RATES OF REACTION DETERMINING RATE OF REACTION The


![RELATIVE RATE CALCULATIONS A -1 a -∆[A] ∆t + = B -1 b C RELATIVE RATE CALCULATIONS A -1 a -∆[A] ∆t + = B -1 b C](https://slidetodoc.com/presentation_image_h/cf33e0b1c9da31eb0c84810e77f6d14c/image-15.jpg)




![Experiment [A] (M) Rate (mol dm-3 s-1) 1 1. 0 0. 60 2 2. Experiment [A] (M) Rate (mol dm-3 s-1) 1 1. 0 0. 60 2 2.](https://slidetodoc.com/presentation_image_h/cf33e0b1c9da31eb0c84810e77f6d14c/image-35.jpg)
![3 A + B C + D Experiment [A] (M) [B] (M) Rate (mol 3 A + B C + D Experiment [A] (M) [B] (M) Rate (mol](https://slidetodoc.com/presentation_image_h/cf33e0b1c9da31eb0c84810e77f6d14c/image-36.jpg)

![f) The rate when [A] = 1. 60 M and [B] = 0. 30 f) The rate when [A] = 1. 60 M and [B] = 0. 30](https://slidetodoc.com/presentation_image_h/cf33e0b1c9da31eb0c84810e77f6d14c/image-39.jpg)
![PRACTICE PROBLEMS Experiment [A] (M) [B] (M) Rate (mol dm-3 s-1) 1 1. 20 PRACTICE PROBLEMS Experiment [A] (M) [B] (M) Rate (mol dm-3 s-1) 1 1. 20](https://slidetodoc.com/presentation_image_h/cf33e0b1c9da31eb0c84810e77f6d14c/image-40.jpg)



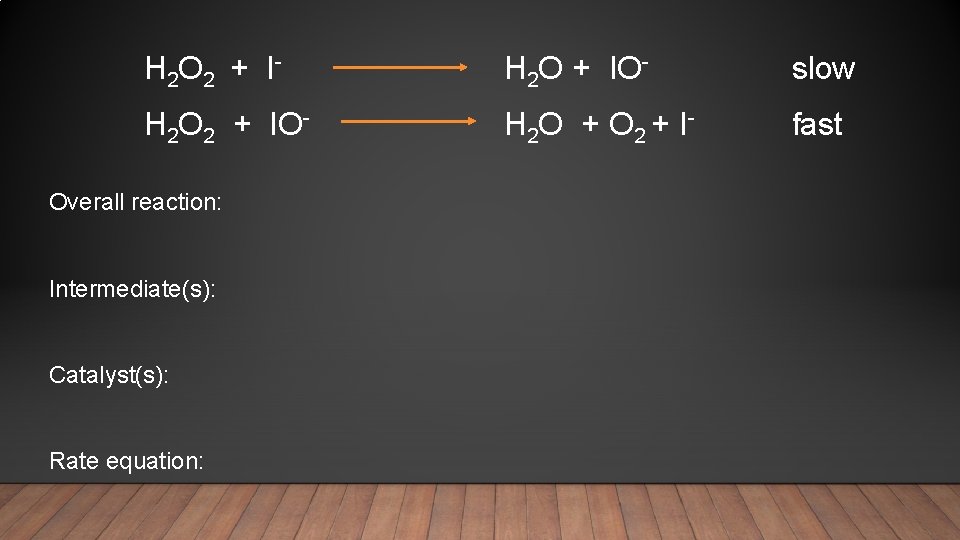
- Slides: 59

RATES OF REACTION

DETERMINING RATE OF REACTION • The rate of a chemical reaction is the speed at which reactants are used up or products are formed • Rate of reaction is measured experimentally. The method is dependant on the type of chemical reaction

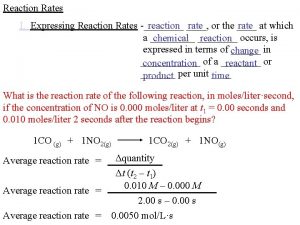
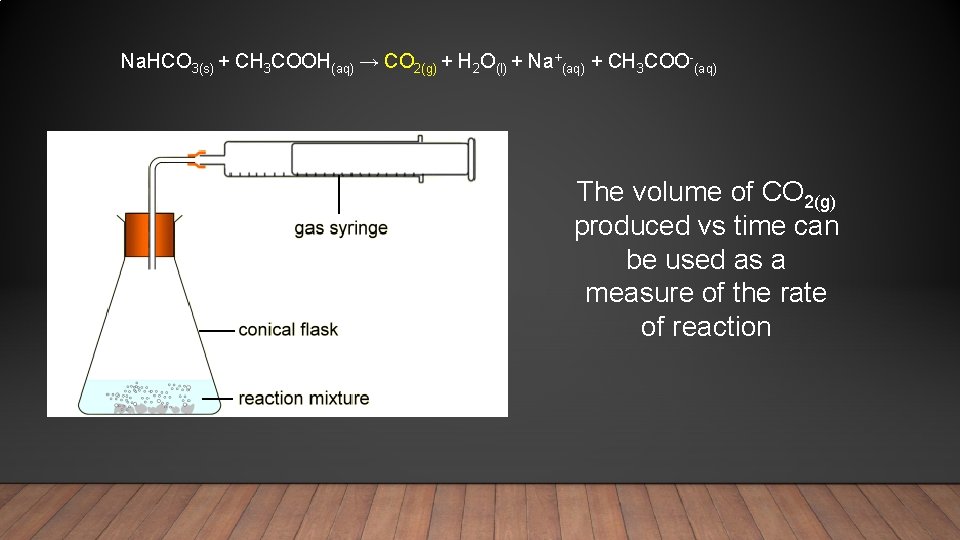
Na. HCO 3(s) + CH 3 COOH(aq) → CO 2(g) + H 2 O(l) + Na+(aq) + CH 3 COO-(aq) The volume of CO 2(g) produced vs time can be used as a measure of the rate of reaction

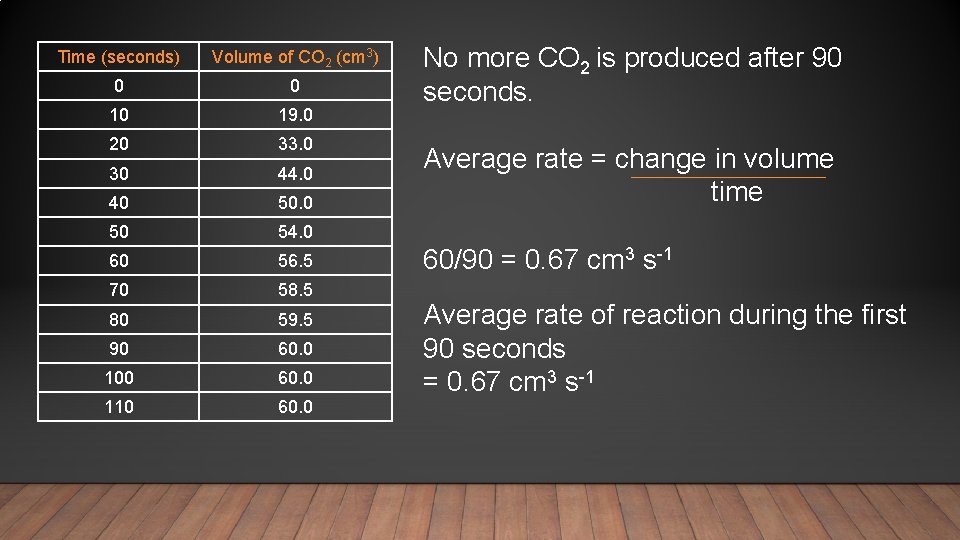
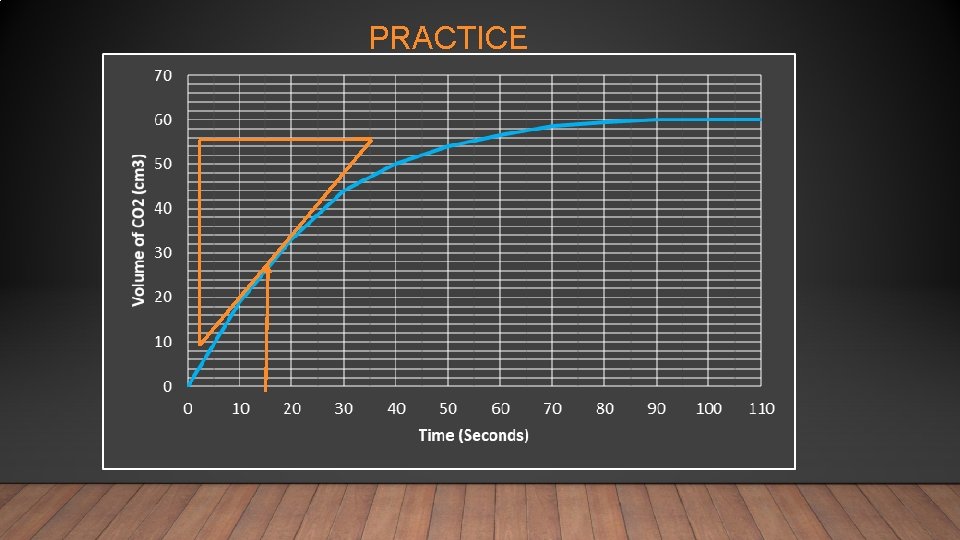
Time (seconds) Volume of CO 2 (cm 3) 0 0 10 19. 0 20 33. 0 30 44. 0 40 50 54. 0 60 56. 5 70 58. 5 80 59. 5 90 60. 0 100 60. 0 110 60. 0 No more CO 2 is produced after 90 seconds. Average rate = change in volume time 60/90 = 0. 67 cm 3 s-1 Average rate of reaction during the first 90 seconds = 0. 67 cm 3 s-1

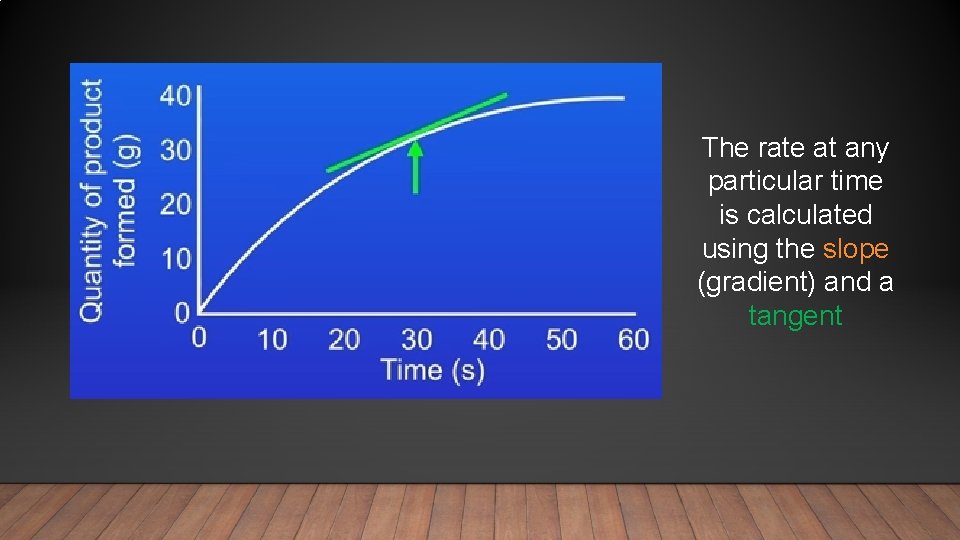
The rate at any particular time is calculated using the slope (gradient) and a tangent

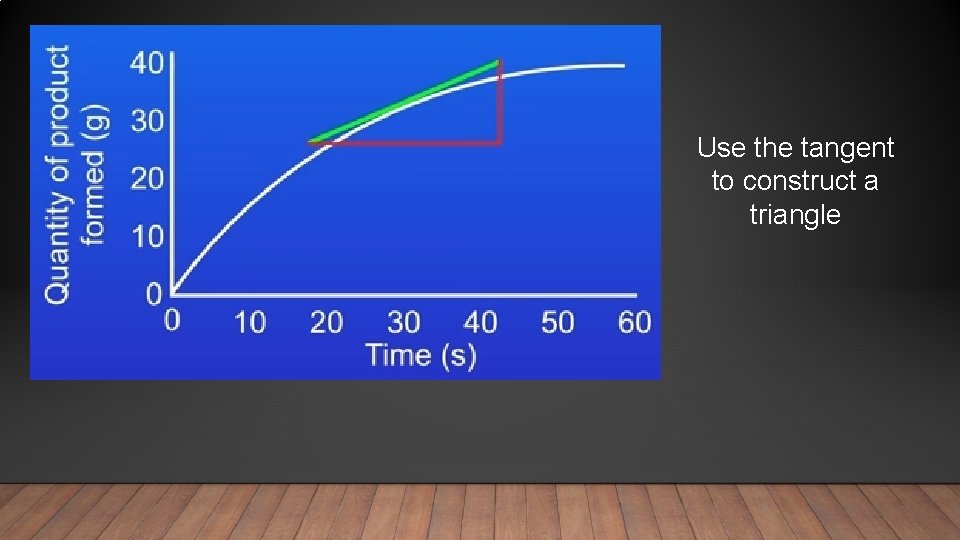
Use the tangent to construct a triangle

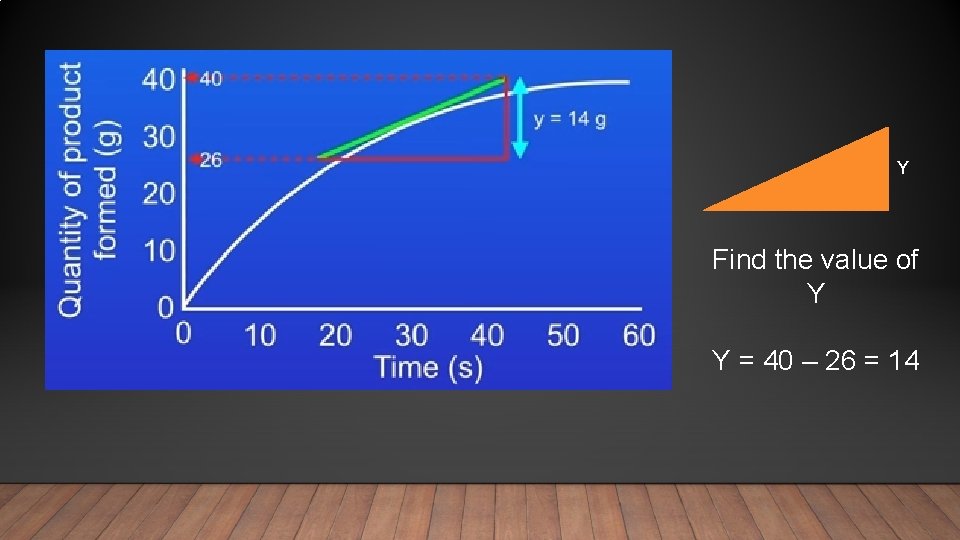
Y Find the value of Y Y = 40 – 26 = 14

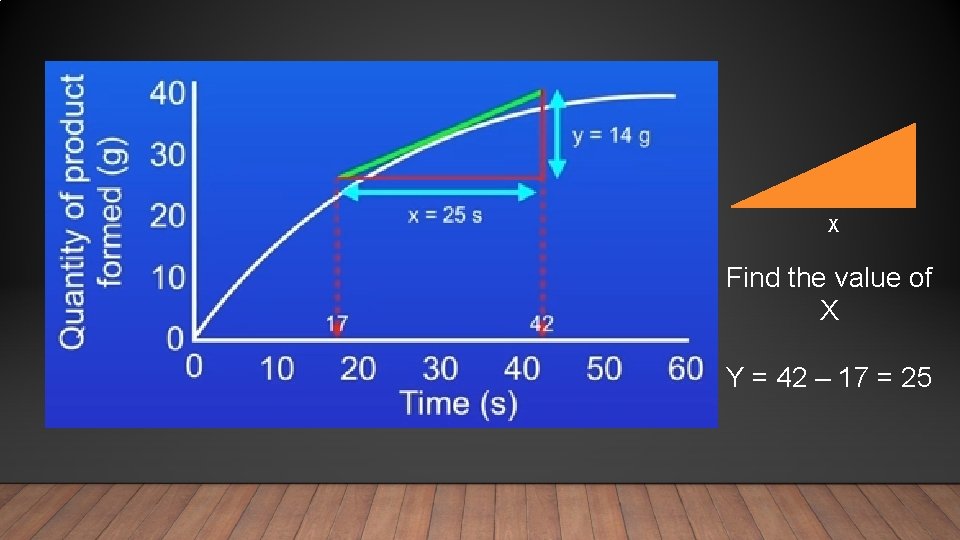
X Find the value of X Y = 42 – 17 = 25

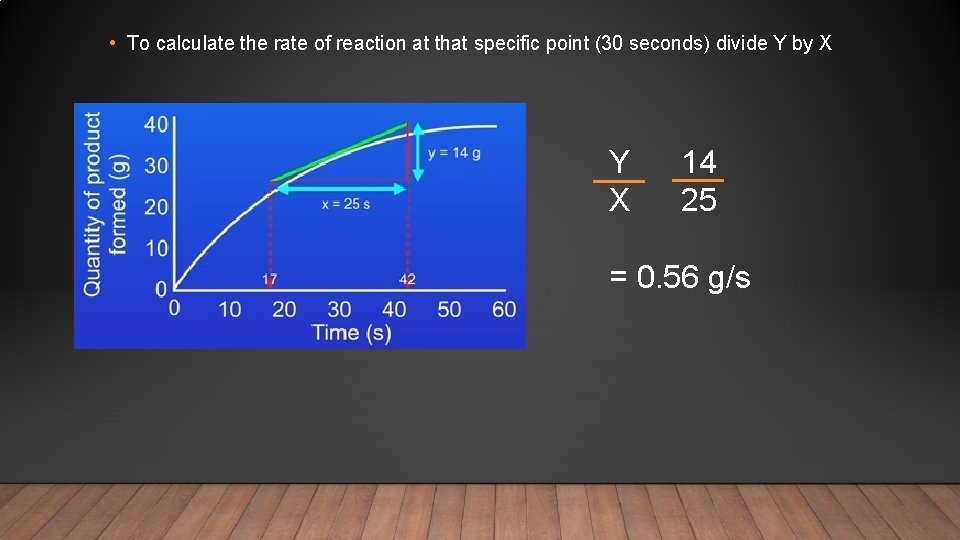
• To calculate the rate of reaction at that specific point (30 seconds) divide Y by X Y X 14 25 = 0. 56 g/s

PRACTICE

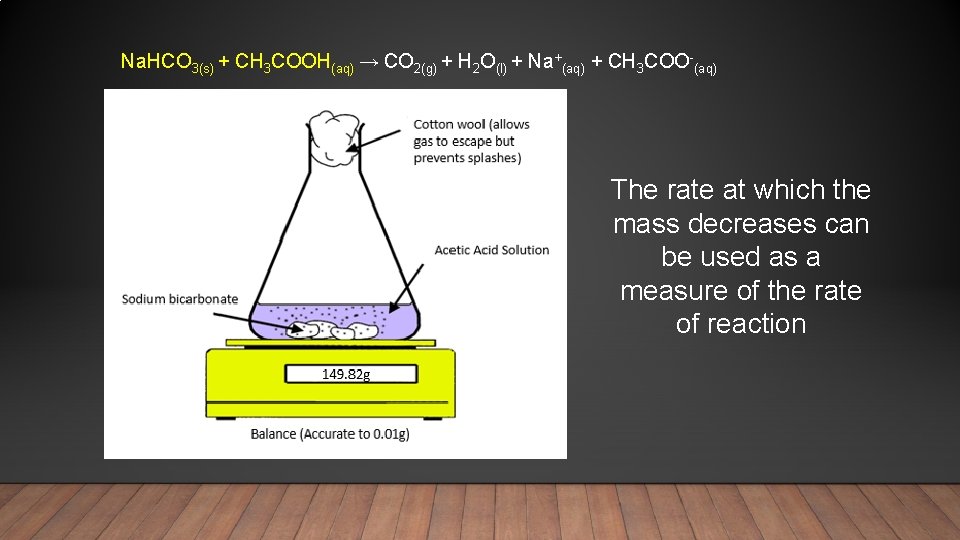
Na. HCO 3(s) + CH 3 COOH(aq) → CO 2(g) + H 2 O(l) + Na+(aq) + CH 3 COO-(aq) The rate at which the mass decreases can be used as a measure of the rate of reaction

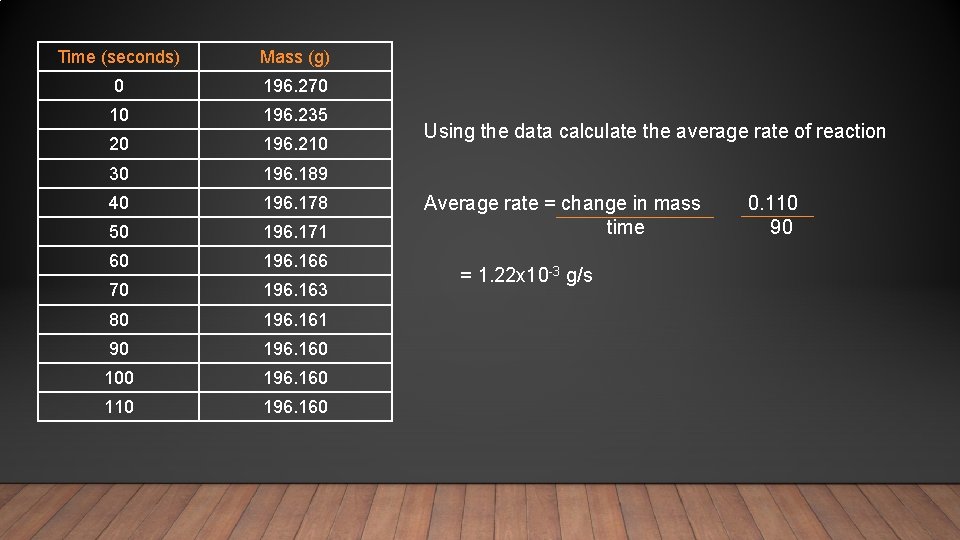
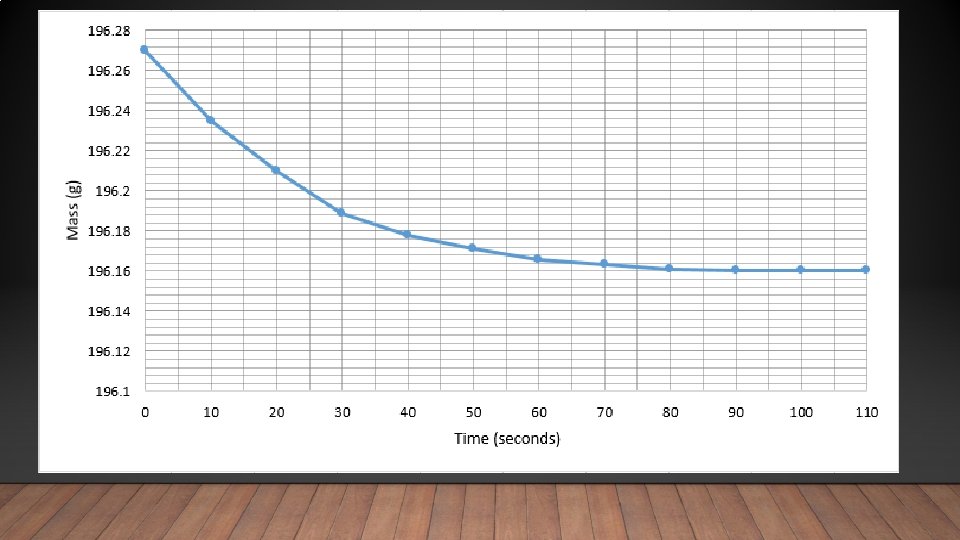
Time (seconds) Mass (g) 0 196. 270 10 196. 235 20 196. 210 30 196. 189 40 196. 178 50 196. 171 60 196. 166 70 196. 163 80 196. 161 90 196. 160 100 196. 160 110 196. 160 Using the data calculate the average rate of reaction Average rate = change in mass time = 1. 22 x 10 -3 g/s 0. 110 90


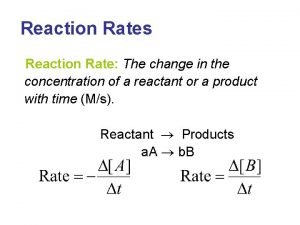
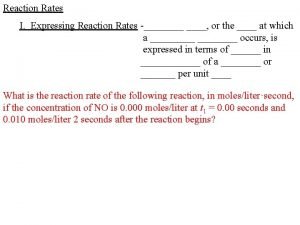
RATE OF REACTION DEFINED • Although the previous methods (volume and mass) are ways that the rate of a reaction can be measured, the change in concentration is the most common. • Rate of reaction is the change in concentration of reactants or products per unit time • Common units: mol dm-3 s-1 mol dm-3 min-1 • Average rate = change in concentration / time • As before a tangent can be used to calculate the rate at a given time
![RELATIVE RATE CALCULATIONS A 1 a A t B 1 b C RELATIVE RATE CALCULATIONS A -1 a -∆[A] ∆t + = B -1 b C](https://slidetodoc.com/presentation_image_h/cf33e0b1c9da31eb0c84810e77f6d14c/image-15.jpg)
RELATIVE RATE CALCULATIONS A -1 a -∆[A] ∆t + = B -1 b C -∆[B] ∆t 1 c = 4 NH 3 + 5 O 2 -1 4 -∆[A] ∆t = -1 5 ∆[C] ∆t D ∆[D] ∆t 1 d = 4 NO + 6 H 2 O -∆[B] ∆t = 1 4 ∆[C] ∆t = 1 6 ∆[D] ∆t

4 NH 3 + 5 O 2 4 NO + 6 H 2 O In the first 10 seconds the [NH 3] decreased from 0. 8 M to 0. 4 M. What is the average reaction rate? -1 a -∆[A] ∆t At what rate is H 2 O being formed? 6 moles of H 2 O are formed for every 4 moles of NH 3 that react with oxygen -1 4 - 0. 4 10 = 0. 01 mol dm-3 s-1 Hint: look at the ratios (6/4) x 0. 02 = 0. 015 mol dm-3 s-1 (d/a)

PRACTICE PROBLEMS 2 Na. OH(aq) + Cu. SO 4(aq) Na 2 SO 4(aq) + Cu(OH)2(s) In the first 15 seconds [Na. OH] decreased from 2 M to 1. 4 M. What is the average rate of reaction? ( -1 / 2 ) x ( - 0. 6 / 15) = 0. 02 mol/dm-3/s At what rate is Cu(OH)2(s) formed? For every 2 moles of Na. OH used 1 mole of Cu(OH)2 is produced 0. 02 / 2 = 0. 01 mol/dm-3/s

PRACTICE PROBLEMS 4 NO 2(g) + O 2(g) 2 N 2 O 5(g) At a particular moment during the reaction, molecular oxygen is reacting at a rate of 0. 024 mol dm-3 s-1. (a) At what rate is N 2 O 5 being formed? (b) At what rate is NO 2 reacting

PRACTICE PROBLEMS 2 Na 3 PO 4 + Ca. Cl 3 2 After 18 seconds the [Ca. Cl 2] decreases by 0. 9 M. (a) At what rate is Na. Cl being formed? (b) At what rate is Na 3 PO 4 reacting? 6 Na. Cl + Ca 3(PO 4)2

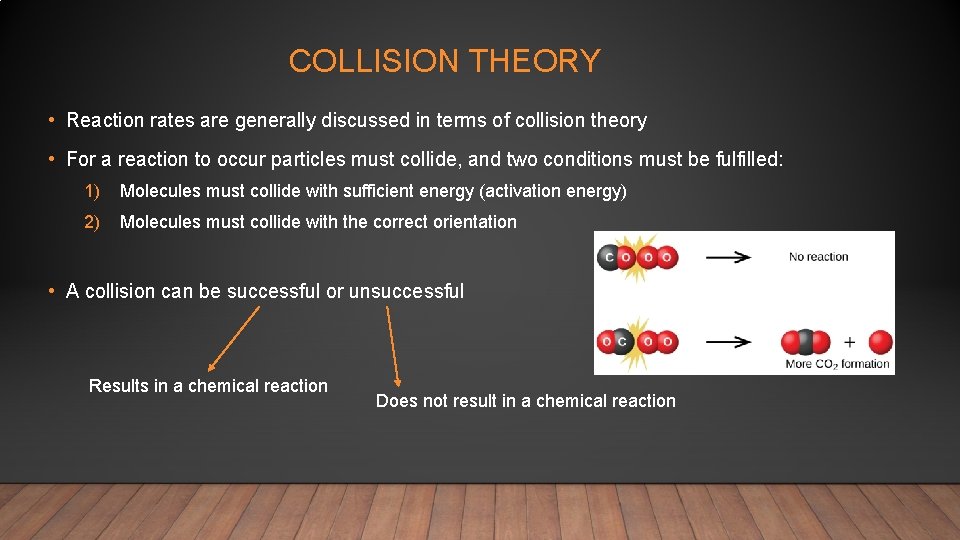
COLLISION THEORY • Reaction rates are generally discussed in terms of collision theory • For a reaction to occur particles must collide, and two conditions must be fulfilled: 1) Molecules must collide with sufficient energy (activation energy) 2) Molecules must collide with the correct orientation • A collision can be successful or unsuccessful Results in a chemical reaction Does not result in a chemical reaction

The main factors that affect the rate of a chemical reaction are: 1) The concentration of reactants 2) Pressure (for reactions involving gases) 3) Surface area (solid reactants) 4) Temperature 5) Catalysts

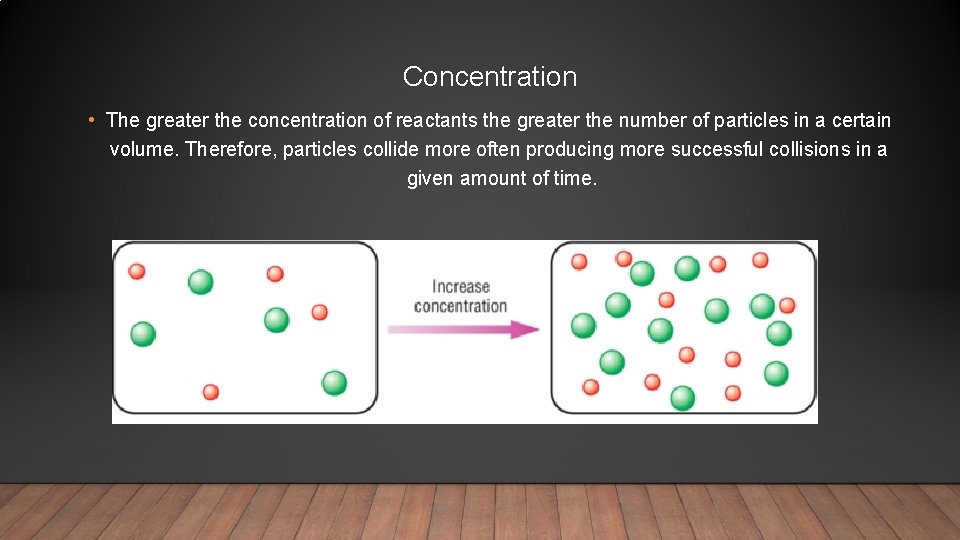
Concentration • The greater the concentration of reactants the greater the number of particles in a certain volume. Therefore, particles collide more often producing more successful collisions in a given amount of time.

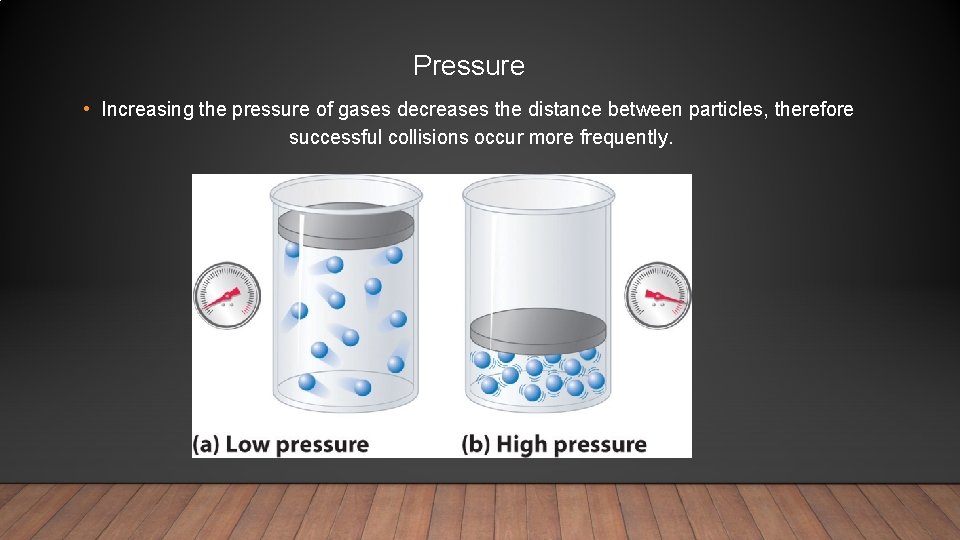
Pressure • Increasing the pressure of gases decreases the distance between particles, therefore successful collisions occur more frequently.

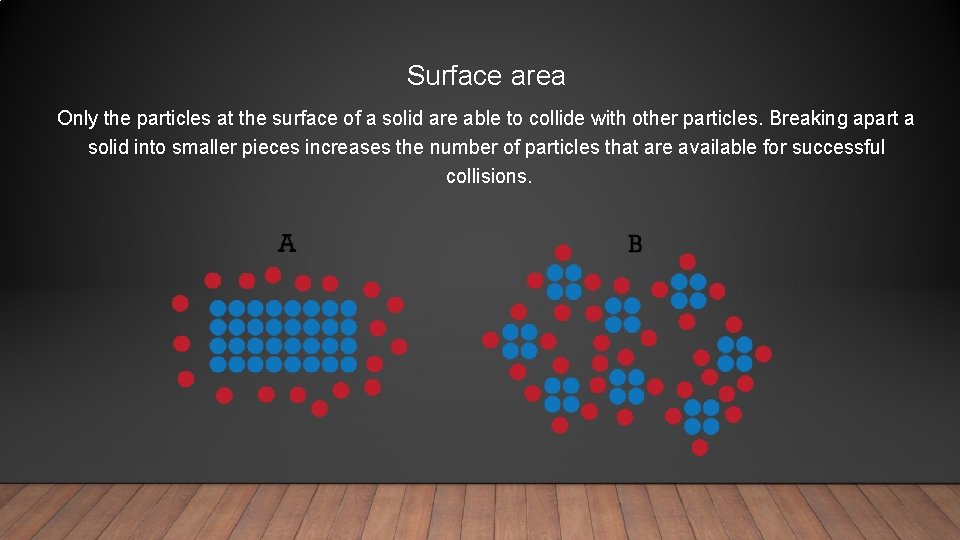
Surface area Only the particles at the surface of a solid are able to collide with other particles. Breaking apart a solid into smaller pieces increases the number of particles that are available for successful collisions.

Temperature Increasing the temperature has a major effect on the rate of reaction. • As temperature increases the average kinetic energy of the particles increases and collision occurs more frequently. However, the mass of the particles needs to be considered. • Example – oxygen and helium are heated to 600 K. The mass of an O 2 molecule is 8 times the mass of a helium atom. Therefore, the helium atoms will be travelling substantially faster at the same temperature. • Increasing the temperature means that the particles collide more often and with greater energy, therefore there is a greater probability of a successful collision

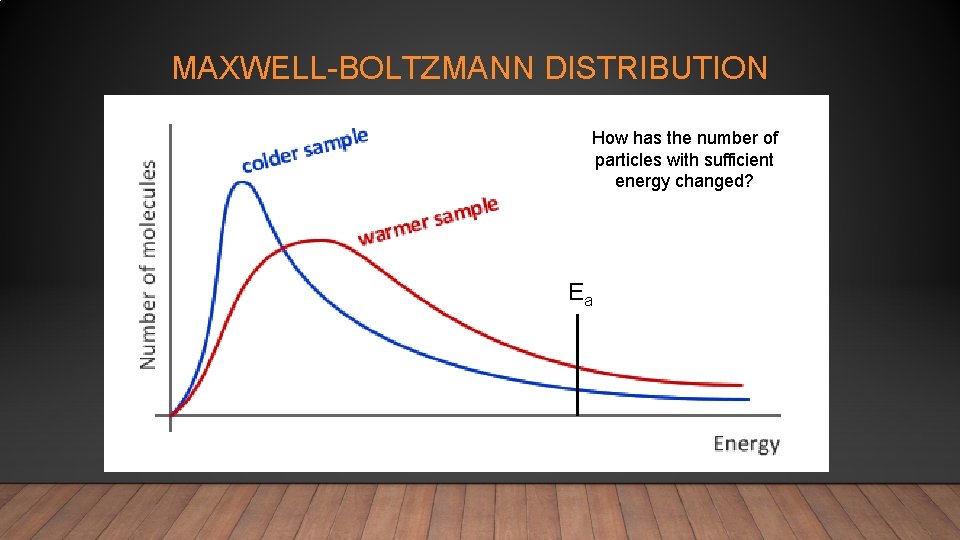
MAXWELL-BOLTZMANN DISTRIBUTION How has the number of particles with sufficient energy changed? Ea

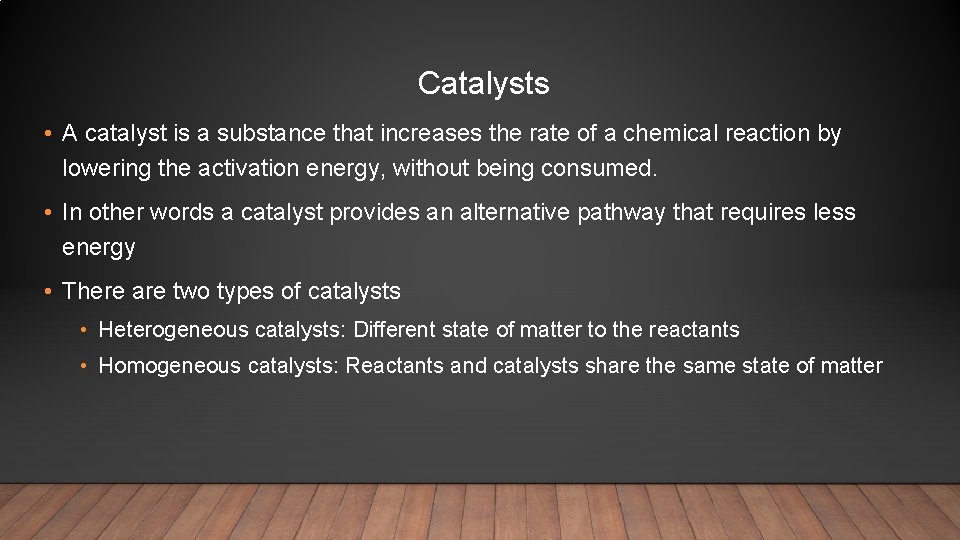
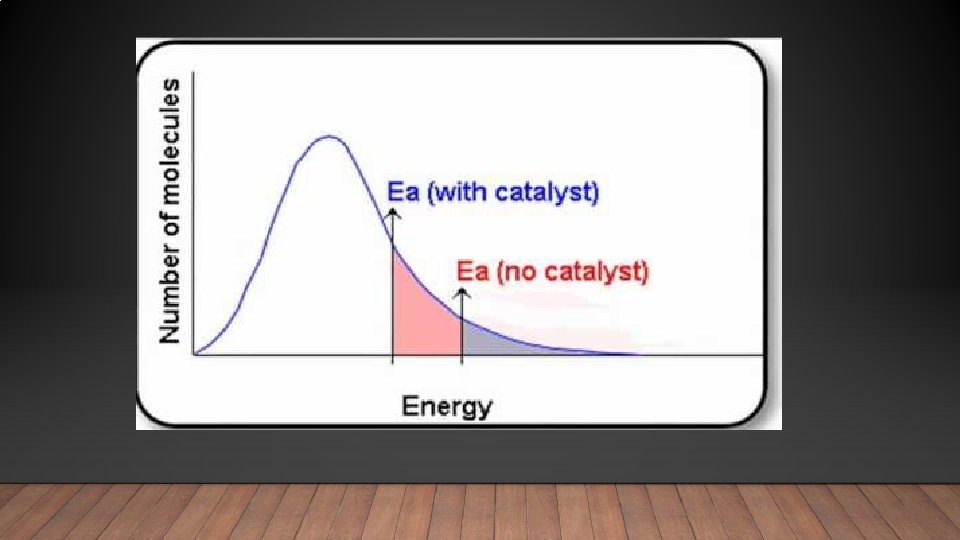
Catalysts • A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy, without being consumed. • In other words a catalyst provides an alternative pathway that requires less energy • There are two types of catalysts • Heterogeneous catalysts: Different state of matter to the reactants • Homogeneous catalysts: Reactants and catalysts share the same state of matter


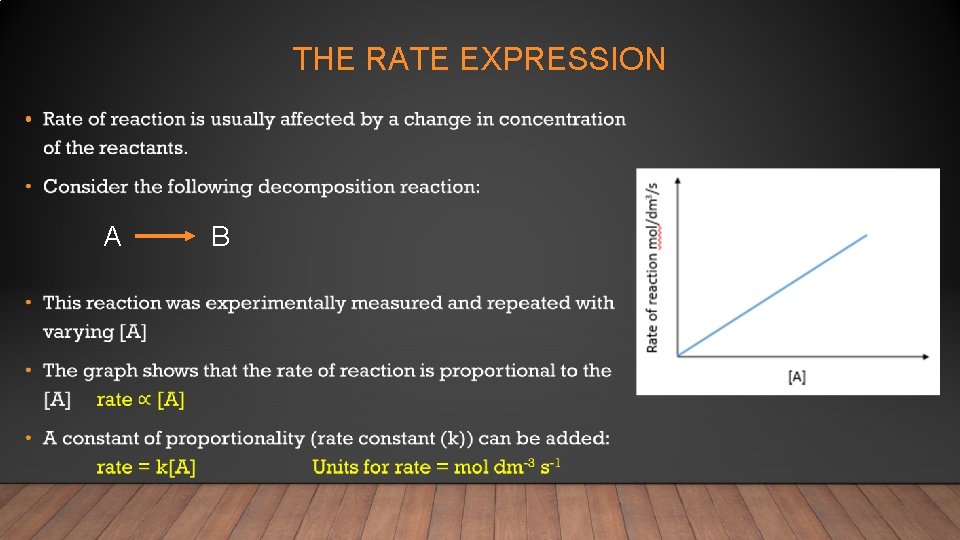
THE RATE EXPRESSION • A B

• A rate expression can be written for any reaction: a. A + b. B Rate = k[A]x [B]y c. C + d. D Note that the rate expression is only for reactants and that x and y are not the coefficients of A and B • The x and y exponents must be determined experimentally: x = the order with respect to A y = the order with respect to B • In order to know the value of x experiments have to be completed using a fixed amount of B and varying [A] • In order to know the value of y experiments have to be completed using a fixed amount of A and varying [B] *** There is no connection between the chemical equation and the rate expression***

ORDER OF REACTIONS • A reaction can be zero order, first order or second order with respect to the concentration of reactants • Zero order : the rate of reaction does not vary with reactant concentration 2 NH 3(g) N 2(g) + 3 H 2(g) Rate = k[A]0 Rate = k Units for k = mol dm-3 s-1

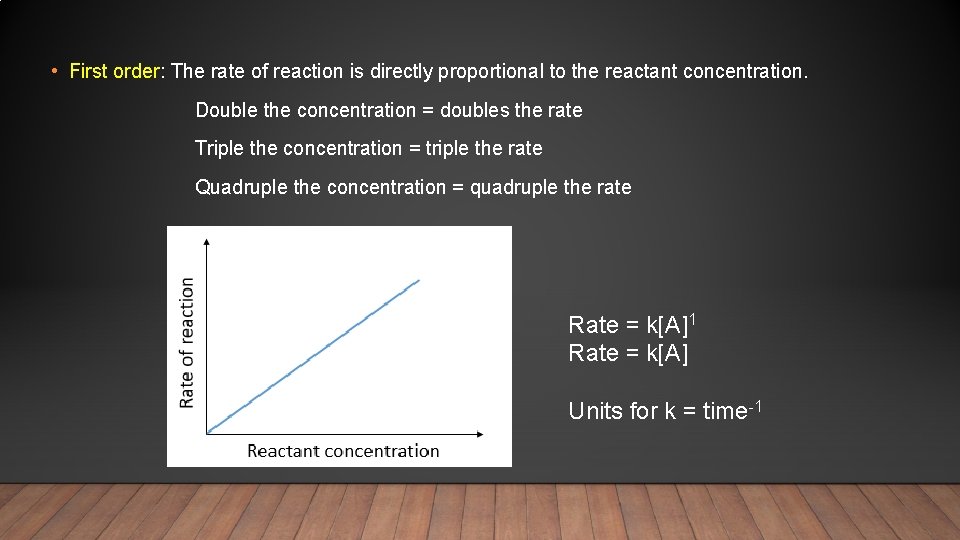
• First order: The rate of reaction is directly proportional to the reactant concentration. Double the concentration = doubles the rate Triple the concentration = triple the rate Quadruple the concentration = quadruple the rate Rate = k[A]1 Rate = k[A] Units for k = time-1

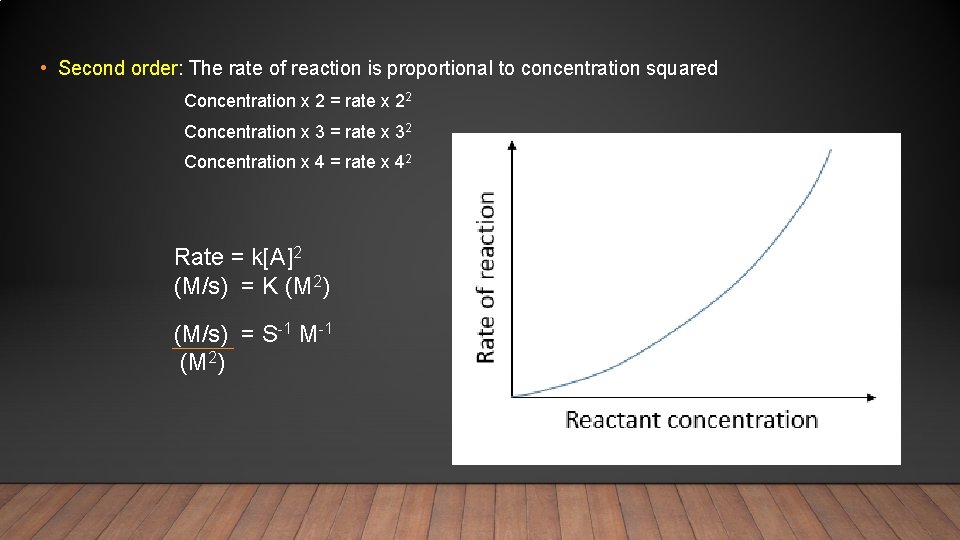
• Second order: The rate of reaction is proportional to concentration squared Concentration x 2 = rate x 22 Concentration x 3 = rate x 32 Concentration x 4 = rate x 42 Rate = k[A]2 (M/s) = K (M 2) (M/s) = S-1 M-1 (M 2)

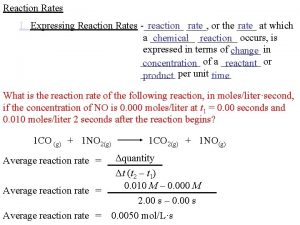
EXPERIMENTAL DATA Reaction : 2 A B We want to determine: 1) The order of reaction with respect to A 2) The rate expression 3) The value of the rate constant (k), with untits 4) The rate of reaction when [A] = 1. 3 mol/dm 3
![Experiment A M Rate mol dm3 s1 1 1 0 0 60 2 2 Experiment [A] (M) Rate (mol dm-3 s-1) 1 1. 0 0. 60 2 2.](https://slidetodoc.com/presentation_image_h/cf33e0b1c9da31eb0c84810e77f6d14c/image-35.jpg)
Experiment [A] (M) Rate (mol dm-3 s-1) 1 1. 0 0. 60 2 2. 0 1. 2 3 5. 0 3. 0 1) The experimental data shows that the rate of reaction is proportional to the [A]. = First order 2) The rate expression is: rate = k[A] As it is first order [A] = [A]1 3) The find the value of k we can use the data from any of the experiments, wherein k = rate / [A] Experiment 1: k = 0. 60 1. 0 mol dm-3 s-1 mol dm-3 4) The rate when [A] = 1. 3 mol dm-3 rate = k[A] rate = 0. 60 x 1. 3 = 0. 78 mol dm-3 s-1
![3 A B C D Experiment A M B M Rate mol 3 A + B C + D Experiment [A] (M) [B] (M) Rate (mol](https://slidetodoc.com/presentation_image_h/cf33e0b1c9da31eb0c84810e77f6d14c/image-36.jpg)
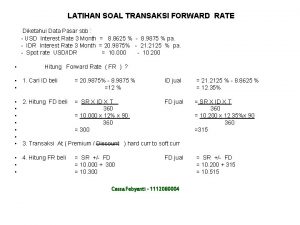
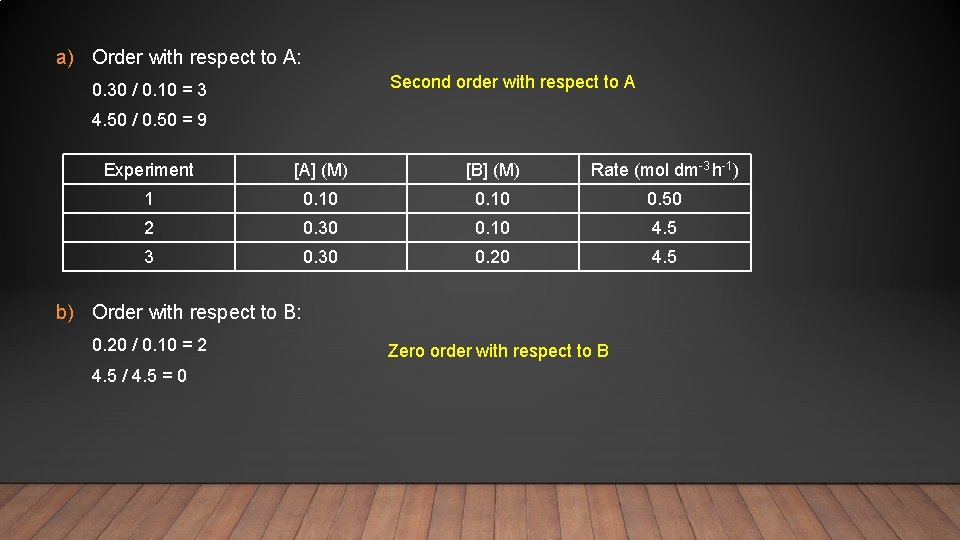
3 A + B C + D Experiment [A] (M) [B] (M) Rate (mol dm-3 h-1) 1 0. 10 0. 50 2 0. 30 0. 10 4. 5 3 0. 30 0. 20 4. 5 • Given the experimental data above, calculate each of the following for the reaction: a) The order with respect to A b) The order with respect to B c) The overall order of reaction d) The rate expression e) The value of k f) The rate when [A] = 1. 60 M and [B] = 0. 30 M

a) Order with respect to A: Second order with respect to A 0. 30 / 0. 10 = 3 4. 50 / 0. 50 = 9 Experiment [A] (M) [B] (M) Rate (mol dm-3 h-1) 1 0. 10 0. 50 2 0. 30 0. 10 4. 5 3 0. 30 0. 20 4. 5 b) Order with respect to B: 0. 20 / 0. 10 = 2 4. 5 / 4. 5 = 0 Zero order with respect to B

c) The overall order of reaction is the sum of the orders with respect to A and B 2 + 0 = 2 Therefore, the overall order is 2 d) The rate expression: rate = k[A]2[B]0 rate = k[A]2 e) The value of the rate constant (k) Substitute in the values for rate and [A] from any of the experiments Rate = k[A]2 0. 50 = k 0. 102 k = 0. 50 / (0. 102) = 50 4. 50 = k 0. 302 k = 4. 50/ (0. 302) = 50 Units = mol dm-3 h-1 (mol dm-3)2 Experiment [A] (M) Rate (mol dm-3 h-1) 1 0. 10 0. 50 2 0. 30 4. 5 3 0. 30 4. 5
![f The rate when A 1 60 M and B 0 30 f) The rate when [A] = 1. 60 M and [B] = 0. 30](https://slidetodoc.com/presentation_image_h/cf33e0b1c9da31eb0c84810e77f6d14c/image-39.jpg)
f) The rate when [A] = 1. 60 M and [B] = 0. 30 M Rate = k[A]2 Rate = 50 x 1. 602 = 128 mol dm-3 h-1 Recall that [B] is not required to calculate the rate as it is zero order with respect to B
![PRACTICE PROBLEMS Experiment A M B M Rate mol dm3 s1 1 1 20 PRACTICE PROBLEMS Experiment [A] (M) [B] (M) Rate (mol dm-3 s-1) 1 1. 20](https://slidetodoc.com/presentation_image_h/cf33e0b1c9da31eb0c84810e77f6d14c/image-40.jpg)
PRACTICE PROBLEMS Experiment [A] (M) [B] (M) Rate (mol dm-3 s-1) 1 1. 20 2. 00 5. 00 x 10 -3 2 2. 40 2. 00 1. 00 x 10 -2 3 2. 40 8. 00 0. 16 Determine the following: a) The order with respect to A b) The order with respect to B c) The overall order of reaction d) The rate expression e) The value of K (with units) f) The rate of reaction when [A] = 3. 2 M and [B] = 2. 4 M

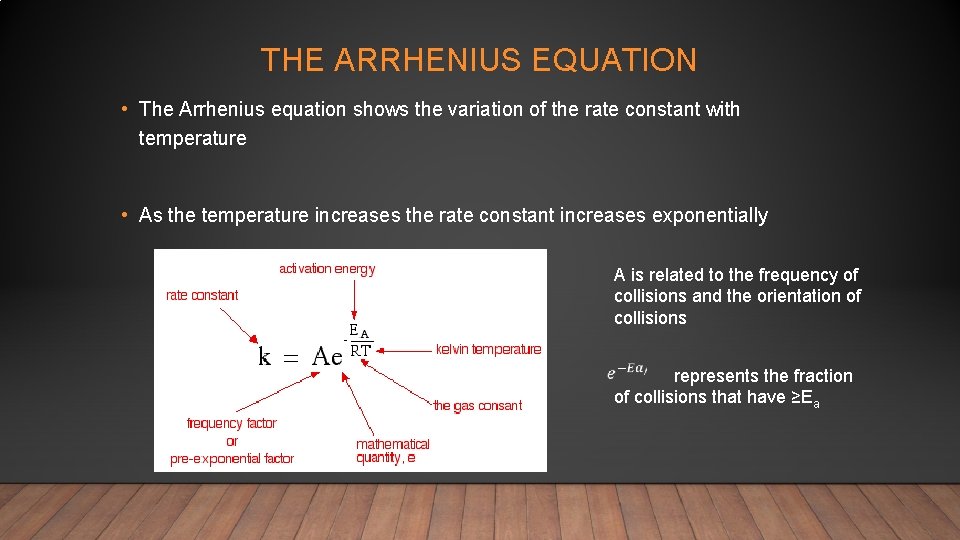
THE ARRHENIUS EQUATION • The Arrhenius equation shows the variation of the rate constant with temperature • As the temperature increases the rate constant increases exponentially A is related to the frequency of collisions and the orientation of collisions represents the fraction of collisions that have ≥Ea

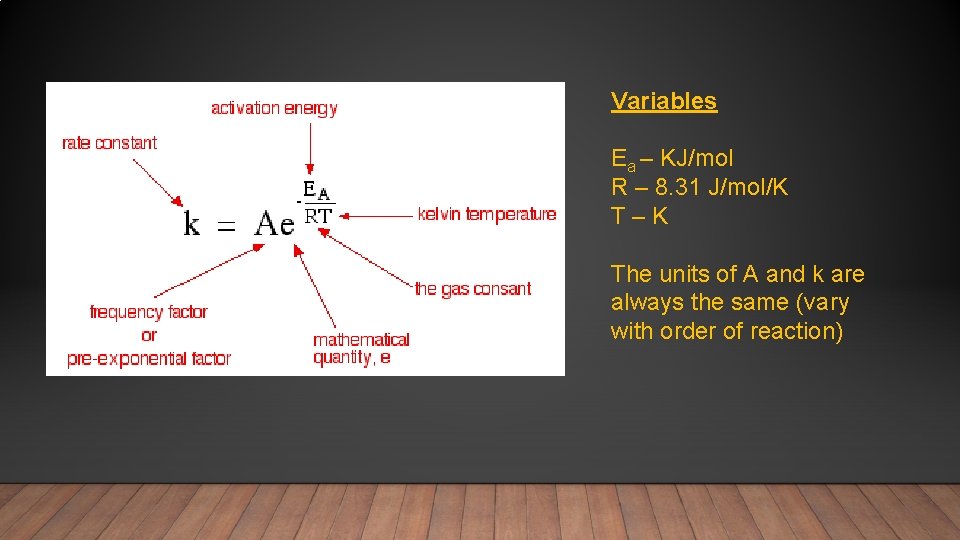
Variables Ea – KJ/mol R – 8. 31 J/mol/K T – K The units of A and k are always the same (vary with order of reaction)

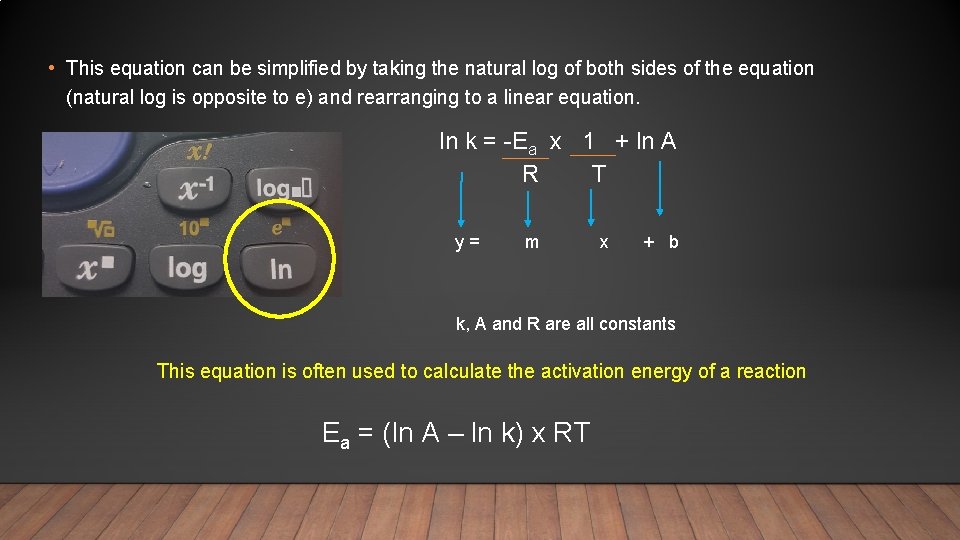
• This equation can be simplified by taking the natural log of both sides of the equation (natural log is opposite to e) and rearranging to a linear equation. ln k = -Ea x 1 + ln A R T y = m x + b k, A and R are all constants This equation is often used to calculate the activation energy of a reaction Ea = (ln A – ln k) x RT

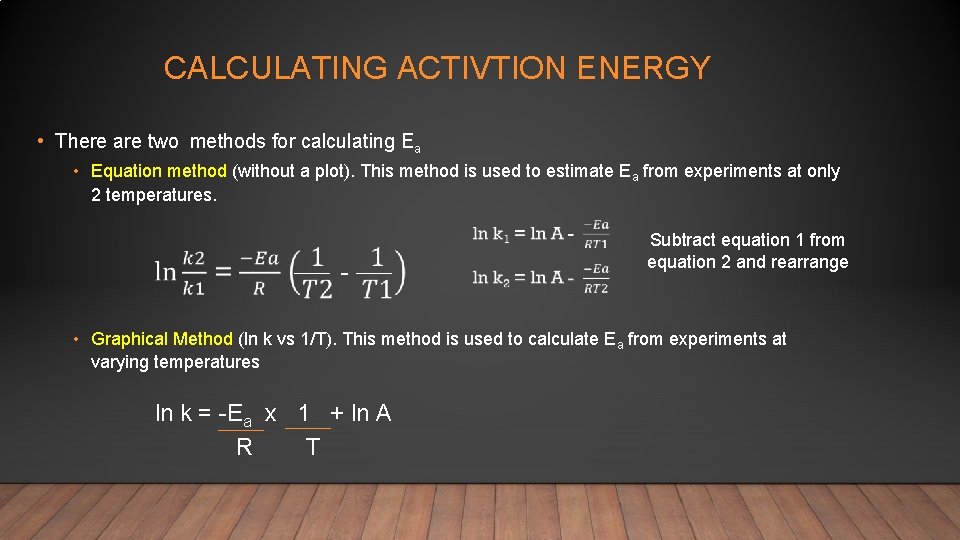
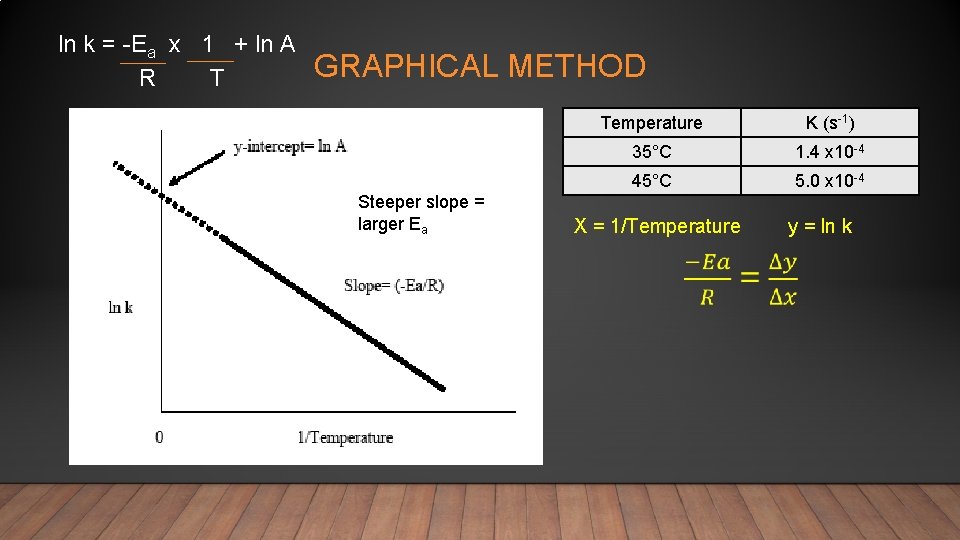
CALCULATING ACTIVTION ENERGY • There are two methods for calculating Ea • Equation method (without a plot). This method is used to estimate Ea from experiments at only 2 temperatures. - Subtract equation 1 from equation 2 and rearrange • Graphical Method (ln k vs 1/T). This method is used to calculate Ea from experiments at varying temperatures ln k = -Ea x 1 + ln A R T

Step 3 – Rewrite with calculated values Temperature K (s-1) 35°C 1. 4 x 10 -4 45°C 5. 0 x 10 -4 Step 4 – Solve for Ea Step 1 – convert temperatures to kelvin T 1 = 35 + 273. 15 = 308 K T 2 = 45 + 273. 15 = 318 K Step 2 – Put values in the equation Step 5 – Units

GRAPHICAL METHOD

ln k = -Ea x 1 + ln A R T GRAPHICAL METHOD Steeper slope = larger Ea Temperature K (s-1) 35°C 1. 4 x 10 -4 45°C 5. 0 x 10 -4 X = 1/Temperature y = ln k

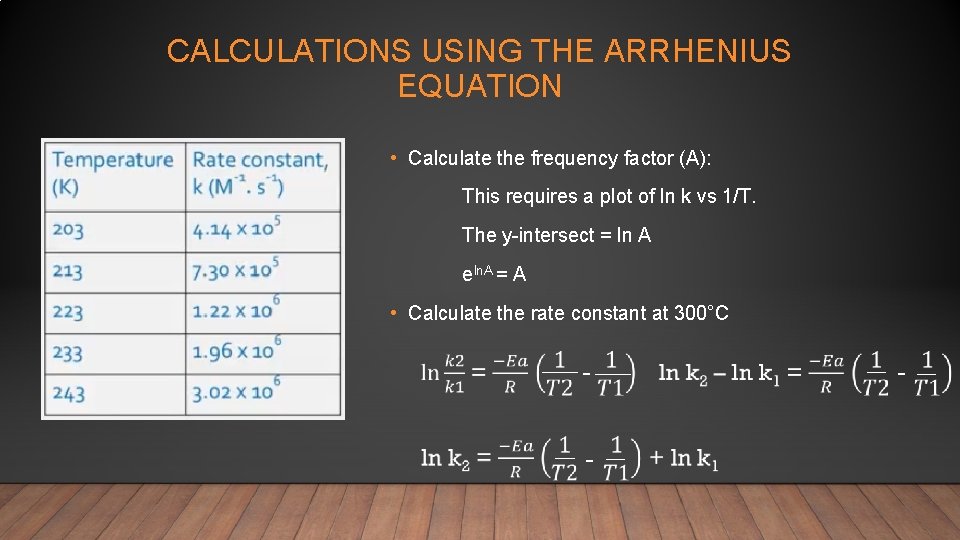
CALCULATIONS USING THE ARRHENIUS EQUATION • Calculate the frequency factor (A): This requires a plot of ln k vs 1/T. The y-intersect = ln A eln. A = A • Calculate the rate constant at 300°C - - -

• Calculating the fraction of molecules with energy > activation energy F = e-Ea/RT • Calculate the temperature at which k has a specific value: ln k – ln A = -Ea/RT T = -Ea / ((ln k – ln A) R)

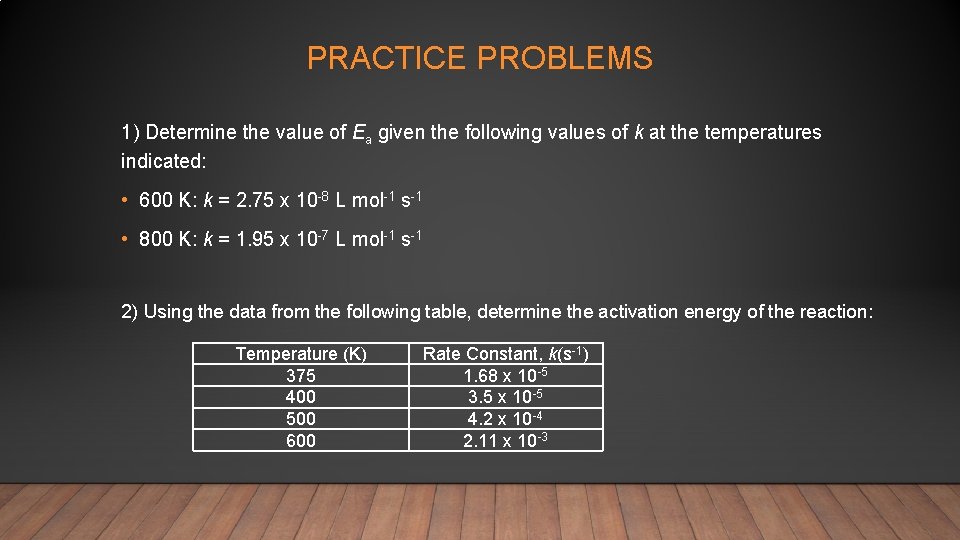
PRACTICE PROBLEMS 1) Determine the value of Ea given the following values of k at the temperatures indicated: • 600 K: k = 2. 75 x 10 -8 L mol-1 s-1 • 800 K: k = 1. 95 x 10 -7 L mol-1 s-1 2) Using the data from the following table, determine the activation energy of the reaction: Temperature (K) 375 400 500 600 Rate Constant, k(s-1) 1. 68 x 10 -5 3. 5 x 10 -5 4. 2 x 10 -4 2. 11 x 10 -3

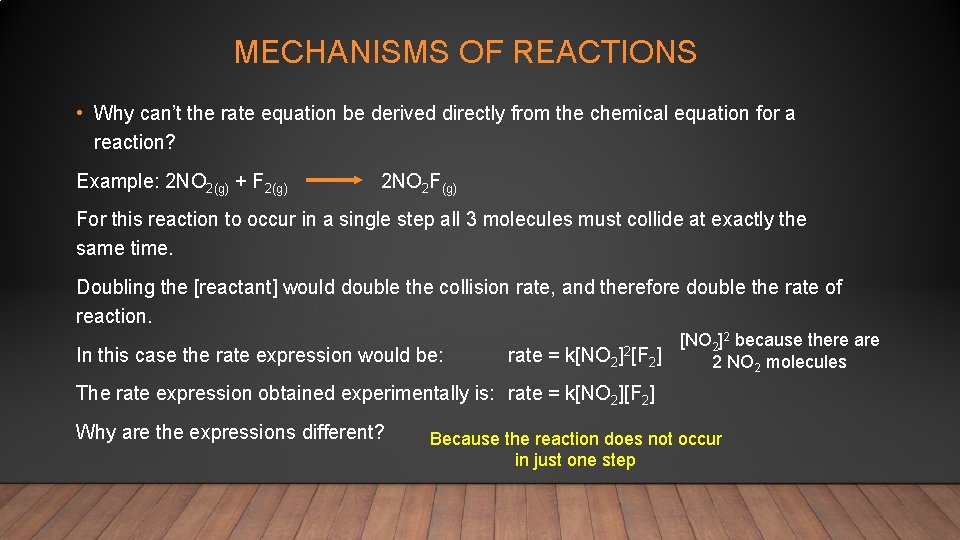
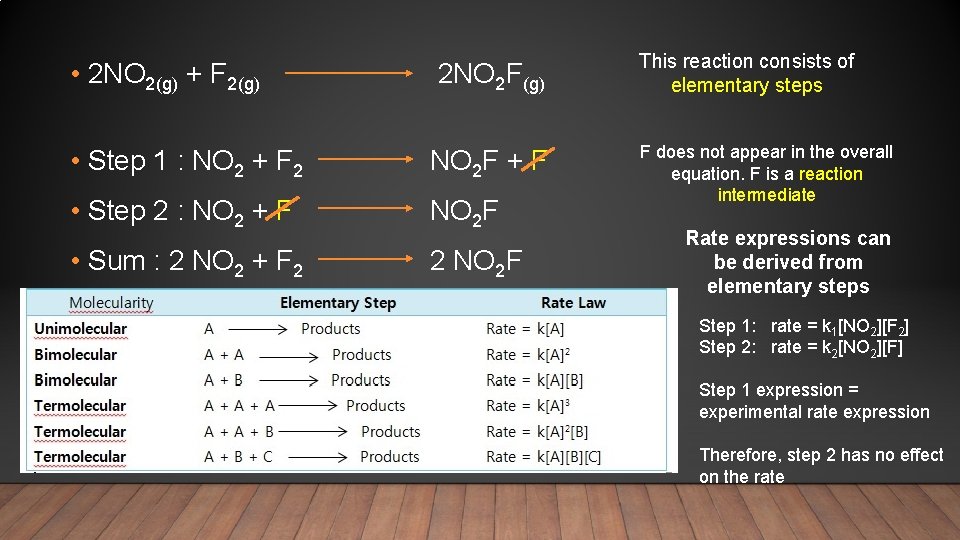
MECHANISMS OF REACTIONS • Why can’t the rate equation be derived directly from the chemical equation for a reaction? Example: 2 NO 2(g) + F 2(g) 2 NO 2 F(g) For this reaction to occur in a single step all 3 molecules must collide at exactly the same time. Doubling the [reactant] would double the collision rate, and therefore double the rate of reaction. In this case the rate expression would be: rate = k[NO 2 ]2[F [NO 2]2 because there are 2] 2 NO 2 molecules The rate expression obtained experimentally is: rate = k[NO 2][F 2] Why are the expressions different? Because the reaction does not occur in just one step

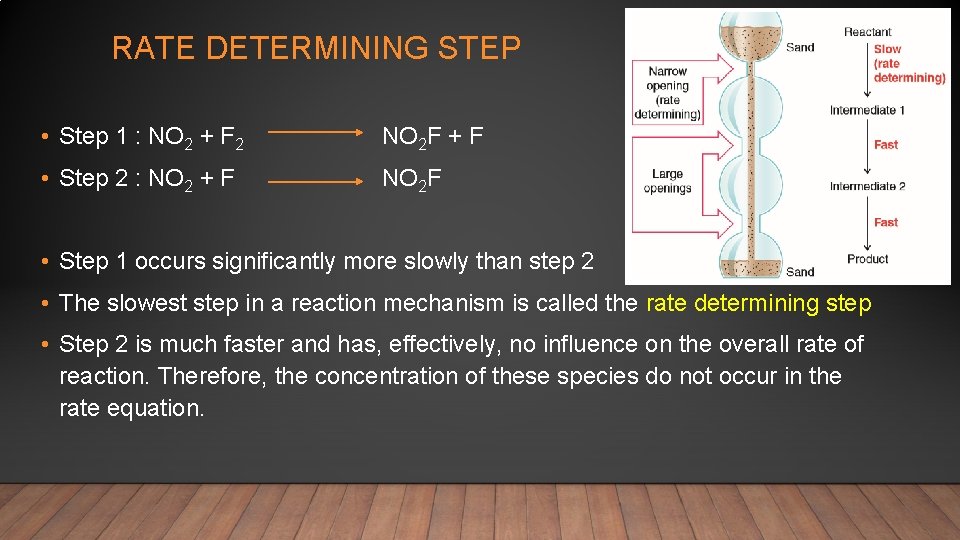
• 2 NO 2(g) + F 2(g) 2 NO 2 F(g) This reaction consists of elementary steps • Step 1 : NO 2 + F 2 NO 2 F + F • Step 2 : NO 2 + F NO 2 F F does not appear in the overall equation. F is a reaction intermediate • Sum : 2 NO 2 + F 2 2 NO 2 F Rate expressions can be derived from elementary steps Step 1: rate = k 1[NO 2][F 2] Step 2: rate = k 2[NO 2][F] Step 1 expression = experimental rate expression Therefore, step 2 has no effect on the rate

RATE DETERMINING STEP • Step 1 : NO 2 + F 2 NO 2 F + F • Step 2 : NO 2 + F NO 2 F • Step 1 occurs significantly more slowly than step 2 • The slowest step in a reaction mechanism is called the rate determining step • Step 2 is much faster and has, effectively, no influence on the overall rate of reaction. Therefore, the concentration of these species do not occur in the rate equation.

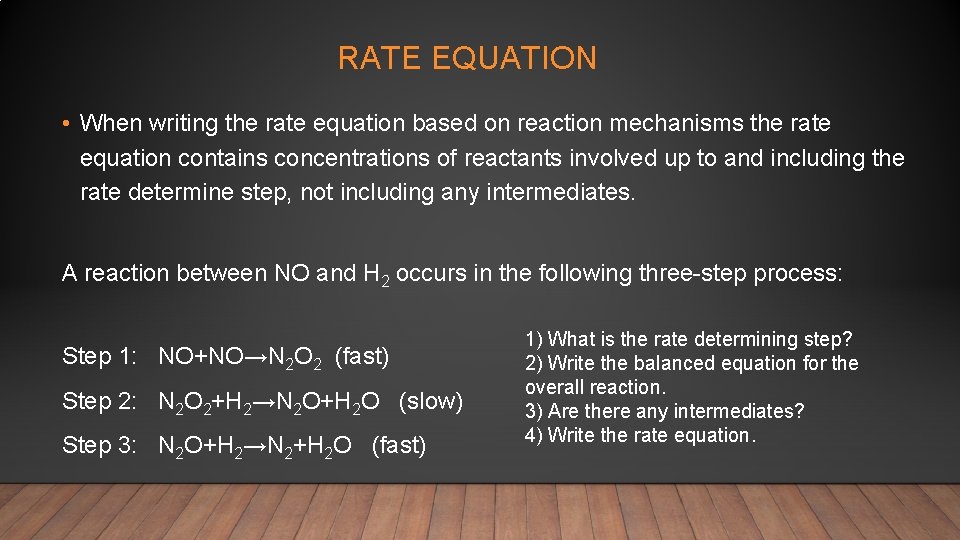
RATE EQUATION • When writing the rate equation based on reaction mechanisms the rate equation contains concentrations of reactants involved up to and including the rate determine step, not including any intermediates. A reaction between NO and H 2 occurs in the following three-step process: Step 1: NO+NO→N 2 O 2 (fast) Step 2: N 2 O 2+H 2→N 2 O+H 2 O (slow) Step 3: N 2 O+H 2→N 2+H 2 O (fast) 1) What is the rate determining step? 2) Write the balanced equation for the overall reaction. 3) Are there any intermediates? 4) Write the rate equation.

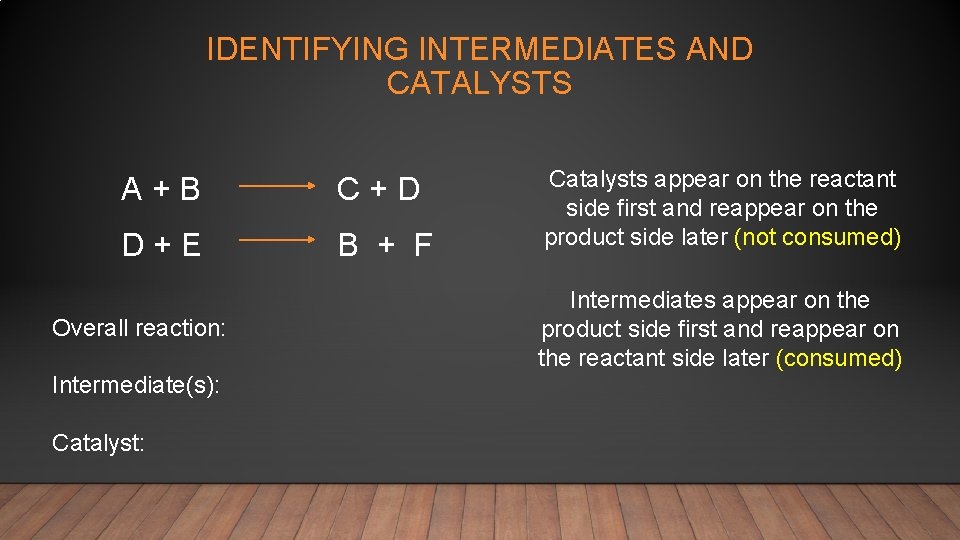
IDENTIFYING INTERMEDIATES AND CATALYSTS A + B C + D D + E B + F Overall reaction: Intermediate(s): Catalyst: Catalysts appear on the reactant side first and reappear on the product side later (not consumed) Intermediates appear on the product side first and reappear on the reactant side later (consumed)

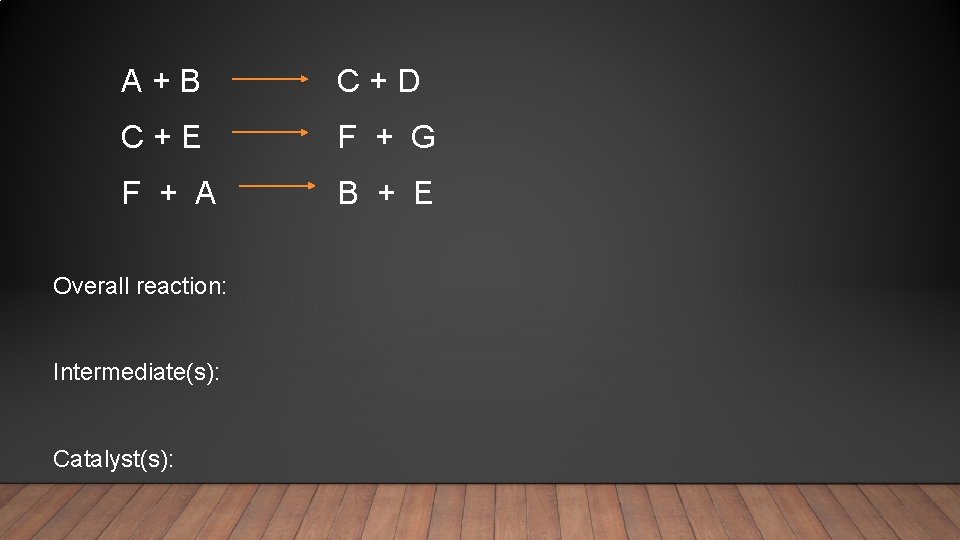
A + B C + D C + E F + G F + A B + E Overall reaction: Intermediate(s): Catalyst(s):
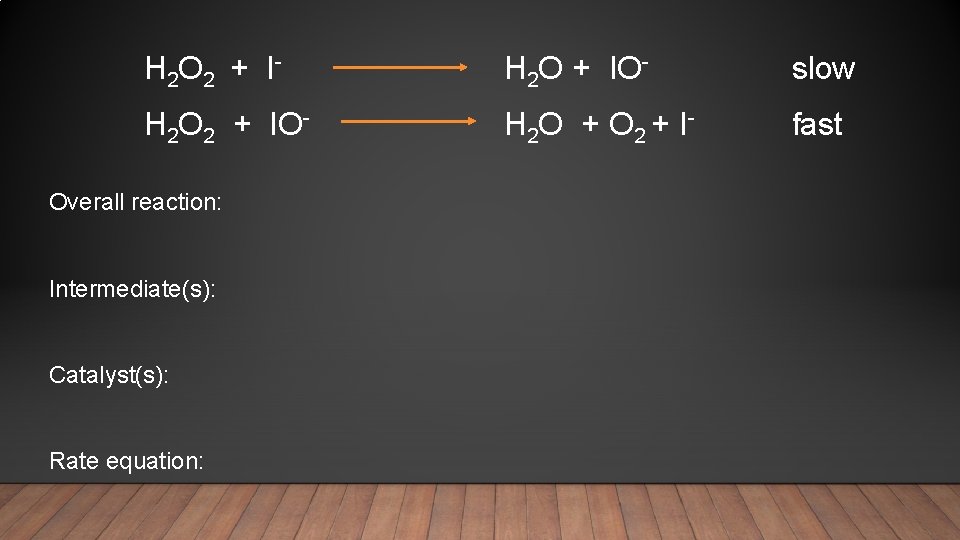
H 2 O 2 + I- H 2 O + IO- slow H 2 O 2 + IO- H 2 O + O 2 + I- fast Overall reaction: Intermediate(s): Catalyst(s): Rate equation:

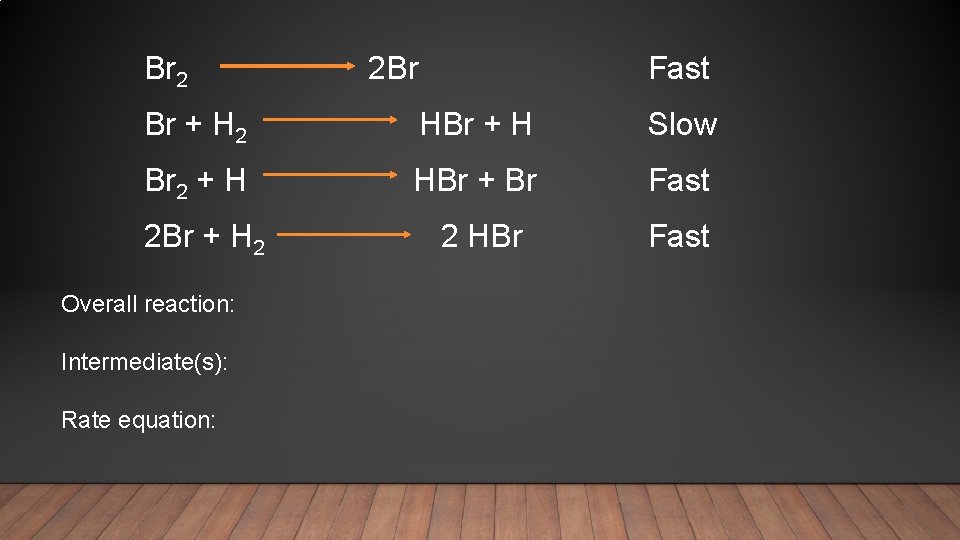
Br 2 2 Br Fast Br + H 2 HBr + H Slow Br 2 + H HBr + Br Fast 2 Br + H 2 2 HBr Overall reaction: Intermediate(s): Rate equation: Fast

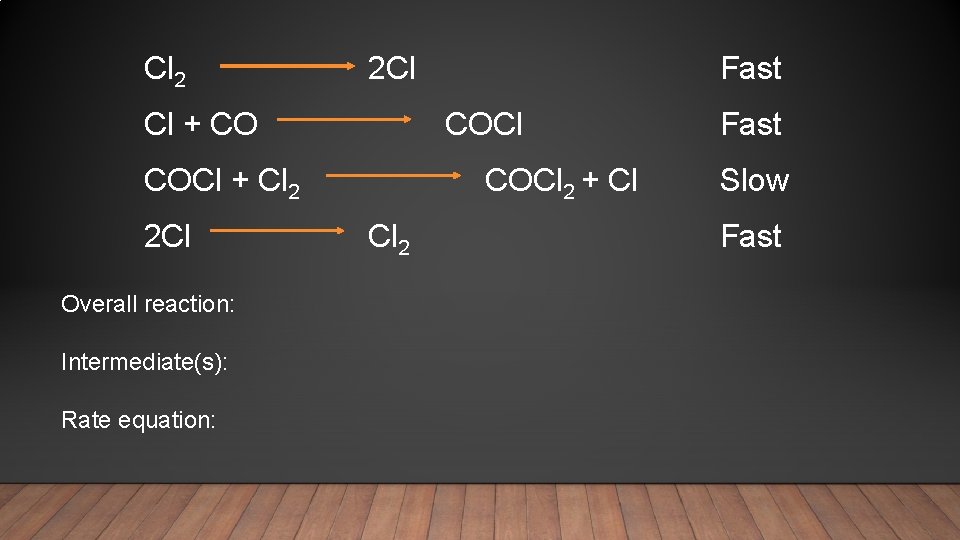
Cl 2 2 Cl Fast Cl + CO COCl Fast COCl + Cl 2 2 Cl Cl 2 Overall reaction: Intermediate(s): Rate equation: COCl 2 + Cl Slow Fast
 Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates Unit rate
Unit rate Ratios guided notes
Ratios guided notes Rate determining step
Rate determining step How to determine the rate determining step
How to determine the rate determining step Rate determining step
Rate determining step Reaction rate equation
Reaction rate equation Reaction rates
Reaction rates Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Reaction rates and equilibrium worksheet answers chapter 19
Reaction rates and equilibrium worksheet answers chapter 19 Reaction rate
Reaction rate Expressing reaction rates
Expressing reaction rates Section 4 reaction rates and equilibrium
Section 4 reaction rates and equilibrium Expressing reaction rates
Expressing reaction rates Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Contoh soal forward rate
Contoh soal forward rate What is the difference between rate and unit rate
What is the difference between rate and unit rate Cap rate interest rate relationship
Cap rate interest rate relationship Determination of exchange rate
Determination of exchange rate Nominal v. real interest rates
Nominal v. real interest rates Forward rate example
Forward rate example What is growth analysis
What is growth analysis Spot rate and forward rate
Spot rate and forward rate Determining customer needs
Determining customer needs Determining comparative advantage output method
Determining comparative advantage output method Constant
Constant Polymer molecular weight determination methods
Polymer molecular weight determination methods Chardakov
Chardakov Margin of safety ratio formula
Margin of safety ratio formula Determining how costs behave
Determining how costs behave Contemporary methods for determining system requirements
Contemporary methods for determining system requirements Determining ionization energy
Determining ionization energy Determining proportionality with tables
Determining proportionality with tables Determining human information requirements
Determining human information requirements Polarity ap chem
Polarity ap chem What is feasibility analysis in entrepreneurship
What is feasibility analysis in entrepreneurship Estimating parameters and determining sample sizes
Estimating parameters and determining sample sizes Problem 3-5 accounting answers
Problem 3-5 accounting answers Oxidation number rules
Oxidation number rules Depletion journal entry
Depletion journal entry How can ngt be used for requirements determination
How can ngt be used for requirements determination A good layout requires determining
A good layout requires determining Empirical formula of copper chloride lab
Empirical formula of copper chloride lab Pearson education south asia pte ltd
Pearson education south asia pte ltd How many scales are there
How many scales are there Traditional methods for determining requirements
Traditional methods for determining requirements Define limiting reactant
Define limiting reactant Determining optimal capital structure
Determining optimal capital structure How to find horizontal asymptotes
How to find horizontal asymptotes Calculating time of death using algor mortis
Calculating time of death using algor mortis Claims of value examples
Claims of value examples Determining importance
Determining importance Determining system requirements
Determining system requirements P and s wave chart
P and s wave chart Hittorf law
Hittorf law Methods for determining time of death
Methods for determining time of death Determining height from bone length
Determining height from bone length