Chemical Kinetics The Rate of Reaction Chemical kinetics

![The Rate Law • Solution: In the second and third experiments, the initial [B] The Rate Law • Solution: In the second and third experiments, the initial [B]](https://slidetodoc.com/presentation_image_h2/6d761f17ae166db74b020f68cf2ae8fb/image-11.jpg)




- Slides: 30

Chemical Kinetics

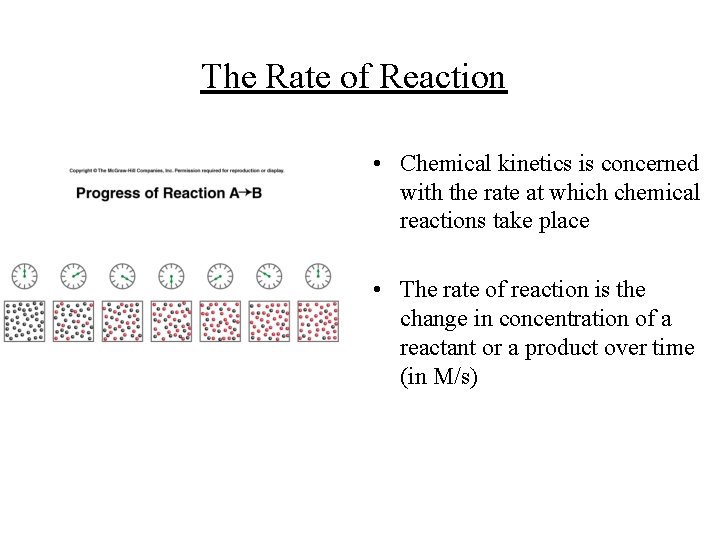
The Rate of Reaction • Chemical kinetics is concerned with the rate at which chemical reactions take place • The rate of reaction is the change in concentration of a reactant or a product over time (in M/s)

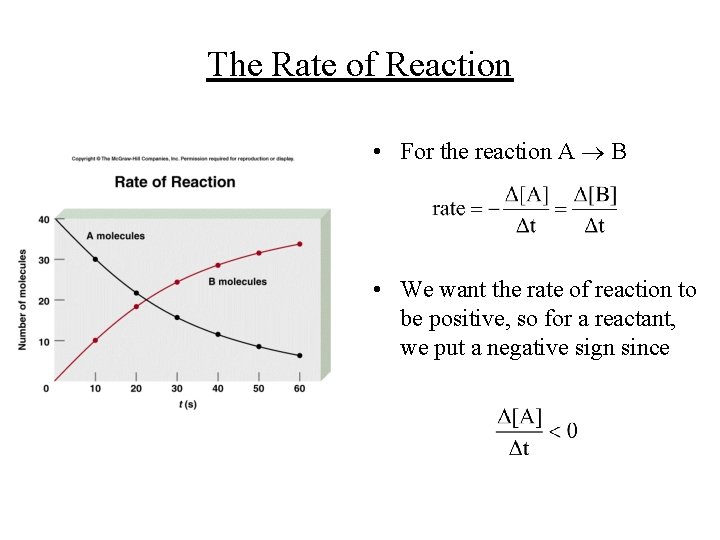
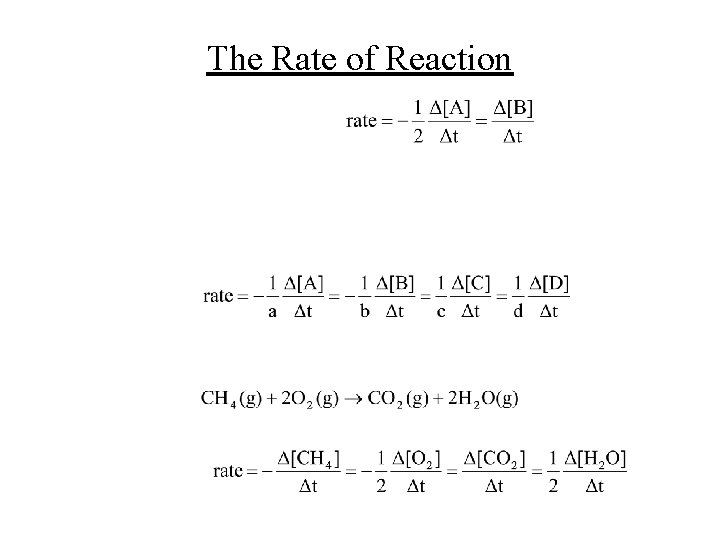
The Rate of Reaction • For the reaction A B • We want the rate of reaction to be positive, so for a reactant, we put a negative sign since

The Rate of Reaction

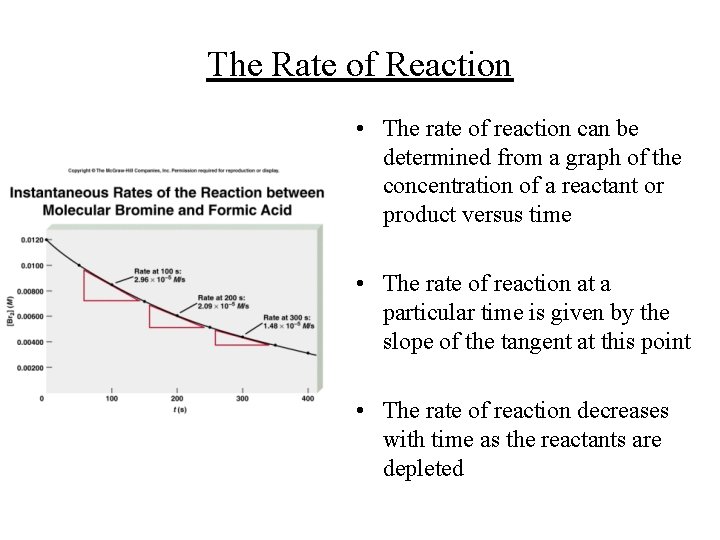
The Rate of Reaction • The rate of reaction can be determined from a graph of the concentration of a reactant or product versus time • The rate of reaction at a particular time is given by the slope of the tangent at this point • The rate of reaction decreases with time as the reactants are depleted

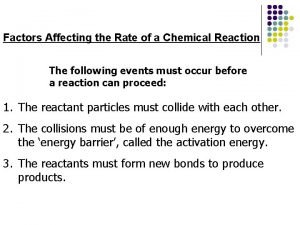
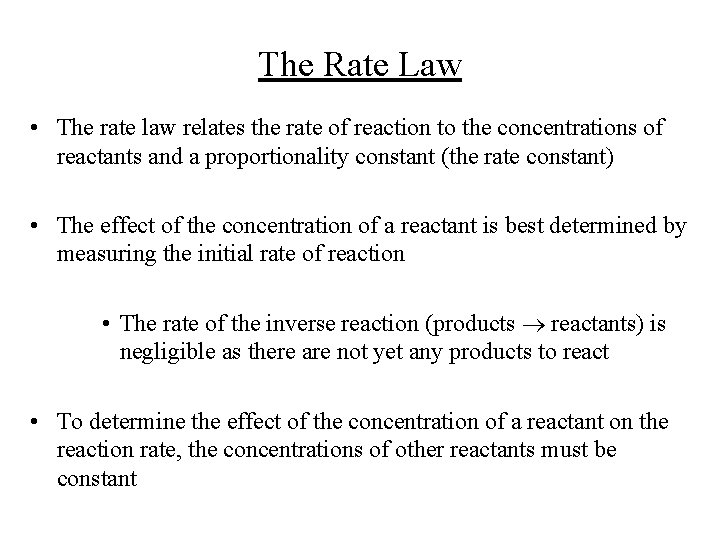
The Rate Law • The rate law relates the rate of reaction to the concentrations of reactants and a proportionality constant (the rate constant) • The effect of the concentration of a reactant is best determined by measuring the initial rate of reaction • The rate of the inverse reaction (products reactants) is negligible as there are not yet any products to react • To determine the effect of the concentration of a reactant on the reaction rate, the concentrations of other reactants must be constant

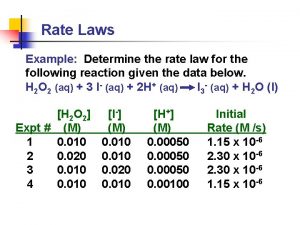
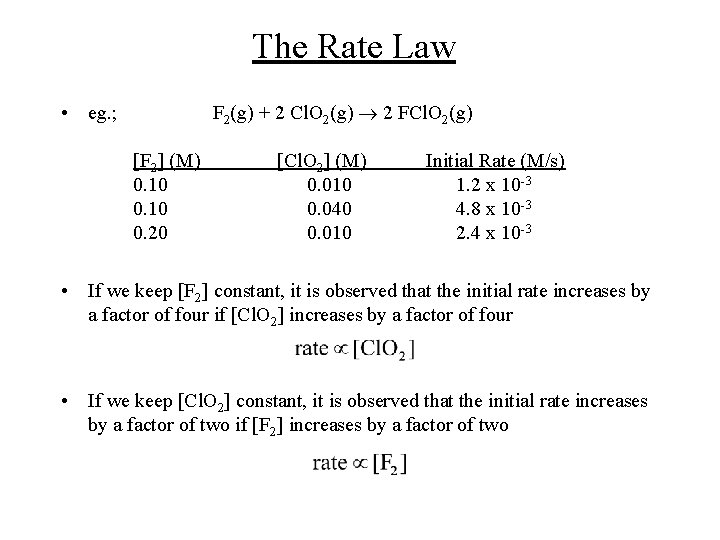
The Rate Law F 2(g) + 2 Cl. O 2(g) 2 FCl. O 2(g) • eg. ; [F 2] (M) 0. 10 0. 20 [Cl. O 2] (M) 0. 010 0. 040 0. 010 Initial Rate (M/s) 1. 2 x 10 -3 4. 8 x 10 -3 2. 4 x 10 -3 • If we keep [F 2] constant, it is observed that the initial rate increases by a factor of four if [Cl. O 2] increases by a factor of four • If we keep [Cl. O 2] constant, it is observed that the initial rate increases by a factor of two if [F 2] increases by a factor of two

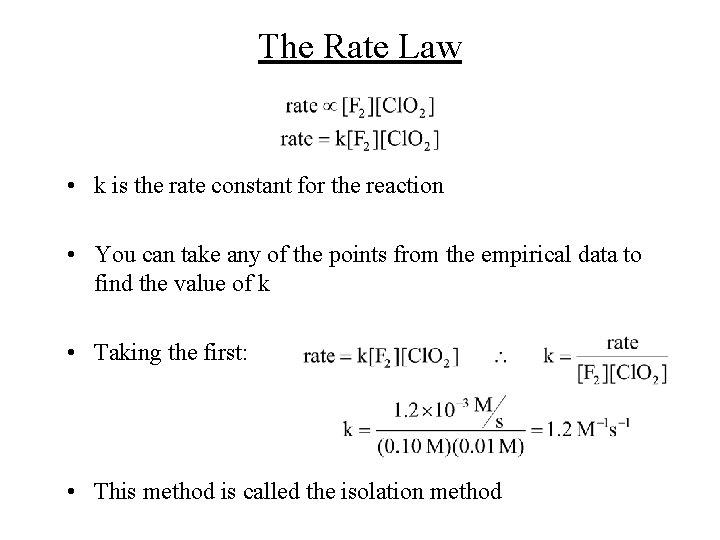
The Rate Law • k is the rate constant for the reaction • You can take any of the points from the empirical data to find the value of k • Taking the first: • This method is called the isolation method

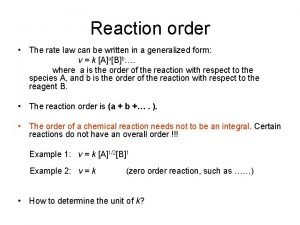
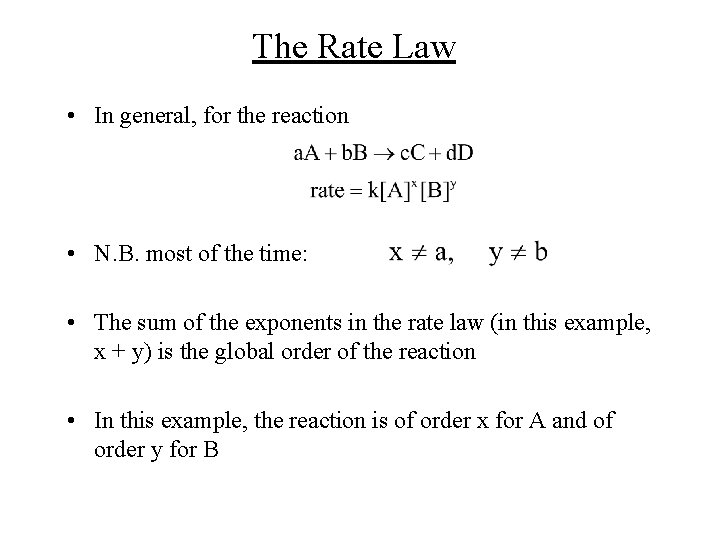
The Rate Law • In general, for the reaction • N. B. most of the time: • The sum of the exponents in the rate law (in this example, x + y) is the global order of the reaction • In this example, the reaction is of order x for A and of order y for B

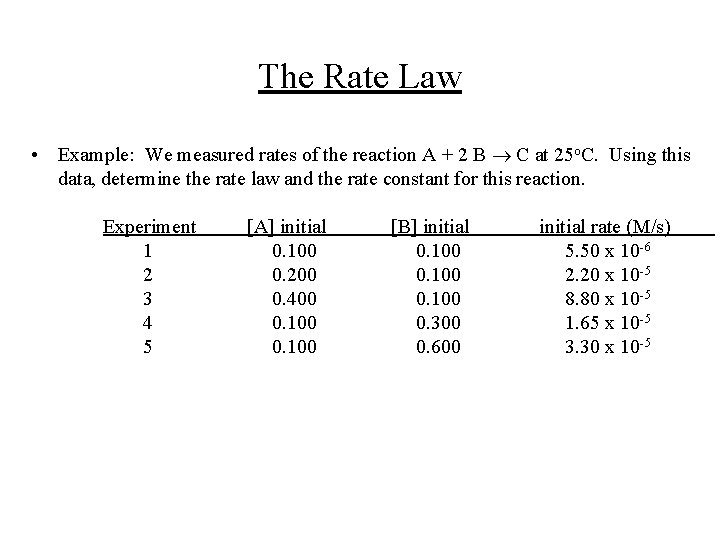
The Rate Law • Example: We measured rates of the reaction A + 2 B C at 25 o. C. Using this data, determine the rate law and the rate constant for this reaction. Experiment 1 2 3 4 5 [A] initial 0. 100 0. 200 0. 400 0. 100 [B] initial 0. 100 0. 300 0. 600 initial rate (M/s) 5. 50 x 10 -6 2. 20 x 10 -5 8. 80 x 10 -5 1. 65 x 10 -5 3. 30 x 10 -5
![The Rate Law Solution In the second and third experiments the initial B The Rate Law • Solution: In the second and third experiments, the initial [B]](https://slidetodoc.com/presentation_image_h2/6d761f17ae166db74b020f68cf2ae8fb/image-11.jpg)
The Rate Law • Solution: In the second and third experiments, the initial [B] is constant, and it is seen that the rate quadruples if we double [A]: In the fourth and fifth experiment, the initial [A] is constant, and it is seen that the rate doubles if we double [B]: Thus, the rate law is: N. B. We could have used other pairs of experiments too. To determine the value of k, we use the first experiment:

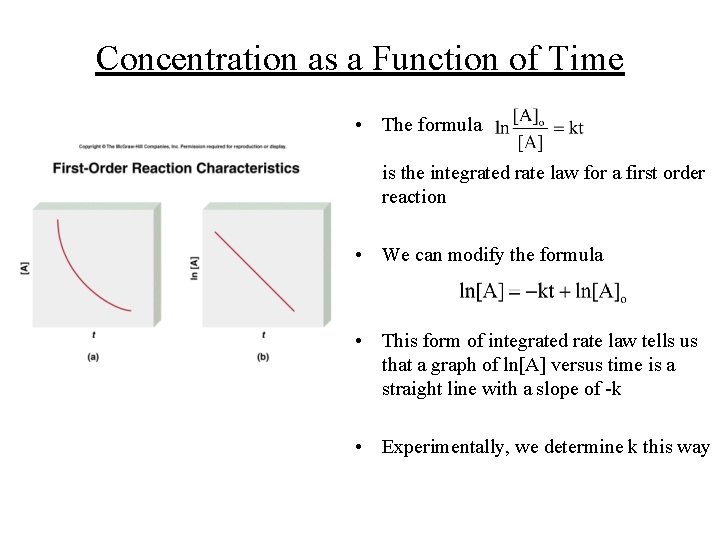
Concentration as a Function of Time • Rate laws allow us to determine the concentrations of reactants at any moment in time • For example, for a reaction of global first order that is first order with respect to A: where [A]o is the concentration of A at t=0 (which need not necessarily the beginning of the experiment)

Concentration as a Function of Time • The formula is the integrated rate law for a first order reaction • We can modify the formula • This form of integrated rate law tells us that a graph of ln[A] versus time is a straight line with a slope of -k • Experimentally, we determine k this way

Concentration as a Function of Time • Example: The reaction 2 A B is first order with respect to A, and the rate constant is 2. 8 x 10 -2 s-1 at 80 o. C. How much time (in seconds) would it take for [A] to decrease from 0. 88 M to 0. 14 M?

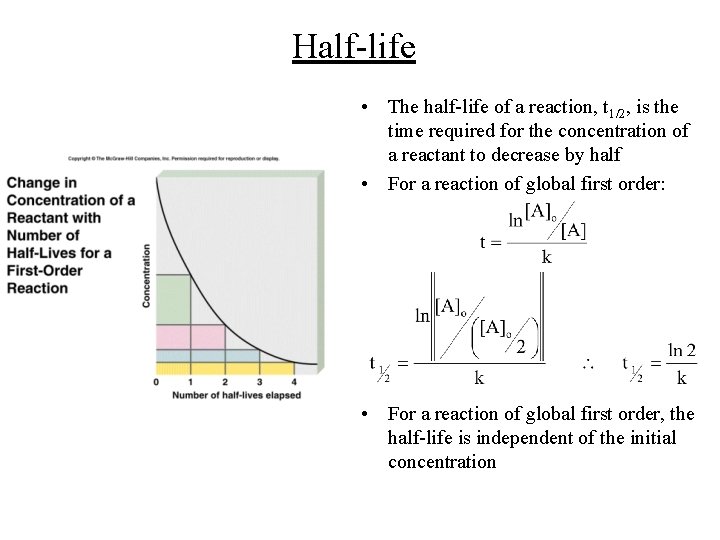
Half-life • The half-life of a reaction, t 1/2, is the time required for the concentration of a reactant to decrease by half • For a reaction of global first order: • For a reaction of global first order, the half-life is independent of the initial concentration

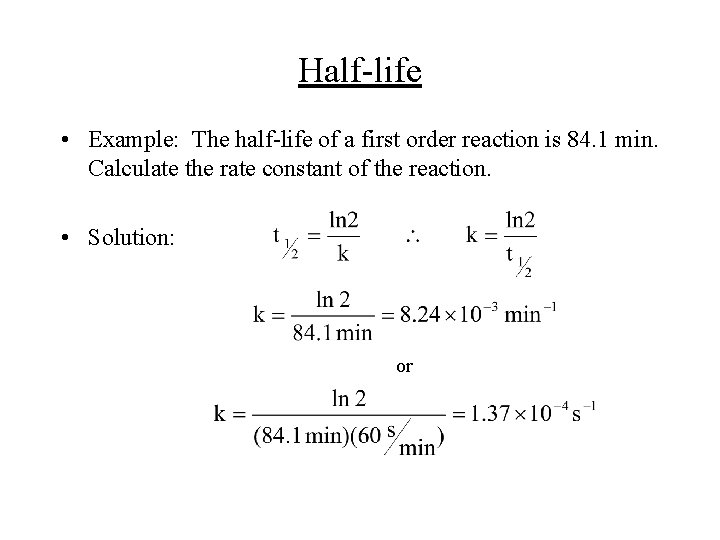
Half-life • Example: The half-life of a first order reaction is 84. 1 min. Calculate the rate constant of the reaction. • Solution: or

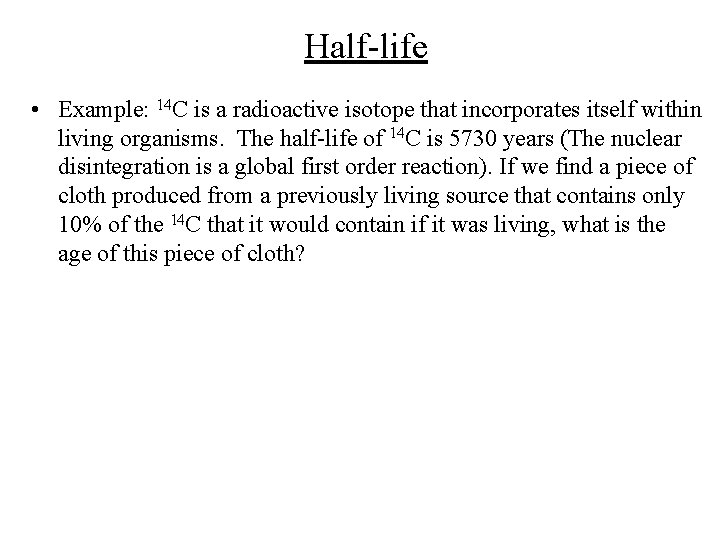
Half-life • Example: 14 C is a radioactive isotope that incorporates itself within living organisms. The half-life of 14 C is 5730 years (The nuclear disintegration is a global first order reaction). If we find a piece of cloth produced from a previously living source that contains only 10% of the 14 C that it would contain if it was living, what is the age of this piece of cloth?

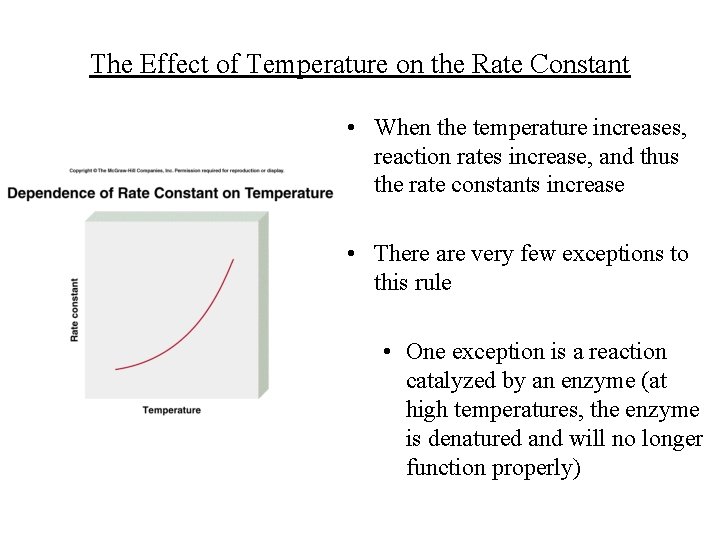
The Effect of Temperature on the Rate Constant • When the temperature increases, reaction rates increase, and thus the rate constants increase • There are very few exceptions to this rule • One exception is a reaction catalyzed by an enzyme (at high temperatures, the enzyme is denatured and will no longer function properly)

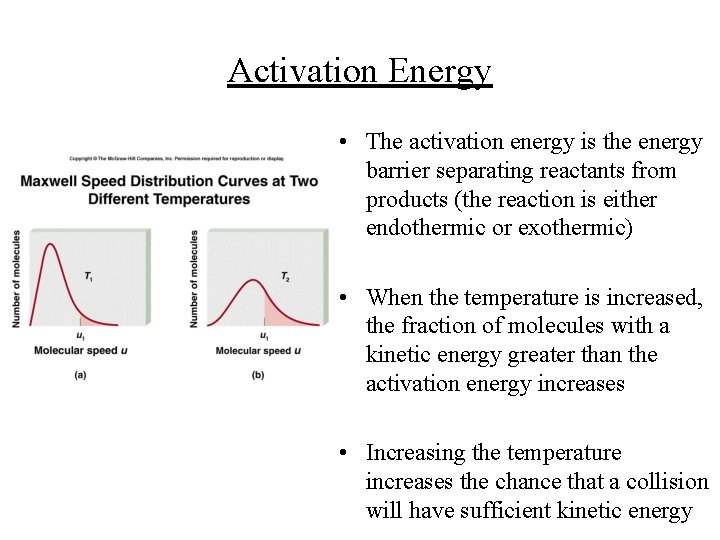
Activation Energy • The activation energy is the energy barrier separating reactants from products (the reaction is either endothermic or exothermic) • When the temperature is increased, the fraction of molecules with a kinetic energy greater than the activation energy increases • Increasing the temperature increases the chance that a collision will have sufficient kinetic energy

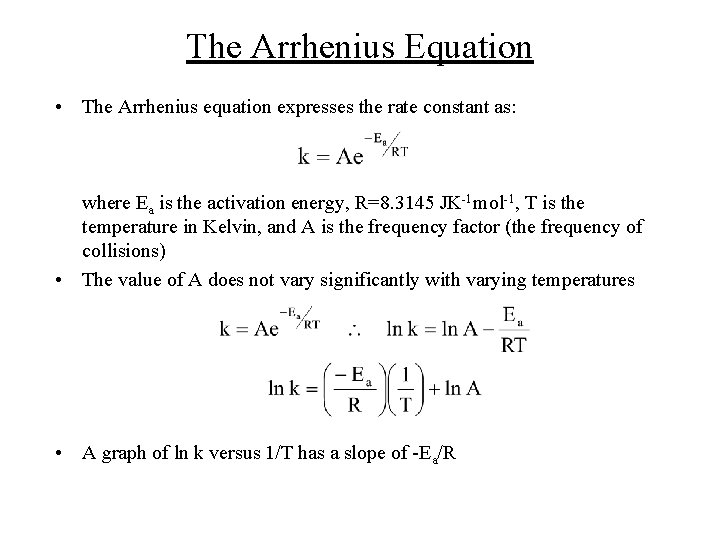
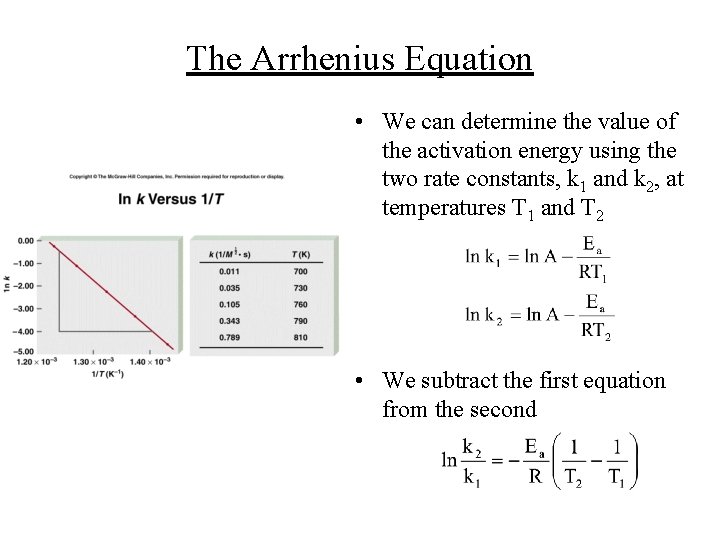
The Arrhenius Equation • The Arrhenius equation expresses the rate constant as: where Ea is the activation energy, R=8. 3145 JK-1 mol-1, T is the temperature in Kelvin, and A is the frequency factor (the frequency of collisions) • The value of A does not vary significantly with varying temperatures • A graph of ln k versus 1/T has a slope of -Ea/R

The Arrhenius Equation • We can determine the value of the activation energy using the two rate constants, k 1 and k 2, at temperatures T 1 and T 2 • We subtract the first equation from the second

The Arrhenius Equation • Example: The rate constant of the (first order) reaction of methyl chloride (CH 3 Cl) with water to form methanol (CH 3 OH) and hydrochloric acid (HCl) is 3. 32 x 10 -10 s-1 at 25 o. C. Calculate the rate constant at 40 o. C if the activation energy is 116 k. J/mol.

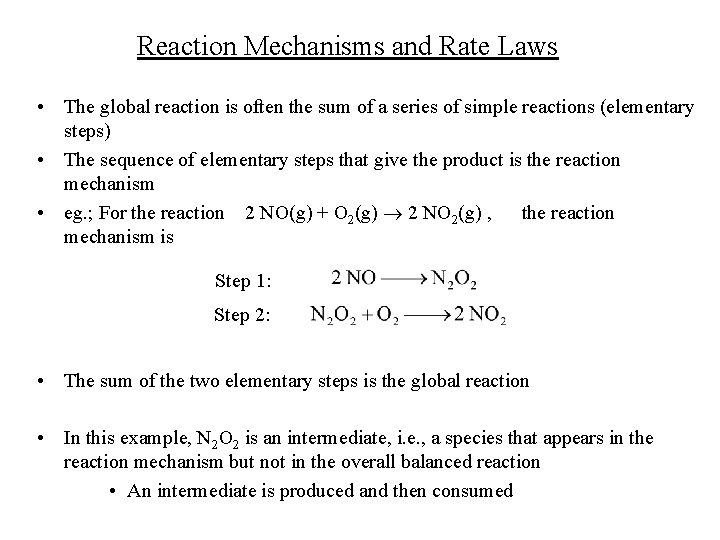
Reaction Mechanisms and Rate Laws • The global reaction is often the sum of a series of simple reactions (elementary steps) • The sequence of elementary steps that give the product is the reaction mechanism • eg. ; For the reaction 2 NO(g) + O 2(g) 2 NO 2(g) , the reaction mechanism is Step 1: Step 2: • The sum of the two elementary steps is the global reaction • In this example, N 2 O 2 is an intermediate, i. e. , a species that appears in the reaction mechanism but not in the overall balanced reaction • An intermediate is produced and then consumed

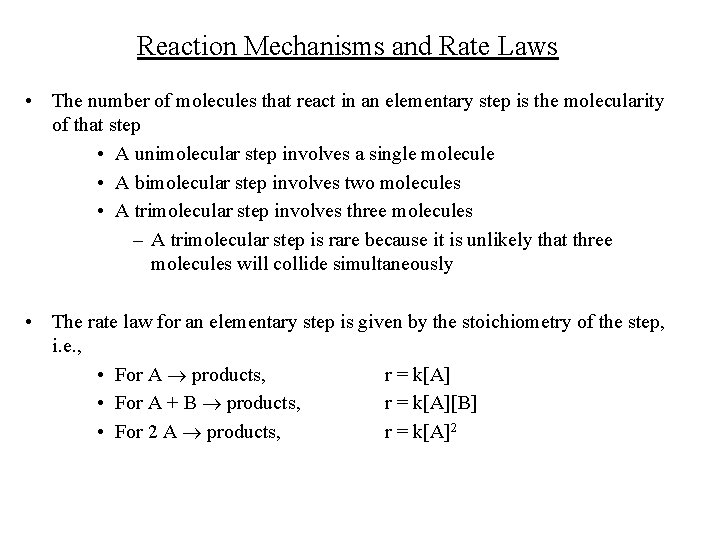
Reaction Mechanisms and Rate Laws • The number of molecules that react in an elementary step is the molecularity of that step • A unimolecular step involves a single molecule • A bimolecular step involves two molecules • A trimolecular step involves three molecules – A trimolecular step is rare because it is unlikely that three molecules will collide simultaneously • The rate law for an elementary step is given by the stoichiometry of the step, i. e. , • For A products, r = k[A] • For A + B products, r = k[A][B] • For 2 A products, r = k[A]2

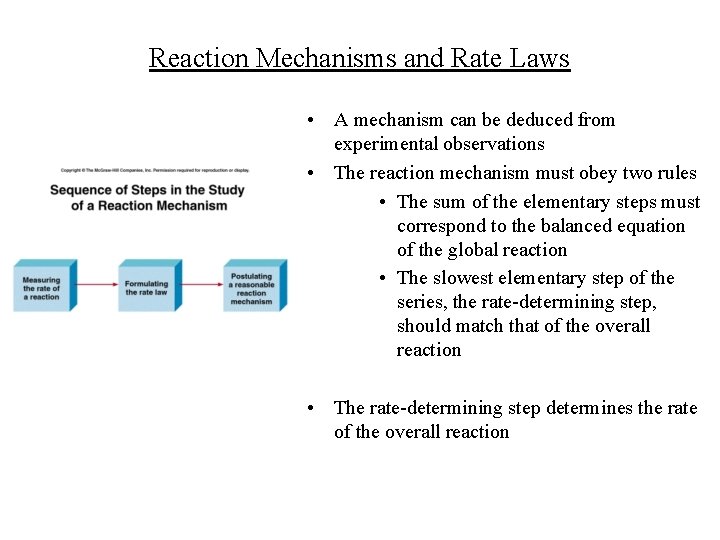
Reaction Mechanisms and Rate Laws • A mechanism can be deduced from experimental observations • The reaction mechanism must obey two rules • The sum of the elementary steps must correspond to the balanced equation of the global reaction • The slowest elementary step of the series, the rate-determining step, should match that of the overall reaction • The rate-determining step determines the rate of the overall reaction

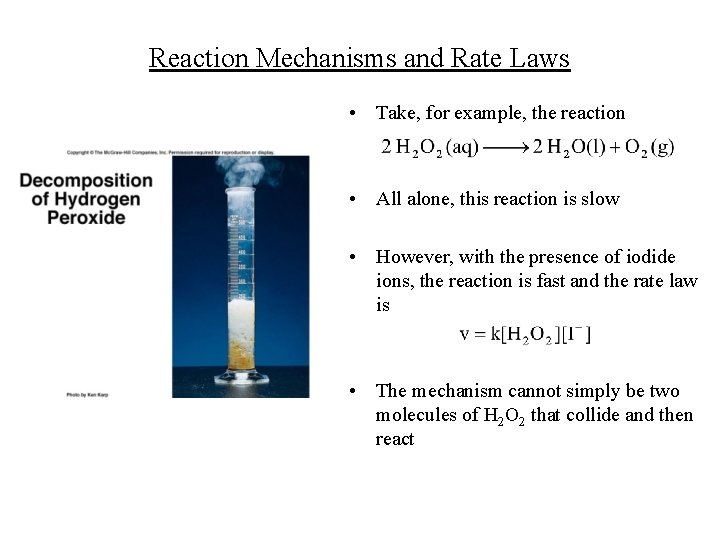
Reaction Mechanisms and Rate Laws • Take, for example, the reaction • All alone, this reaction is slow • However, with the presence of iodide ions, the reaction is fast and the rate law is • The mechanism cannot simply be two molecules of H 2 O 2 that collide and then react

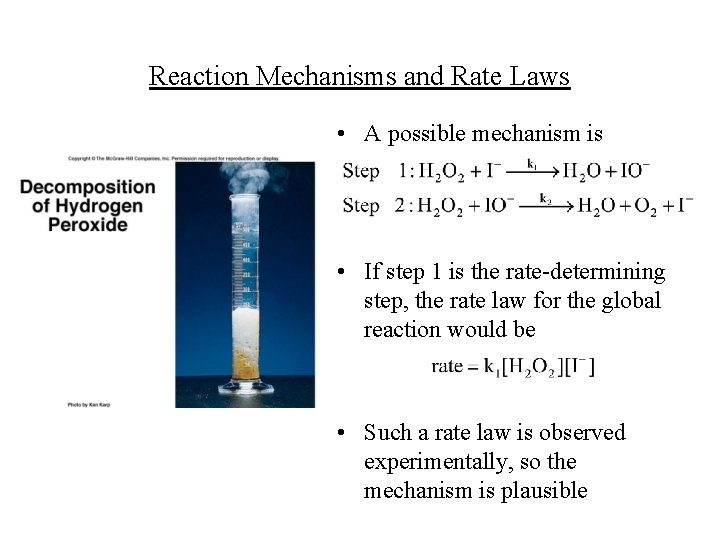
Reaction Mechanisms and Rate Laws • A possible mechanism is • If step 1 is the rate-determining step, the rate law for the global reaction would be • Such a rate law is observed experimentally, so the mechanism is plausible

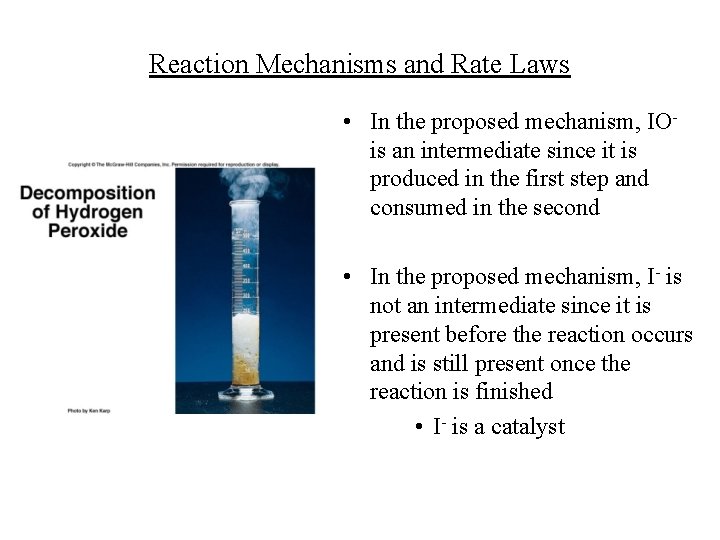
Reaction Mechanisms and Rate Laws • In the proposed mechanism, IOis an intermediate since it is produced in the first step and consumed in the second • In the proposed mechanism, I- is not an intermediate since it is present before the reaction occurs and is still present once the reaction is finished • I- is a catalyst

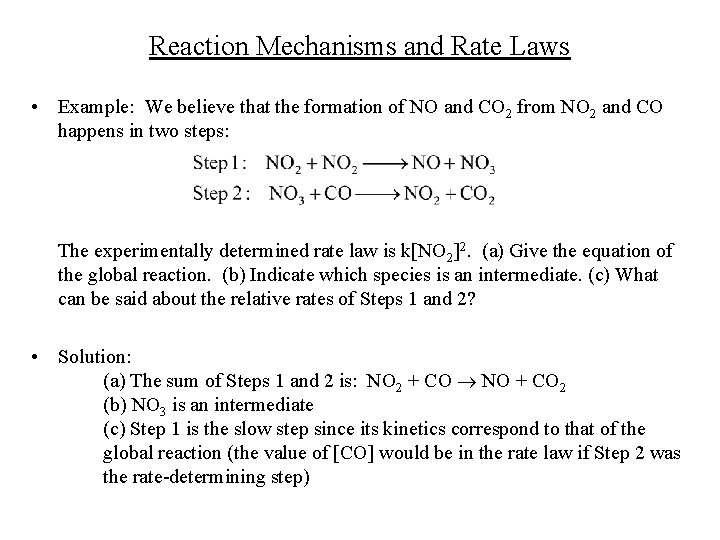
Reaction Mechanisms and Rate Laws • Example: We believe that the formation of NO and CO 2 from NO 2 and CO happens in two steps: The experimentally determined rate law is k[NO 2]2. (a) Give the equation of the global reaction. (b) Indicate which species is an intermediate. (c) What can be said about the relative rates of Steps 1 and 2? • Solution: (a) The sum of Steps 1 and 2 is: NO 2 + CO NO + CO 2 (b) NO 3 is an intermediate (c) Step 1 is the slow step since its kinetics correspond to that of the global reaction (the value of [CO] would be in the rate law if Step 2 was the rate-determining step)

• The reaction A(aq) B(aq) is a first-order reaction with respect to A. The half-life at 25. 0 o. C is 875. 3 s. If the activation energy of this reaction is 35. 1 k. J/mol, what would the concentration of A be after 1000. 0 s if we have an initial concentration of A of 0. 500 M and temperature is held constant at 50. 0 o. C?
 Rate of reaction
Rate of reaction Activity formula
Activity formula Factors affecting rate of chemical reaction
Factors affecting rate of chemical reaction Kinetics reaction
Kinetics reaction Definition of chemical kinetics in chemistry
Definition of chemical kinetics in chemistry Chemistry grade 11 unit 4
Chemistry grade 11 unit 4 Order of kinetics
Order of kinetics Chemical kinetics definition
Chemical kinetics definition Chemical kinetics experiment
Chemical kinetics experiment Applications of chemical kinetics
Applications of chemical kinetics Steady state kinetics
Steady state kinetics E1cb elimination reaction
E1cb elimination reaction Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Equilibrium reaction rate
Equilibrium reaction rate How to determine the rate law of a reaction
How to determine the rate law of a reaction Overall rate law of a reaction
Overall rate law of a reaction How to calculate rate of reaction
How to calculate rate of reaction How to find the rate law
How to find the rate law How to calculate instantaneous rate of reaction
How to calculate instantaneous rate of reaction Rate law for first order reaction
Rate law for first order reaction Initial rate of reaction
Initial rate of reaction Factors affecting rate constant
Factors affecting rate constant Overall rate law of a reaction
Overall rate law of a reaction What is catalyst and how it affects reaction rate
What is catalyst and how it affects reaction rate How does temperature affect rate of reaction
How does temperature affect rate of reaction Average rate of reaction formula
Average rate of reaction formula How to determine the rate law of a reaction
How to determine the rate law of a reaction How to calculate activation energy
How to calculate activation energy How to write reaction rate
How to write reaction rate Rate of reaction quiz
Rate of reaction quiz Surface area affecting rate of reaction
Surface area affecting rate of reaction