Chemical Kinetics How rapidly reactions proceed rate of




![NO 2(g) + CO(g) NO(g) + CO 2 (g) rate = D[NO] D[CO 2] NO 2(g) + CO(g) NO(g) + CO 2 (g) rate = D[NO] D[CO 2]](https://slidetodoc.com/presentation_image_h/20ab02826bb354ccb9b8b7a95aaa6a82/image-12.jpg)
![Rate = - d[N 2 O 5] / dt = k [N 2 O Rate = - d[N 2 O 5] / dt = k [N 2 O](https://slidetodoc.com/presentation_image_h/20ab02826bb354ccb9b8b7a95aaa6a82/image-19.jpg)
![2 NO 2(g) 2 NO(g) + O 2 (g) Rate = k [NO 2]2 2 NO 2(g) 2 NO(g) + O 2 (g) Rate = k [NO 2]2](https://slidetodoc.com/presentation_image_h/20ab02826bb354ccb9b8b7a95aaa6a82/image-20.jpg)

![Order of a Reaction rate = k [A]m [B]n Reaction is mth order in Order of a Reaction rate = k [A]m [B]n Reaction is mth order in](https://slidetodoc.com/presentation_image_h/20ab02826bb354ccb9b8b7a95aaa6a82/image-23.jpg)
![C 2 H 6(g) 2 CH 3(g) rate = k [C 2 H 6]2 C 2 H 6(g) 2 CH 3(g) rate = k [C 2 H 6]2](https://slidetodoc.com/presentation_image_h/20ab02826bb354ccb9b8b7a95aaa6a82/image-24.jpg)

- Slides: 26

Chemical Kinetics How rapidly reactions proceed - rate of reaction Details of process from reactants to products - mechanism Thermodynamics determines the direction in which reactions proceed spontaneously and the conditions at equilibrium, but not the rate at which equilibrium is reached. For a complete picture of a chemical reaction need both thermodynamics and kinetics of a reaction.


N 2(g) + 3 H 2(g) 2 NH 3(g) At 298 K DGo = -33. 0 k. J DHo = -92 k. J; exothermic reaction K = 6. 0 x 105 ; thermodynamically favored at 298 K, Rate is slow at 298 K Commercial production of NH 3 is carried out at temperatures of 800 to 900 K, because the rate is faster even though K is smaller.

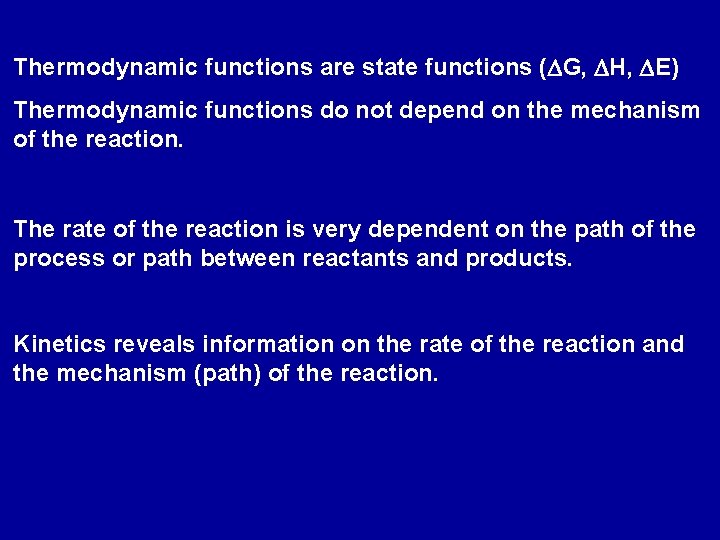
Thermodynamic functions are state functions (DG, DH, DE) Thermodynamic functions do not depend on the mechanism of the reaction. The rate of the reaction is very dependent on the path of the process or path between reactants and products. Kinetics reveals information on the rate of the reaction and the mechanism (path) of the reaction.

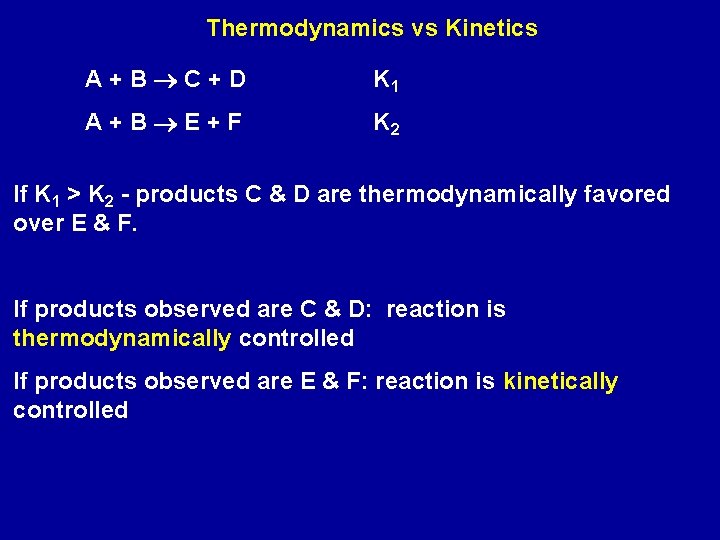
Thermodynamics vs Kinetics A+B C+D K 1 A+B E+F K 2 If K 1 > K 2 - products C & D are thermodynamically favored over E & F. If products observed are C & D: reaction is thermodynamically controlled If products observed are E & F: reaction is kinetically controlled

Zn 2+(aq) + S 2 -(aq) Zn. S(s) K = 1/Ksp = 2. 2 x 1023 Fe 2+(aq) + S 2 -(aq) Fe. S(s) K = 1/Ksp = 2. 7 x 1018 On addition of S 2 - to an aqueous solution containing both Zn 2+ and Fe 2+, Zn. S precipitates first - reaction is thermodynamically controlled.

(1) 2 NO(g) + O 2(g) 2 NO 2(g) (2) 2 CO(g) + O 2(g) 2 CO 2(g) Both have large values of K; both are thermodynamically favored in the forward direction Reaction (1) is very fast; reaction (2) slow Reactions are kinetically controlled Result is brown color of air due NO 2 and buildup of CO in the air

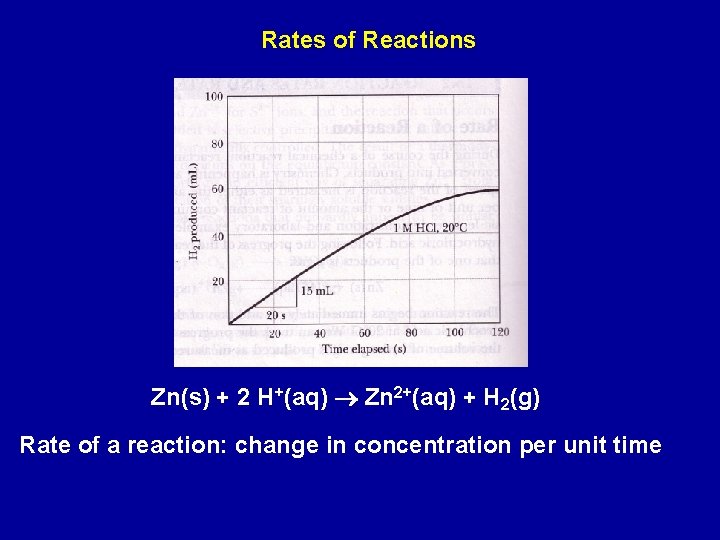
Rates of Reactions Zn(s) + 2 H+(aq) Zn 2+(aq) + H 2(g) Rate of a reaction: change in concentration per unit time

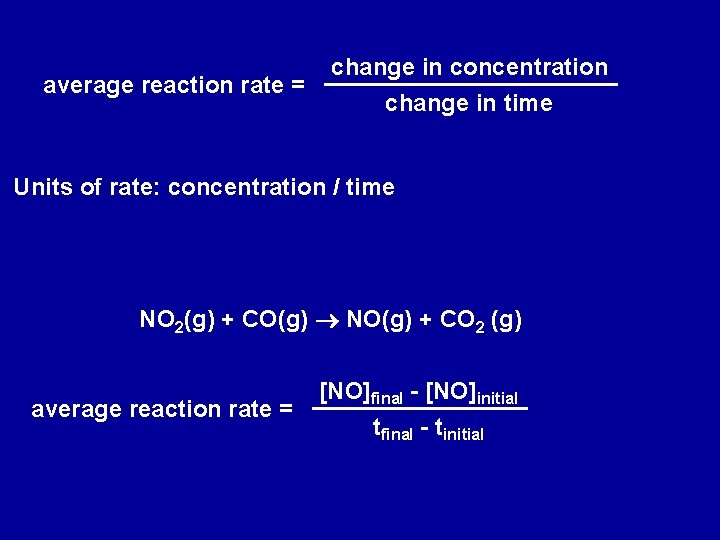
average reaction rate = change in concentration change in time Units of rate: concentration / time NO 2(g) + CO(g) NO(g) + CO 2 (g) average reaction rate = [NO]final - [NO]initial tfinal - tinitial

NO 2(g) + CO(g) NO(g) + CO 2 (g) Time (s) 0 50 100 150 200 [NO] mol L-1 0 0. 0160 0. 0240 0. 0288 0. 0320 Average rate 1 st 50 seconds = 3. 2 x 10 -4 mol L-1 Average rate 2 nd 50 seconds = 1. 6 x 10 -4 mol L-1 Average rate 3 rd 50 seconds = 9. 6 x 10 -5 mol L-1

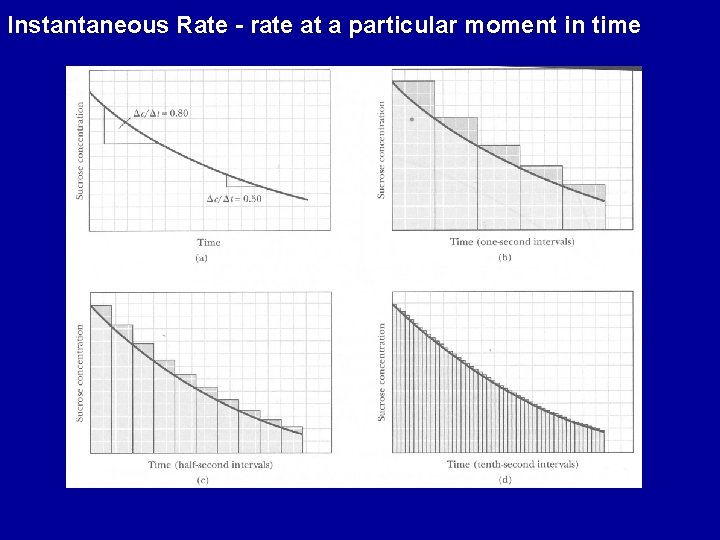
Instantaneous Rate - rate at a particular moment in time
![NO 2g COg NOg CO 2 g rate DNO DCO 2 NO 2(g) + CO(g) NO(g) + CO 2 (g) rate = D[NO] D[CO 2]](https://slidetodoc.com/presentation_image_h/20ab02826bb354ccb9b8b7a95aaa6a82/image-12.jpg)
NO 2(g) + CO(g) NO(g) + CO 2 (g) rate = D[NO] D[CO 2] - D[NO 2] - D[CO] = = = Dt Dt For an infinitesimally small changes, the instantaneous rate = d[NO] d[CO 2] d[NO 2] - d[CO] = dt dt For a general reaction: a. A + b. B x. C + y. D 1 d[D] = 1 d[C] rate = = y dt x dt - 1 d[A] = a dt 1 d[B] b dt

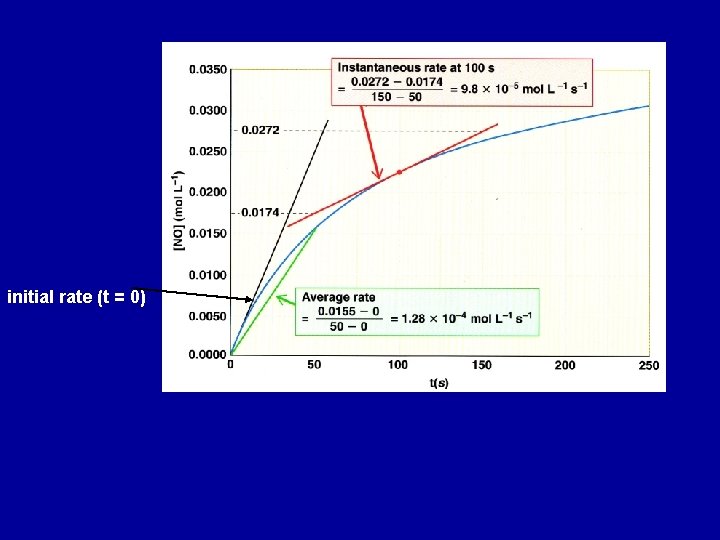
initial rate (t = 0)

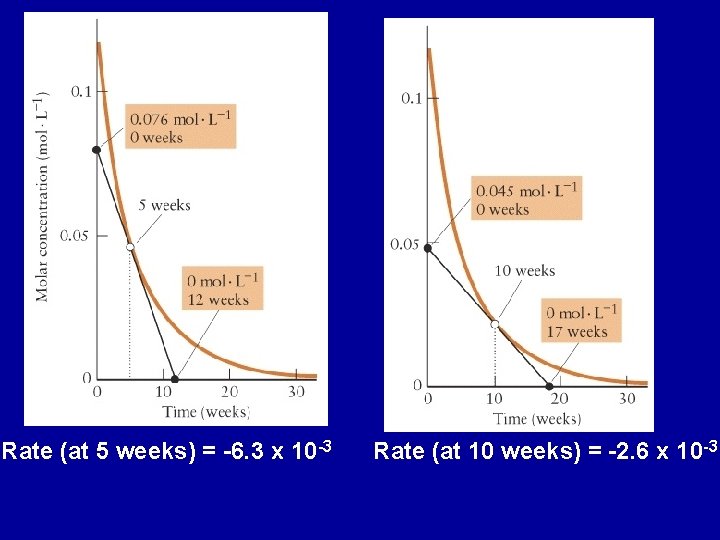
Rate (at 5 weeks) = -6. 3 x 10 -3 Rate (at 10 weeks) = -2. 6 x 10 -3

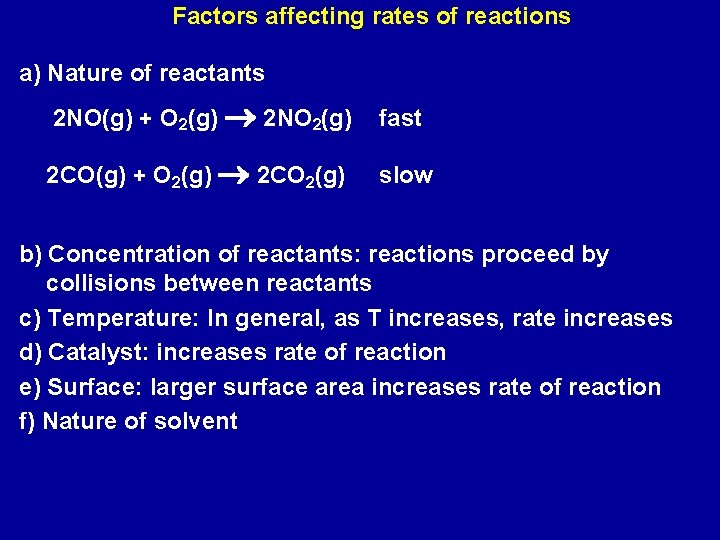
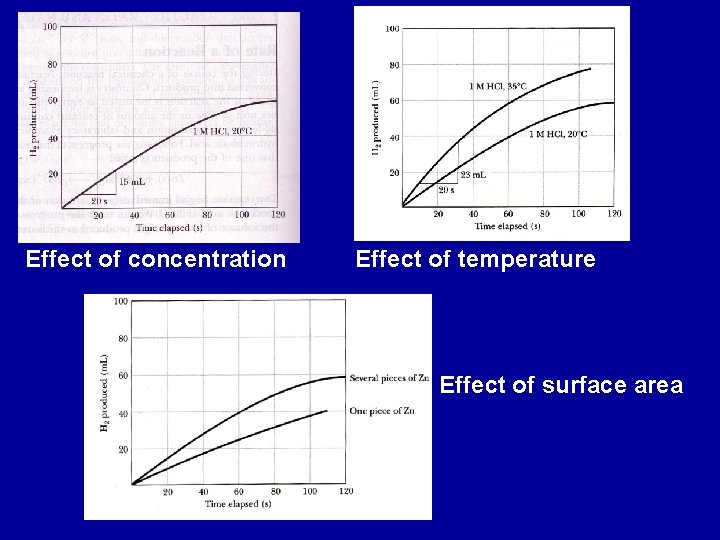
Factors affecting rates of reactions a) Nature of reactants 2 NO(g) + O 2(g) 2 NO 2(g) fast 2 CO(g) + O 2(g) 2 CO 2(g) slow b) Concentration of reactants: reactions proceed by collisions between reactants c) Temperature: In general, as T increases, rate increases d) Catalyst: increases rate of reaction e) Surface: larger surface area increases rate of reaction f) Nature of solvent

Effect of concentration Effect of temperature Effect of surface area

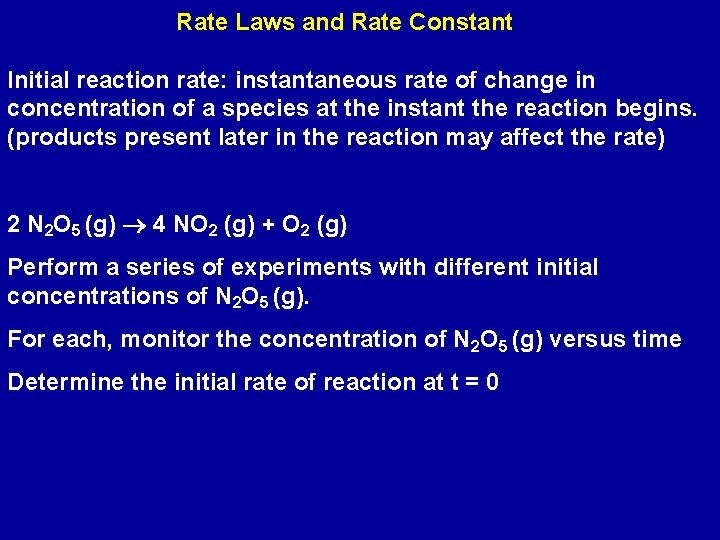
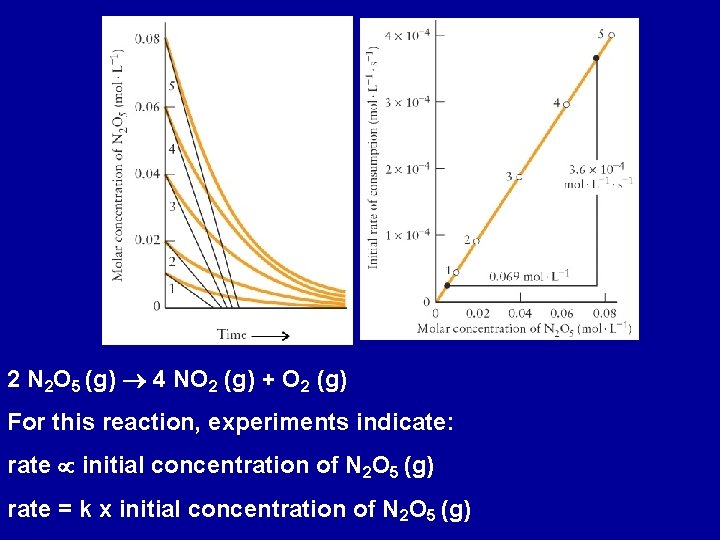
Rate Laws and Rate Constant Initial reaction rate: instantaneous rate of change in concentration of a species at the instant the reaction begins. (products present later in the reaction may affect the rate) 2 N 2 O 5 (g) 4 NO 2 (g) + O 2 (g) Perform a series of experiments with different initial concentrations of N 2 O 5 (g). For each, monitor the concentration of N 2 O 5 (g) versus time Determine the initial rate of reaction at t = 0

2 N 2 O 5 (g) 4 NO 2 (g) + O 2 (g) For this reaction, experiments indicate: rate initial concentration of N 2 O 5 (g) rate = k x initial concentration of N 2 O 5 (g)
![Rate dN 2 O 5 dt k N 2 O Rate = - d[N 2 O 5] / dt = k [N 2 O](https://slidetodoc.com/presentation_image_h/20ab02826bb354ccb9b8b7a95aaa6a82/image-19.jpg)
Rate = - d[N 2 O 5] / dt = k [N 2 O 5] Rate law k = 5. 2 x 10 -3 s-1 k is the specific rate constant Units of k depends on the rate law Rate laws are determined experimentally 2 NO 2(g) 2 NO(g) + O 2 (g) d[NO 2] 2 rate = = k [NO ] 2 dt k = 0. 54 (mol NO 2)-1 s-1 Rate Law
![2 NO 2g 2 NOg O 2 g Rate k NO 22 2 NO 2(g) 2 NO(g) + O 2 (g) Rate = k [NO 2]2](https://slidetodoc.com/presentation_image_h/20ab02826bb354ccb9b8b7a95aaa6a82/image-20.jpg)
2 NO 2(g) 2 NO(g) + O 2 (g) Rate = k [NO 2]2

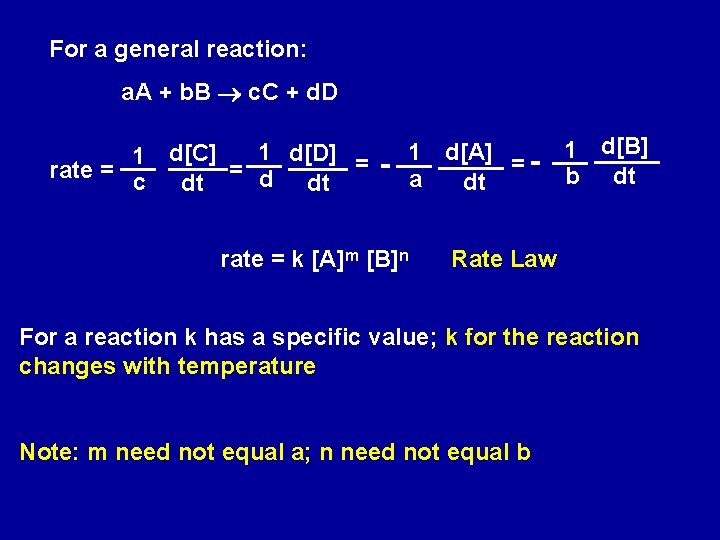
For a general reaction: a. A + b. B c. C + d. D 1 d[D] = 1 d[C] rate = = d c dt dt - 1 d[A] = a dt rate = k [A]m [B]n 1 d[B] b dt Rate Law For a reaction k has a specific value; k for the reaction changes with temperature Note: m need not equal a; n need not equal b

![Order of a Reaction rate k Am Bn Reaction is mth order in Order of a Reaction rate = k [A]m [B]n Reaction is mth order in](https://slidetodoc.com/presentation_image_h/20ab02826bb354ccb9b8b7a95aaa6a82/image-23.jpg)
Order of a Reaction rate = k [A]m [B]n Reaction is mth order in A and nth order in B Overall reaction order = m + n The reaction order is determined by the experimentally determined rate law N 2 O 5(g) N 2 O 4(g) + 1/2 O 2(g) Rate = k [N 2 O 5] First order reaction For a 1 st order reaction, units of k: time-1
![C 2 H 6g 2 CH 3g rate k C 2 H 62 C 2 H 6(g) 2 CH 3(g) rate = k [C 2 H 6]2](https://slidetodoc.com/presentation_image_h/20ab02826bb354ccb9b8b7a95aaa6a82/image-24.jpg)
C 2 H 6(g) 2 CH 3(g) rate = k [C 2 H 6]2 second order reaction 2 NO 2(g) 2 NO(g) + O 2 (g) Rate = k [NO 2]2 second order reaction For 2 nd order reactions, units of k: concentration-1 time-1

S 2 O 82 -(aq) + 3 I-(aq) 2 SO 42 -(aq) + I 3 -(aq) (S 2 O 82 - persulfate) Rate of disappearance of S 2 O 82 - = k [S 2 O 82 -(aq)] [I-(aq)] Overall reaction order = 1 + 1 = 2 1 st order in S 2 O 82 -(aq) and 1 st order in I-(aq) If the above reaction was carried out at a high concentration S 2 O 82 -(aq), and experimentally: Rate of disappearance of S 2 O 82 - = k’ [I-(aq)] pseudo first-order reaction

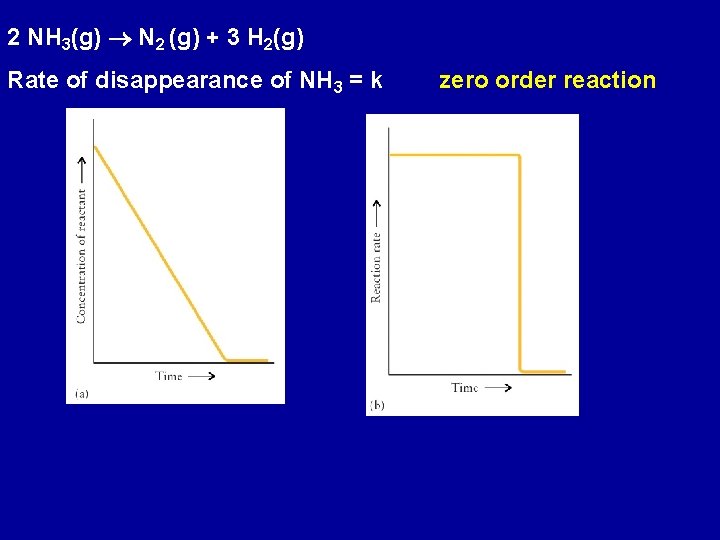
2 NH 3(g) N 2 (g) + 3 H 2(g) Rate of disappearance of NH 3 = k zero order reaction
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Section 1 chemical changes
Section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Molecularity of reaction
Molecularity of reaction Chemistry grade 11 unit 4 chemical kinetics
Chemistry grade 11 unit 4 chemical kinetics Kinetics half life
Kinetics half life Chemical kinetics definition
Chemical kinetics definition Chemical kinetics experiment
Chemical kinetics experiment Applications of chemical kinetics
Applications of chemical kinetics Steady state kinetics
Steady state kinetics Redox half reactions
Redox half reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Precede proceed model lawrence green
Precede proceed model lawrence green Precede proceed model example childhood obesity
Precede proceed model example childhood obesity Precede proceed model example
Precede proceed model example Beogram 1000 historie
Beogram 1000 historie Before we proceed further
Before we proceed further Diagnosis perilaku adalah
Diagnosis perilaku adalah Applications of trigonometry in navigation
Applications of trigonometry in navigation Precede-proceed model
Precede-proceed model From an iron post proceed 500 m northeast
From an iron post proceed 500 m northeast Let us proceed
Let us proceed Factors that affect the rate of reactions
Factors that affect the rate of reactions Stoichiometry island diagram
Stoichiometry island diagram Unit 5 chemical reactions
Unit 5 chemical reactions