction rate or preventing the reaction.

Review: Factors in Reaction Rate �Let’s now recall of the factors together: 1. Nature of Reactants 2. Concentration 3. Surface Area 4. Temperature 5. Catalysts

Rate Law The Mathematical Relationship in Chemical Reactions

Rate Laws �An expression that shows the relationship between the rate of reaction and the concentration of the reactants is called a rate law. �For any reaction, the rate is based on the concentration of reactant(s) and a specific rate constant (represented by k). �For a reaction A B, the rate expression is represented by: Rate = k [A]

Rate Laws (cont’d) �Using the reaction above: if we double the concentration of A, we will double the reaction rate if we increase concentration by 5 times, the rate will increase 5 times. �The rate constant, k, will be unique for a reaction under specific conditions so different temperatures will give a different k value for the exact same reaction.

Rate Laws: (cont’d) �Understanding Question: �How does the value of k affect the rate? What if it is a low value? A low k value means that the reaction rate will be lower. What if it is a high value? A high k value means that the rate of reaction will be higher.

Reaction Order �Reaction Order basically tells us how the concentration of the individual reactants affect the rate of the chemical reaction. First Order – in a first order reaction, the rate is directly proportional to the concentration of the reactant (like our first example above). Second Order – in a second order reaction, the rate will be a square of the change in concentration of the reactant. Zero Order – in this special case, the rate is independent of the concentration of the reactant.

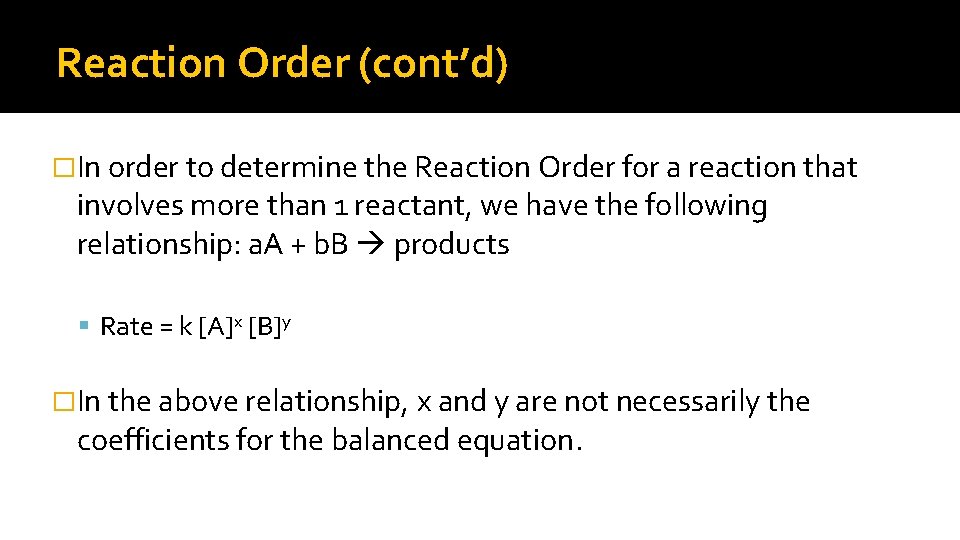
Reaction Order (cont’d) �In order to determine the Reaction Order for a reaction that involves more than 1 reactant, we have the following relationship: a. A + b. B products Rate = k [A]x [B]y �In the above relationship, x and y are not necessarily the coefficients for the balanced equation.

Reaction Order: (cont’d) �The reaction order will be the sum of the values of x + y (sum of the superscripts), or the sum of the orders for the individual reactants (remember that no superscript means 1!). So if we have Rate = k [A]3 [B], our Reaction Order is 4. �The only way to determine an order for each of the reactants is to test it experimentally.

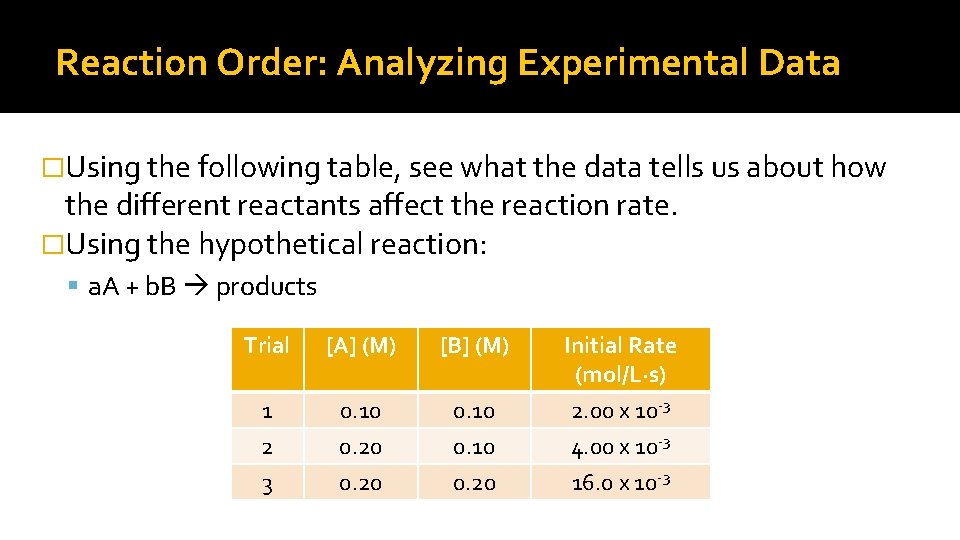
Reaction Order: Analyzing Experimental Data �Using the following table, see what the data tells us about how the different reactants affect the reaction rate. �Using the hypothetical reaction: a. A + b. B products Trial [A] (M) [B] (M) Initial Rate (mol/L∙s) 1 2 3 0. 10 0. 20 2. 00 x 10 -3 4. 00 x 10 -3 16. 0 x 10 -3

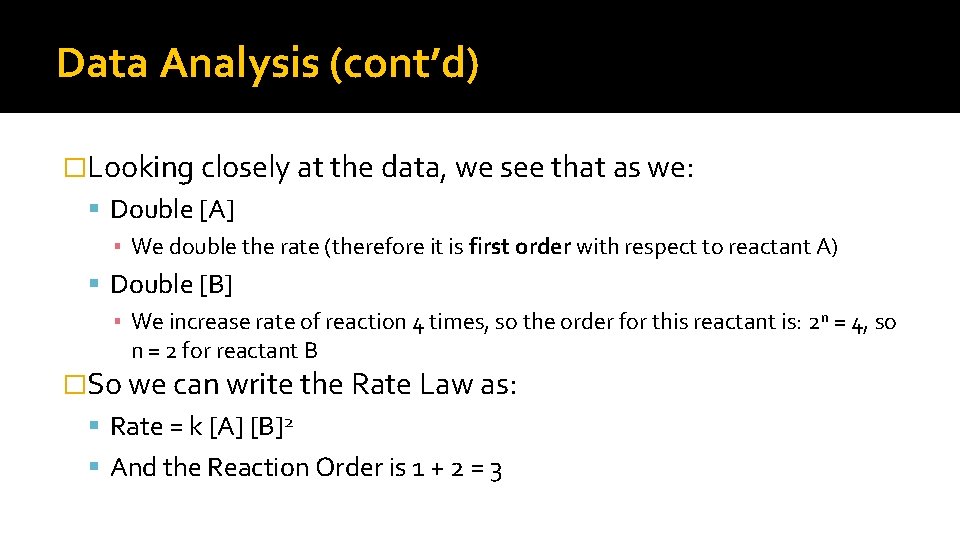
Data Analysis (cont’d) �Looking closely at the data, we see that as we: Double [A] ▪ We double the rate (therefore it is first order with respect to reactant A) Double [B] ▪ We increase rate of reaction 4 times, so the order for this reactant is: 2 n = 4, so n = 2 for reactant B �So we can write the Rate Law as: Rate = k [A] [B]2 And the Reaction Order is 1 + 2 = 3

How Do We Find Order? � We use this principle to find our Order, which is the exponent.

Finding an Order: (cont’d) �

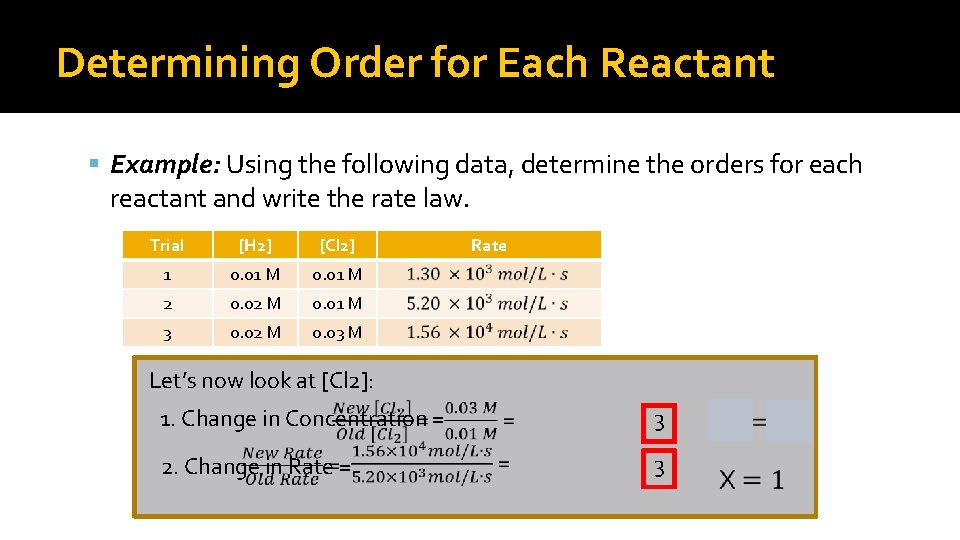
Determining Order for Each Reactant Example: Using the following data, determine the orders for each reactant and write the rate law. Trial [H 2] [Cl 2] 1 0. 01 M 2 0. 02 M 0. 01 M 3 0. 02 M 0. 03 M Rate Let’s look [H 2] now at look at first: [Cl 2]: 1. Change in Concentration = 32 2. Change in in Rate == 2. 34
![Data Analysis contd The Rate Law will then be Rate kH 22Cl 2 Data Analysis (cont’d) �The Rate Law will then be: Rate = k[H 2]2[Cl 2]](https://slidetodoc.com/presentation_image_h2/43225cfd2fae1bab52d376f795085f4d/image-25.jpg)
Data Analysis (cont’d) �The Rate Law will then be: Rate = k[H 2]2[Cl 2] Reaction Order = 2 + 1 = 3 �What if the rate is not affected by the concentration of a reactant? The Order for that reactant is zero, so it will not have a role in both the Rate Law expression and the Reaction Order

Instantaneous Reaction Rate Applying the Rate Law

Instantaneous Reaction Rate �Now that we can write a rate law for a chemical reaction, what can we do with this? �First we need to understand that the law can tell us how fast a reaction is going to occur, given the concentrations for the reactant(s). �So we can use that rate law to determine what is called an instantaneous reaction rate given a specific concentration of the reactant(s).

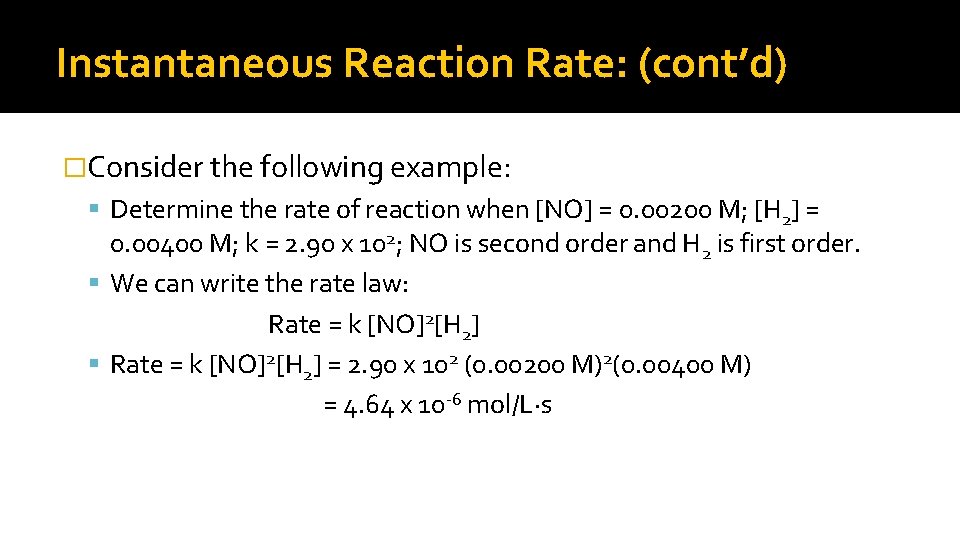
Instantaneous Reaction Rate: (cont’d) �Consider the following example: Determine the rate of reaction when [NO] = 0. 00200 M; [H 2] = 0. 00400 M; k = 2. 90 x 102; NO is second order and H 2 is first order. We can write the rate law: Rate = k [NO]2[H 2] = 2. 90 x 102 (0. 00200 M)2(0. 00400 M) = 4. 64 x 10 -6 mol/L∙s

Reaction Mechanisms Breaking Down a Chemical Reaction, More than Meets the Eye

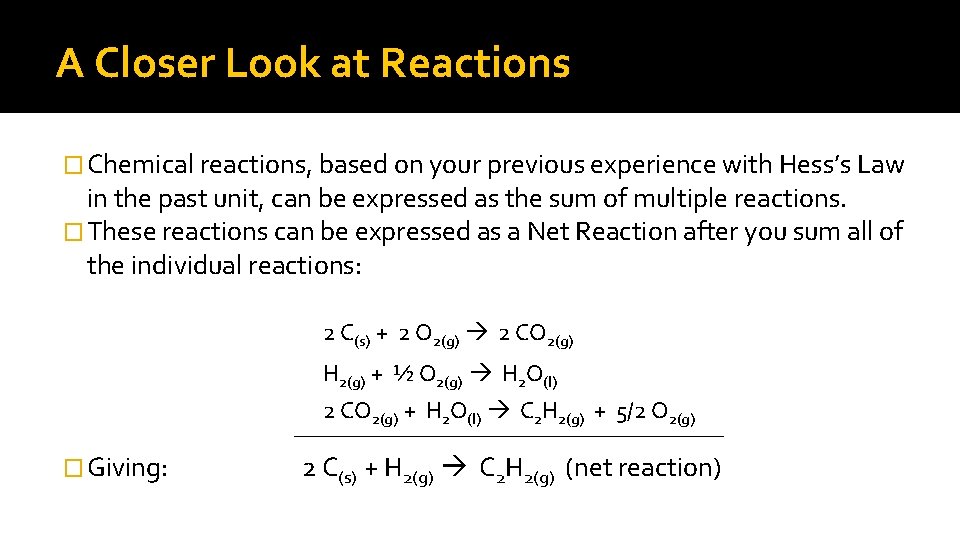
A Closer Look at Reactions � Chemical reactions, based on your previous experience with Hess’s Law in the past unit, can be expressed as the sum of multiple reactions. � These reactions can be expressed as a Net Reaction after you sum all of the individual reactions: 2 C(s) + 2 O 2(g) 2 CO 2(g) H 2(g) + ½ O 2(g) H 2 O(l) 2 CO 2(g) + H 2 O(l) C 2 H 2(g) + 5/2 O 2(g) _________________________ � Giving: 2 C(s) + H 2(g) C 2 H 2(g) (net reaction)

“Step”ping into Reactions �The final, overall reaction is the net reaction that we actually would see. �The individual “step” reactions we wouldn’t really see. �Most chemical reactions actually do occur in more than the one reaction step we observe. �The group of the reactions and the order that they take place is what we call a reaction mechanism.

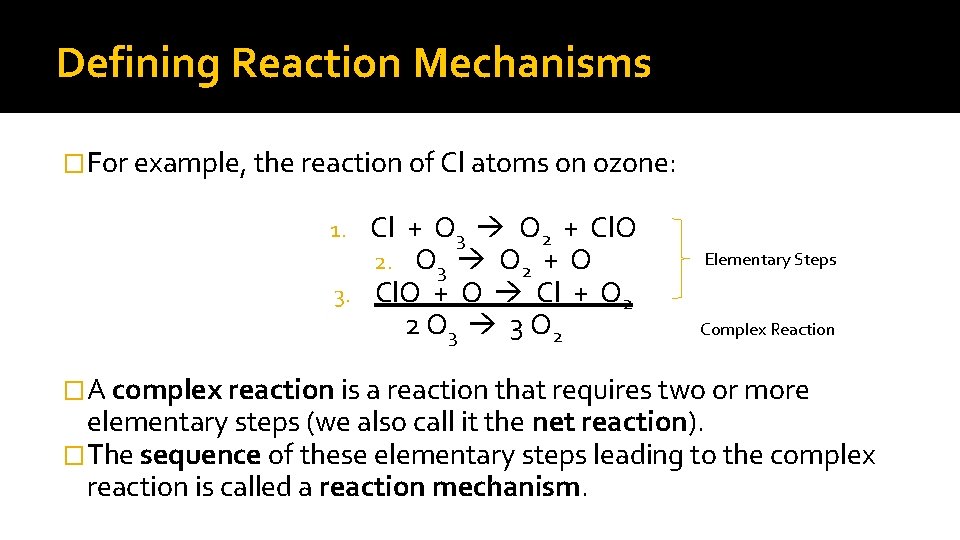
Defining Reaction Mechanisms �For example, the reaction of Cl atoms on ozone: Cl + O 3 O 2 + Cl. O 2. O 3 O 2 + O 3. Cl. O + O Cl + O 2 2 O 3 3 O 2 1. Elementary Steps Complex Reaction �A complex reaction is a reaction that requires two or more elementary steps (we also call it the net reaction). �The sequence of these elementary steps leading to the complex reaction is called a reaction mechanism.

Effect of Reaction Mechanisms �Recall that a chemical reaction occurs at a specific rate (based on its’ k value and on the concentration of its’ “reactants”). �Since a reaction may be the result of several elementary steps that, together, result in the reaction we observe, what does that insight lead us to conclude about reaction rate? Think about it this way, if every reaction has a specific rate, how does this potentially affect a net reaction?

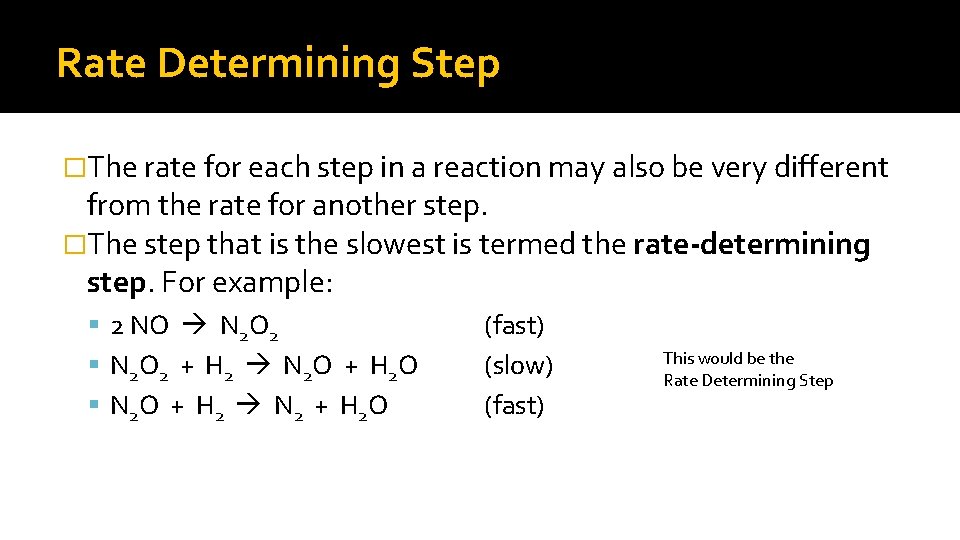
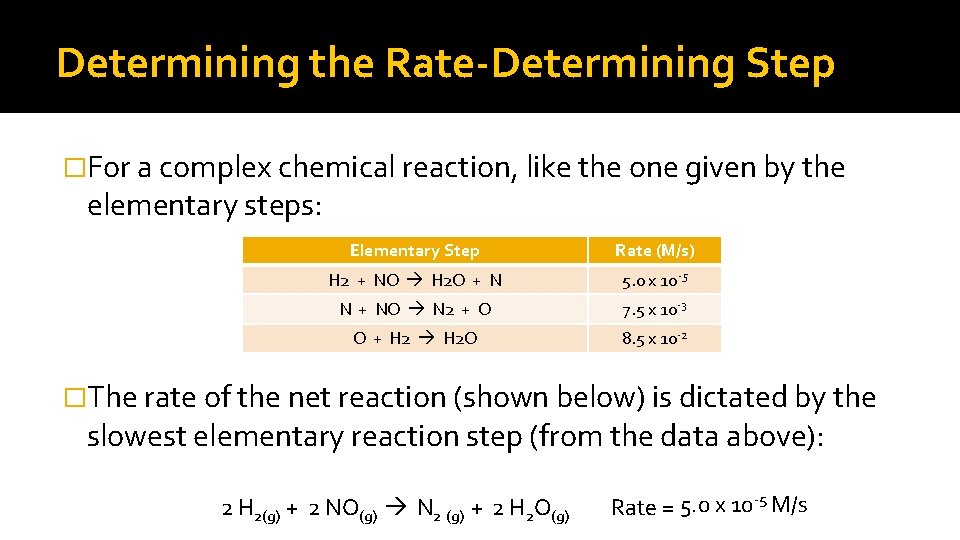
Rate Determining Step �The rate for each step in a reaction may also be very different from the rate for another step. �The step that is the slowest is termed the rate-determining step. For example: 2 NO N 2 O 2 N 2 O 2 + H 2 N 2 O + H 2 O N 2 O + H 2 N 2 + H 2 O (fast) (slow) (fast) This would be the Rate Determining Step

Determining the Rate-Determining Step �For a complex chemical reaction, like the one given by the elementary steps: Elementary Step Rate (M/s) H 2 + NO H 2 O + N 5. 0 x 10 -5 N + NO N 2 + O 7. 5 x 10 -3 O + H 2 O 8. 5 x 10 -2 �The rate of the net reaction (shown below) is dictated by the slowest elementary reaction step (from the data above): 2 H 2(g) + 2 NO(g) N 2 (g) + 2 H 2 O(g) Rate = 5. 0 x 10 -5 M/s

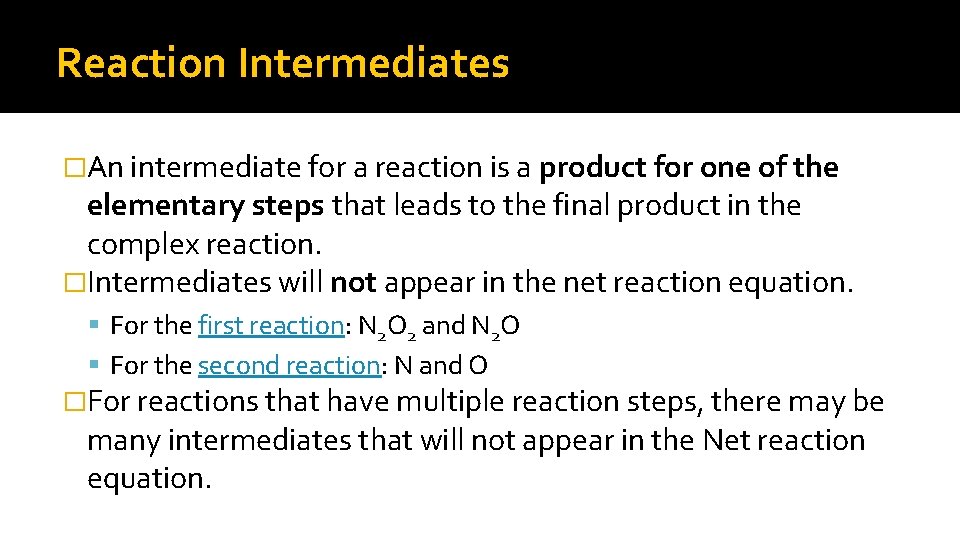
Reaction Intermediates �An intermediate for a reaction is a product for one of the elementary steps that leads to the final product in the complex reaction. �Intermediates will not appear in the net reaction equation. For the first reaction: N 2 O 2 and N 2 O For the second reaction: N and O �For reactions that have multiple reaction steps, there may be many intermediates that will not appear in the Net reaction equation.

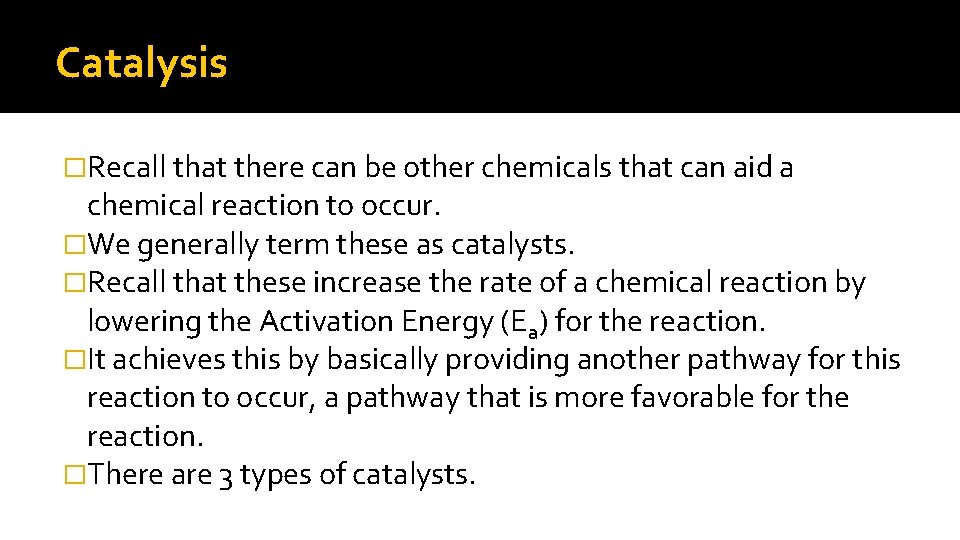
Catalysis �Recall that there can be other chemicals that can aid a chemical reaction to occur. �We generally term these as catalysts. �Recall that these increase the rate of a chemical reaction by lowering the Activation Energy (Ea) for the reaction. �It achieves this by basically providing another pathway for this reaction to occur, a pathway that is more favorable for the reaction. �There are 3 types of catalysts.

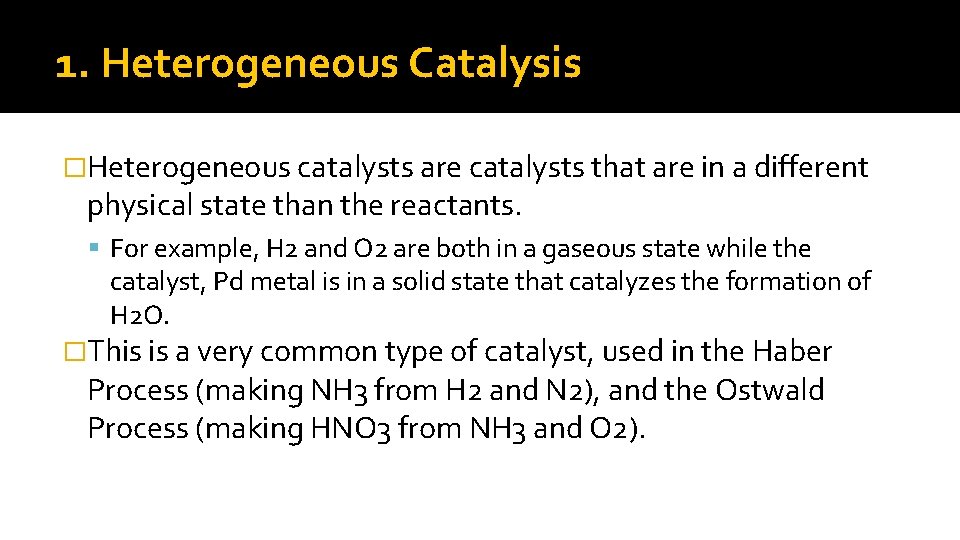
1. Heterogeneous Catalysis �Heterogeneous catalysts are catalysts that are in a different physical state than the reactants. For example, H 2 and O 2 are both in a gaseous state while the catalyst, Pd metal is in a solid state that catalyzes the formation of H 2 O. �This is a very common type of catalyst, used in the Haber Process (making NH 3 from H 2 and N 2), and the Ostwald Process (making HNO 3 from NH 3 and O 2).

2. Homogeneous Catalysis �Homogeneous catalysts exist in the same phase as the reactants. This commonly occurs in a solution. As a general example, many reactions occur in a faster rate in the presence of either an acid or a base. In which case, these chemicals act as a catalyst. A specific example involves the formation of SO 3 from SO 2 and O 2. This reaction is catalyzed by NO 2 (it is not used up in the reaction process).

3. Enzyme Catalysis �A very common, natural catalytic process involves the use of enzymes. �Enzymes are biological catalysts. They can increase the rate of a reaction by 106 or more. �These catalysts are also highly specific, which means that they only work with a specific molecule or group of molecules. �The molecule(s) that the enzyme specifically uses is/are called substrate(s).

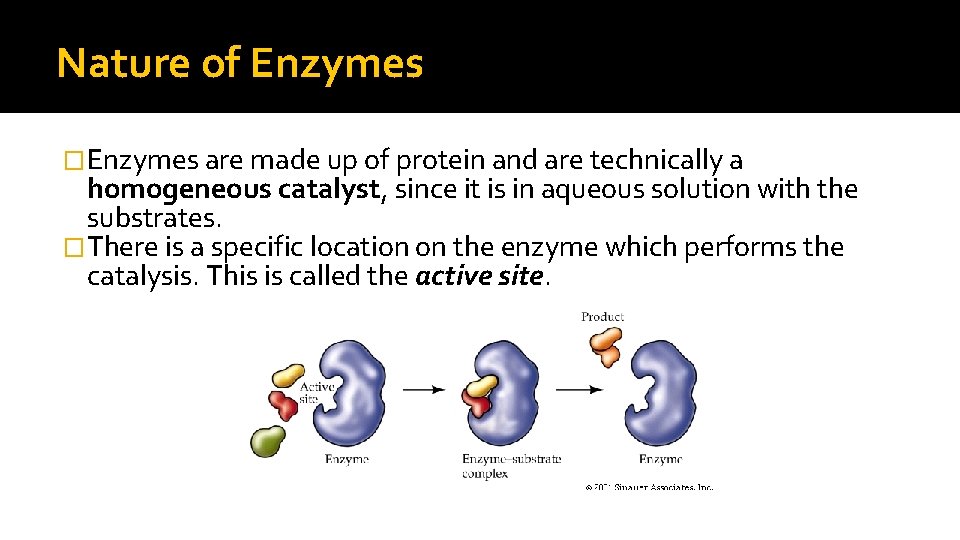
Nature of Enzymes �Enzymes are made up of protein and are technically a homogeneous catalyst, since it is in aqueous solution with the substrates. �There is a specific location on the enzyme which performs the catalysis. This is called the active site.

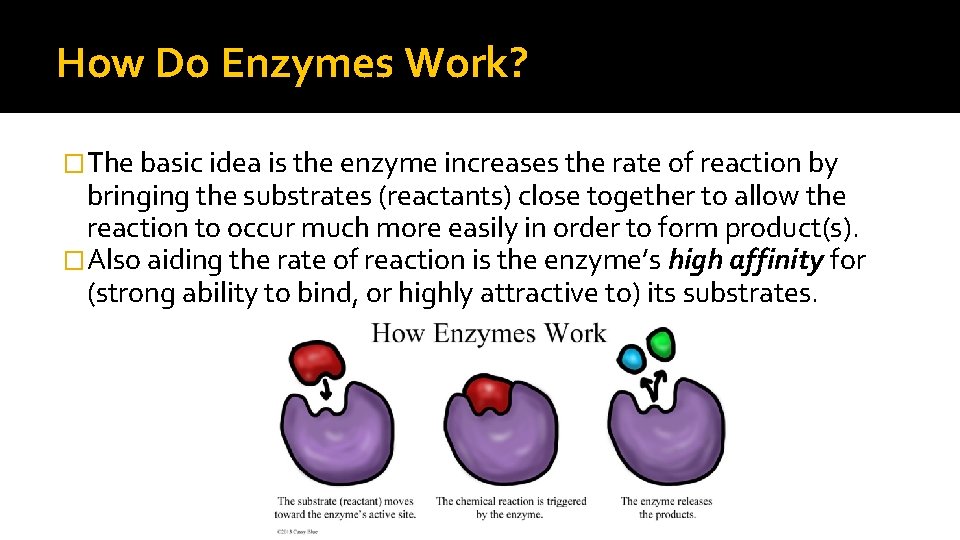
How Do Enzymes Work? �The basic idea is the enzyme increases the rate of reaction by bringing the substrates (reactants) close together to allow the reaction to occur much more easily in order to form product(s). �Also aiding the rate of reaction is the enzyme’s high affinity for (strong ability to bind, or highly attractive to) its substrates.

Unit Review: � 1. Rate of a Reaction (loss of reactant, gain of product over time) � 2. Factors Affecting Rate of Reaction (there are 5) � 3. Rate Law (rate =. . . ) What is k? � 4. Determining a Rate Law (Reactant Order) Using Experimental Data (Concentration vs. Rate change) Initial Rate of Reaction (why? )

Unit Review: (cont’d) � 5. Reaction Order (how to find? ) � 6. Instantaneous Reaction Rate (applying a rate law) � 7. Reaction Mechanism & Intermediates � 8. Catalysts (homogeneous, heterogeneous, enzymes)