Chemical Kinetics Reaction Rates Reaction Rate How fast

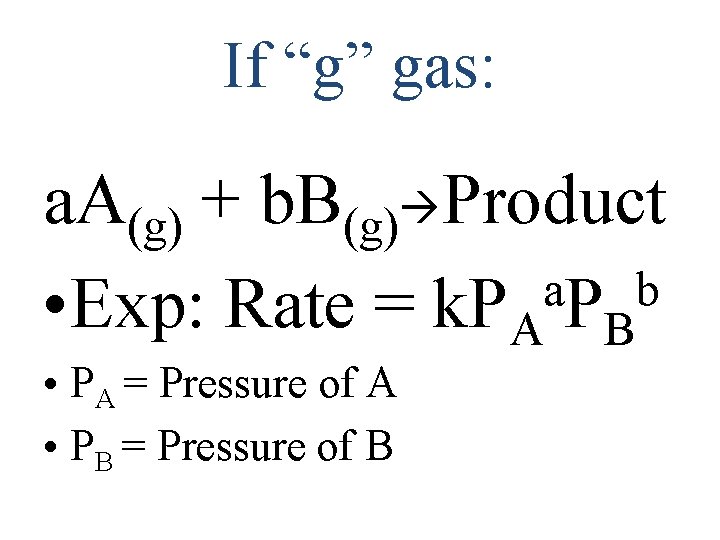

![Think about it. . Rate = [A][B]2 If the initial concentration of B is Think about it. . Rate = [A][B]2 If the initial concentration of B is](https://slidetodoc.com/presentation_image/0374c710da8d37ea1350387dba0a3663/image-10.jpg)

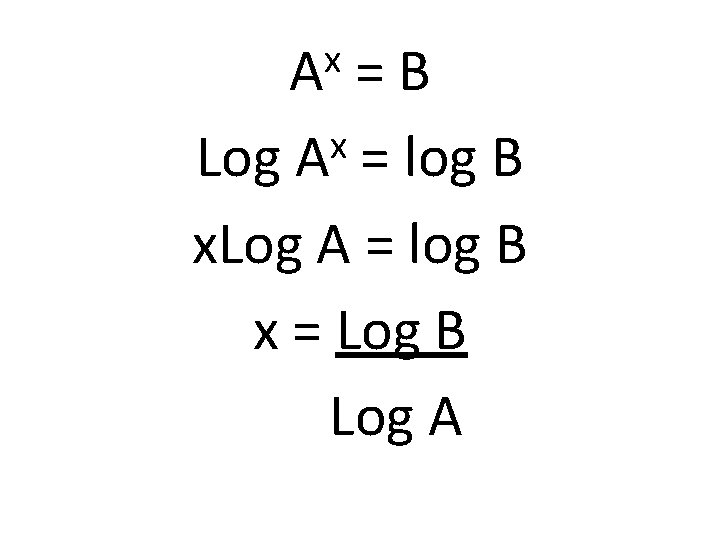

![Experiment [Br. O 3 -] (M) [Br-] (M) [H+] (M) Initial Rate (M/s) 1 Experiment [Br. O 3 -] (M) [Br-] (M) [H+] (M) Initial Rate (M/s) 1](https://slidetodoc.com/presentation_image/0374c710da8d37ea1350387dba0a3663/image-16.jpg)


- Slides: 26

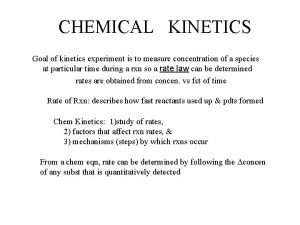
Chemical Kinetics/ Reaction Rates

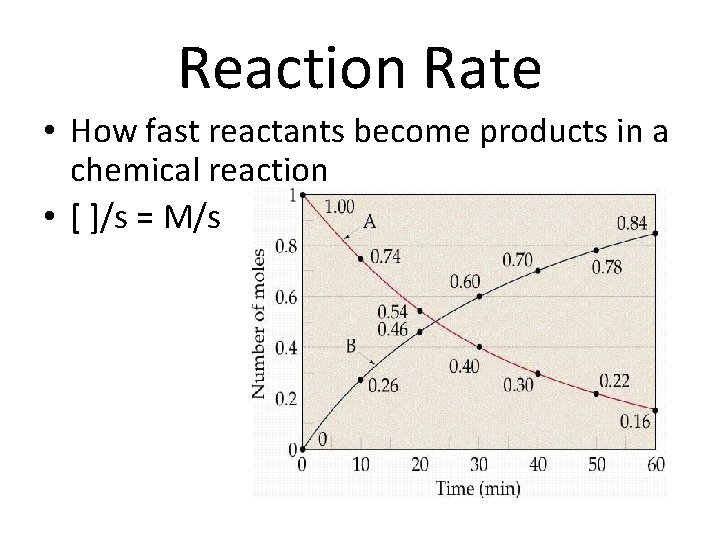
Reaction Rate • How fast reactants become products in a chemical reaction • [ ]/s = M/s

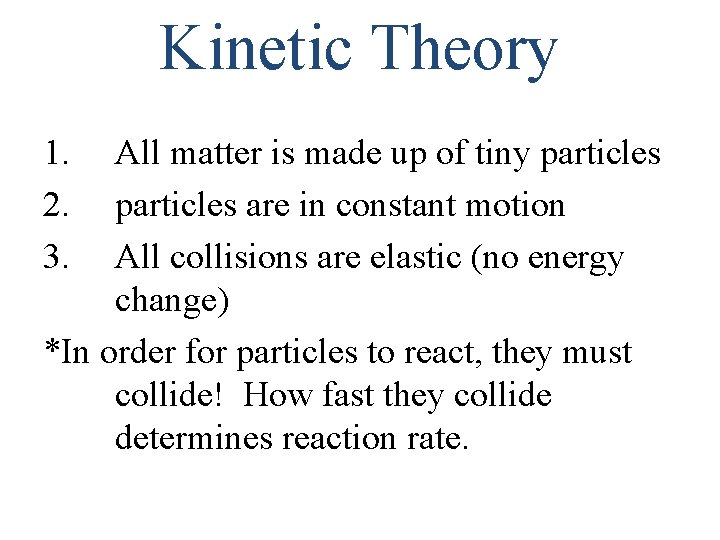
Kinetic Theory 1. 2. 3. All matter is made up of tiny particles are in constant motion All collisions are elastic (no energy change) *In order for particles to react, they must collide! How fast they collide determines reaction rate.

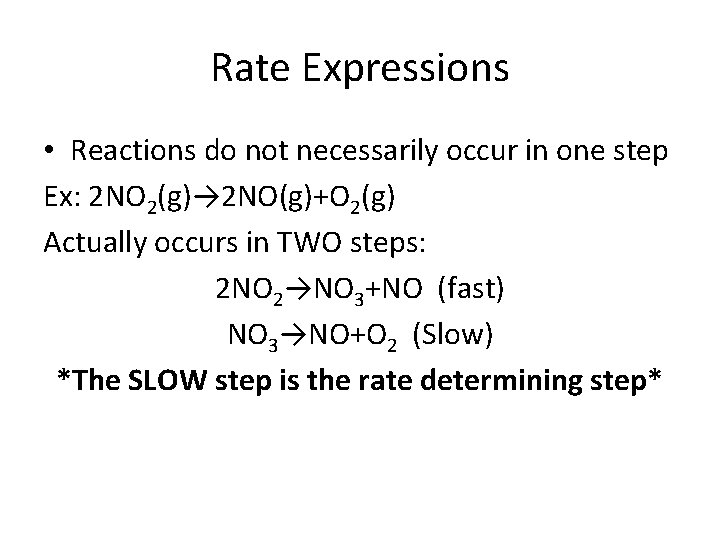
Rate Expressions • Reactions do not necessarily occur in one step Ex: 2 NO 2(g)→ 2 NO(g)+O 2(g) Actually occurs in TWO steps: 2 NO 2→NO 3+NO (fast) NO 3→NO+O 2 (Slow) *The SLOW step is the rate determining step*

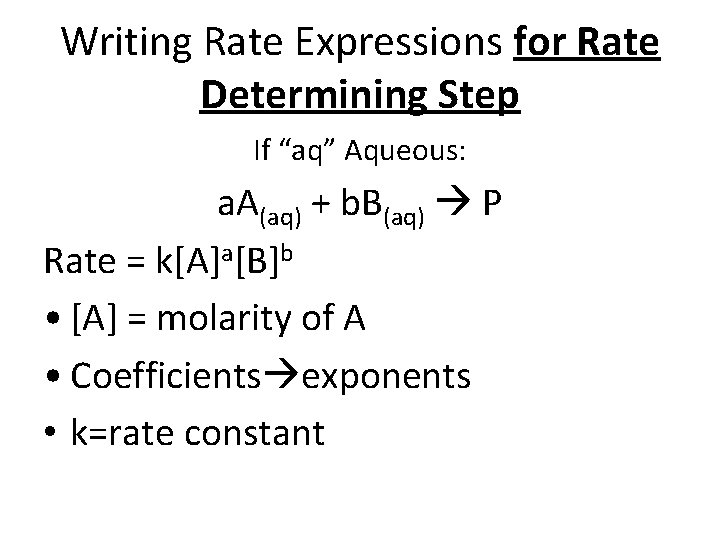
Writing Rate Expressions for Rate Determining Step If “aq” Aqueous: a. A(aq) + b. B(aq) P Rate = k[A]a[B]b • [A] = molarity of A • Coefficients exponents • k=rate constant
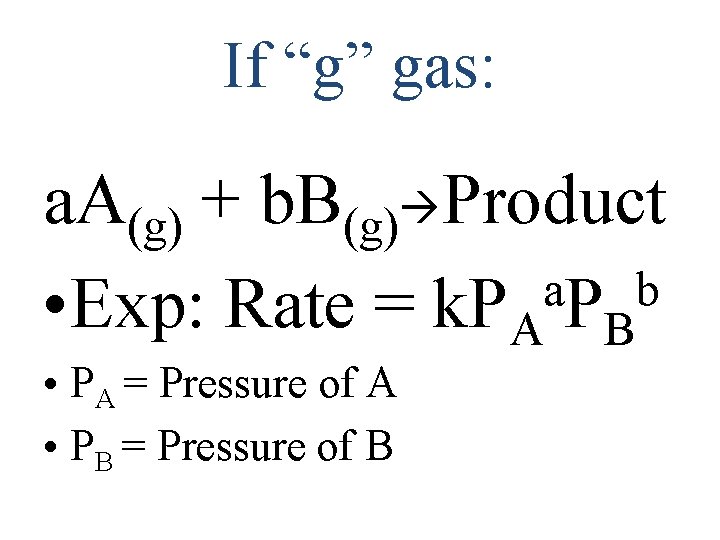
If “g” gas: a. A(g) + b. B(g) Product a b • Exp: Rate = k. PA PB • PA = Pressure of A • PB = Pressure of B

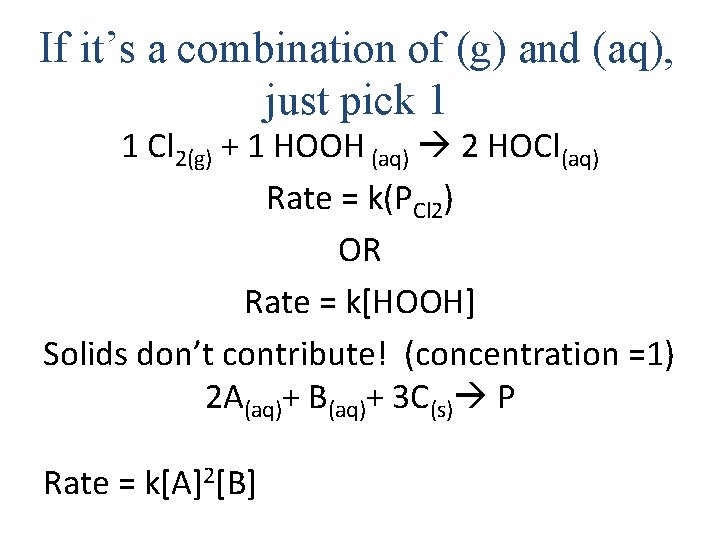
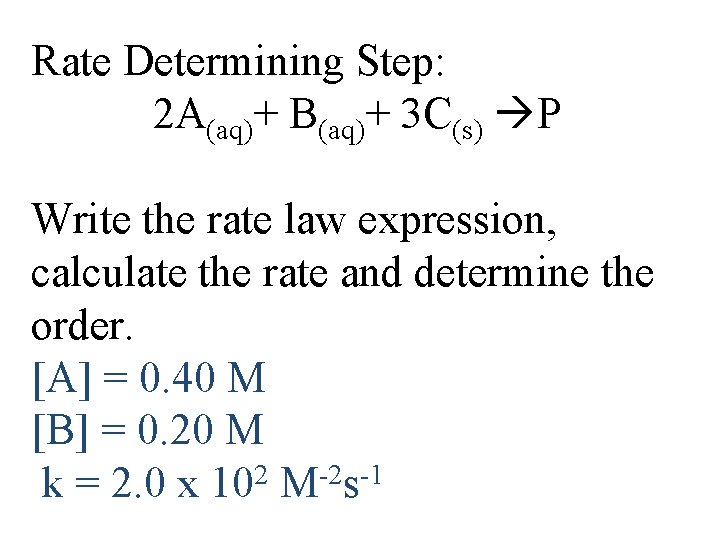
If it’s a combination of (g) and (aq), just pick 1 1 Cl 2(g) + 1 HOOH (aq) 2 HOCl(aq) Rate = k(PCl 2) OR Rate = k[HOOH] Solids don’t contribute! (concentration =1) 2 A(aq)+ B(aq)+ 3 C(s) P Rate = k[A]2[B]

Reaction Order • Reaction Order = total exponents in rate expression (don’t forget 1!) Ex. Rate = k[A][B]2 Reaction Order = 3

Rate Determining Step: 2 A(aq)+ B(aq)+ 3 C(s) P Write the rate law expression, calculate the rate and determine the order. [A] = 0. 40 M [B] = 0. 20 M k = 2. 0 x 102 M-2 s-1
![Think about it Rate AB2 If the initial concentration of B is Think about it. . Rate = [A][B]2 If the initial concentration of B is](https://slidetodoc.com/presentation_image/0374c710da8d37ea1350387dba0a3663/image-10.jpg)
Think about it. . Rate = [A][B]2 If the initial concentration of B is increased from 0. 1 M to 0. 3 M, by what factor will the rate increase?

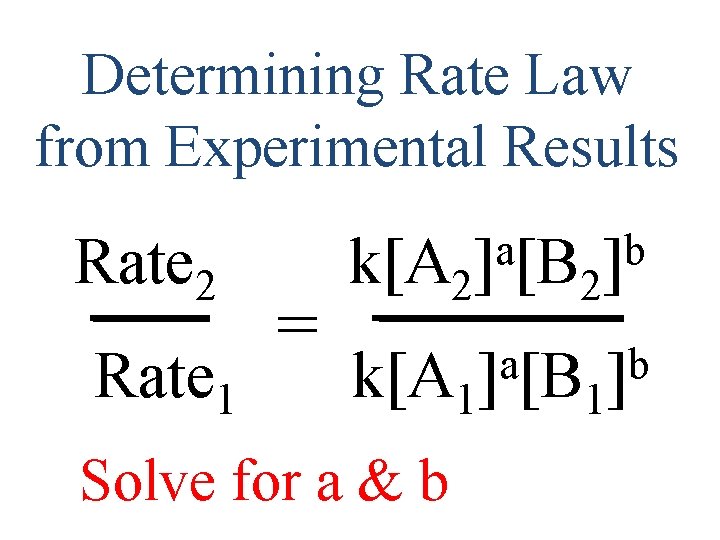
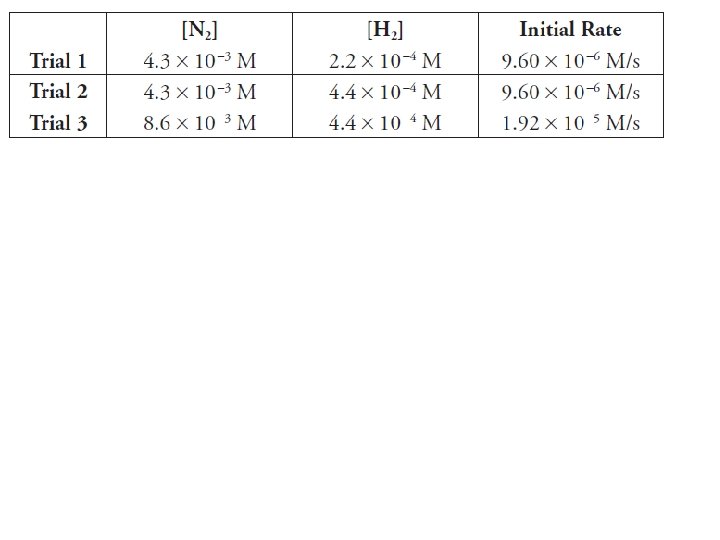
Determining Rate Law from Experimental Results Rate 2 Rate 1 = a b k[A 2] [B 2] a b k[A 1] [B 1] Solve for a & b

How do you solve for x if Ax=B?
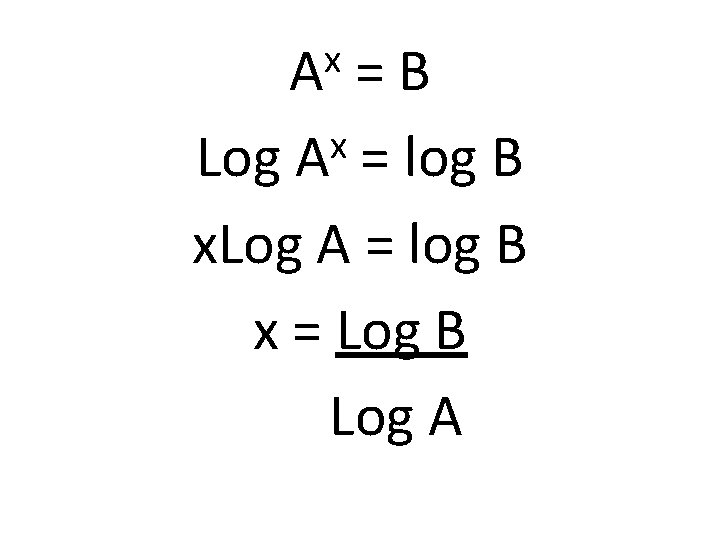
x A =B x Log A = log B x = Log B Log A

Once you have determined rate law, you can use one of the trials to solve for k!

![Experiment Br O 3 M Br M H M Initial Rate Ms 1 Experiment [Br. O 3 -] (M) [Br-] (M) [H+] (M) Initial Rate (M/s) 1](https://slidetodoc.com/presentation_image/0374c710da8d37ea1350387dba0a3663/image-16.jpg)
Experiment [Br. O 3 -] (M) [Br-] (M) [H+] (M) Initial Rate (M/s) 1 0. 10 8. 0 x 10 -4 2 3 4 0. 20 0. 10 0. 20 1. 6 x 10 -3 3. 2 x 10 -3

VIDEO • https: //www. youtube. com/watch? v=Ott. RV 5 yk. P 7 A

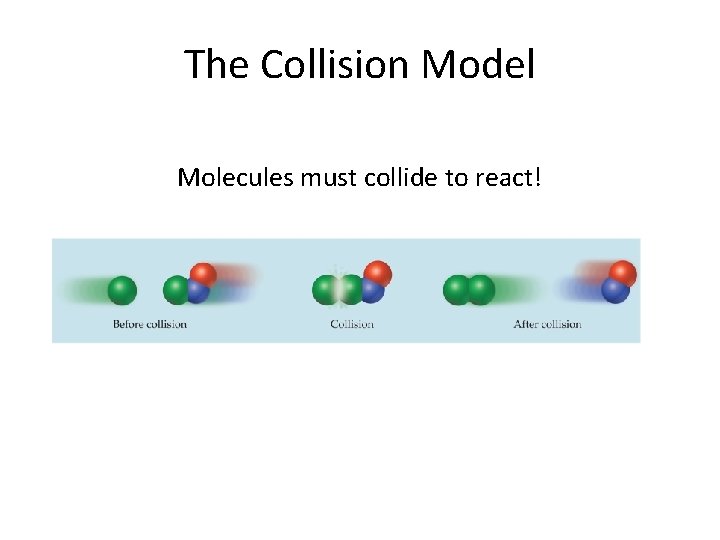
The Collision Model Molecules must collide to react!

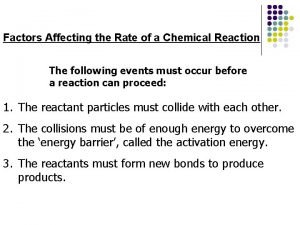
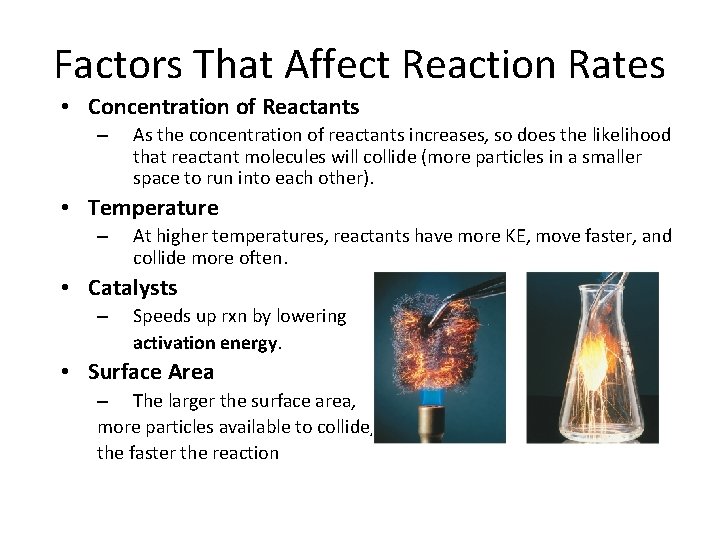
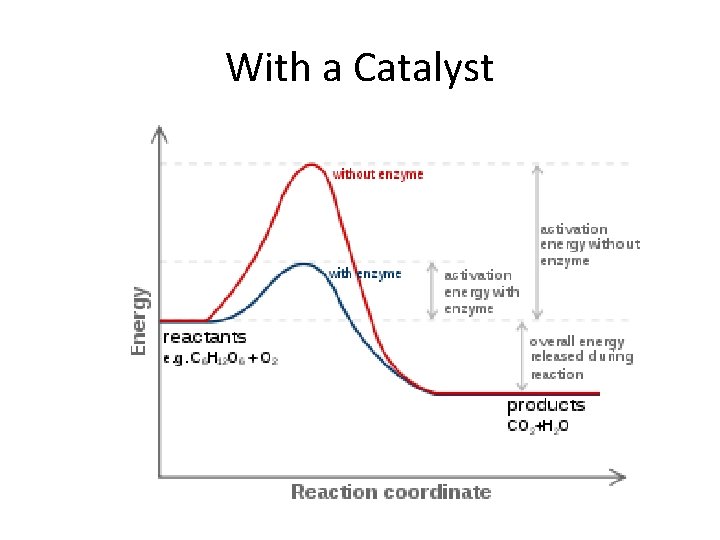
Factors That Affect Reaction Rates • Concentration of Reactants – As the concentration of reactants increases, so does the likelihood that reactant molecules will collide (more particles in a smaller space to run into each other). • Temperature – At higher temperatures, reactants have more KE, move faster, and collide more often. • Catalysts – Speeds up rxn by lowering activation energy. • Surface Area – The larger the surface area, more particles available to collide, the faster the reaction

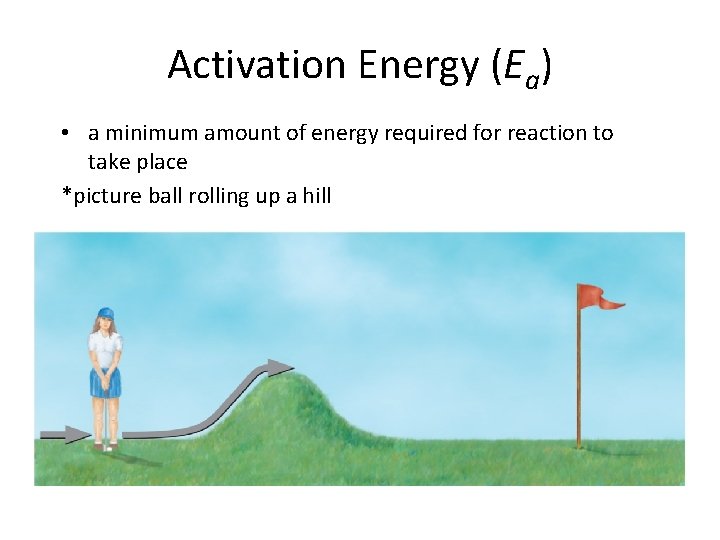
Activation Energy (Ea) • a minimum amount of energy required for reaction to take place *picture ball rolling up a hill

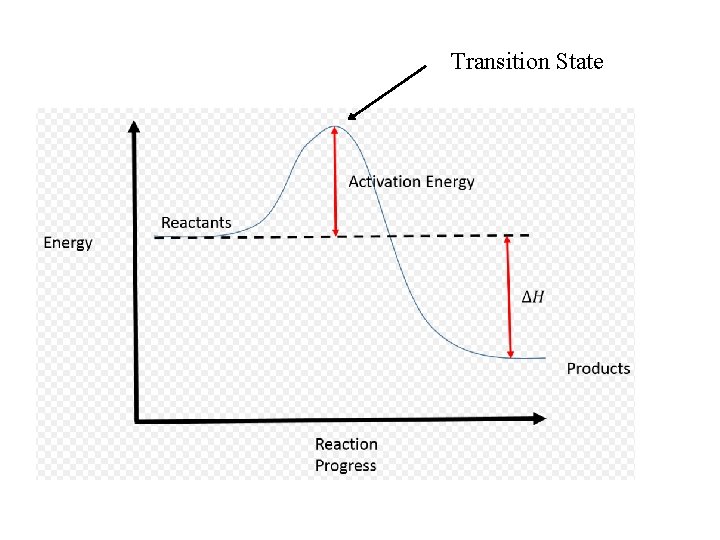
Transition State

With a Catalyst

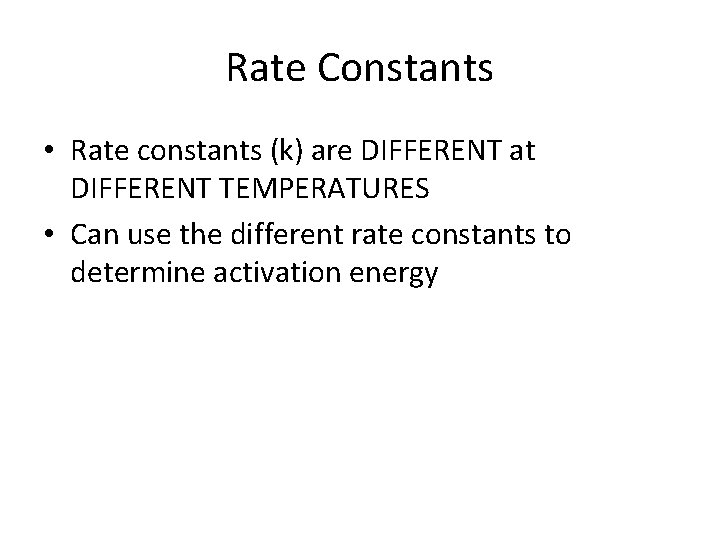
Rate Constants • Rate constants (k) are DIFFERENT at DIFFERENT TEMPERATURES • Can use the different rate constants to determine activation energy

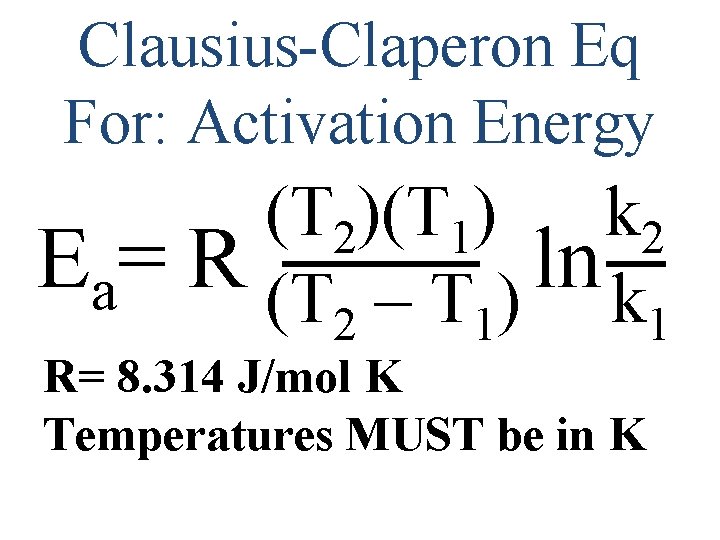
Clausius-Claperon Eq For: Activation Energy (T 2)(T 1) k 2 Ea= R (T – T ) ln k 2 1 1 R= 8. 314 J/mol K Temperatures MUST be in K

Calculate the activation energy of a reaction whose rate constant is 2. 0 x 103 M-2 S-1 at 27 o. C and is 2. 0 x 106 M-2 S-1 at 77 o. C:

Examples
 Is a ratio a rate
Is a ratio a rate Proportions guided notes
Proportions guided notes Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates Molecularity
Molecularity Chemistry grade 11 unit 4
Chemistry grade 11 unit 4 Chemical kinetics half life
Chemical kinetics half life Chemical kinetics definition
Chemical kinetics definition Chemical kinetics experiment
Chemical kinetics experiment Applications of chemical kinetics
Applications of chemical kinetics Steady state kinetics
Steady state kinetics Kinetics reaction
Kinetics reaction What factors influence the rate of a chemical reaction
What factors influence the rate of a chemical reaction Equation for rate of reaction
Equation for rate of reaction Half life formula
Half life formula Acid fast vs non acid fast
Acid fast vs non acid fast Example of acid-fast bacteria
Example of acid-fast bacteria Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Reaction rates and equilibrium worksheet answers chapter 19
Reaction rates and equilibrium worksheet answers chapter 19 Section 4 reaction rates and equilibrium
Section 4 reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Expressing reaction rates
Expressing reaction rates Rate of reaction quiz
Rate of reaction quiz Expressing reaction rates
Expressing reaction rates Did a chemical reaction occur
Did a chemical reaction occur